Non-alcoholic fatty liver disease (NAFLD) is becoming a major cause of liver disease-related morbidity, as well as mortality. Importantly, NAFLD is considered a mediator of systemic diseases including cardiovascular disease. Its prevalence is expected to increase, mainly due to its close association with obesity and type 2 diabetes mellitus (T2D). In addition, T2D and NAFLD share common pathophysiological mechanisms, and one can lead to or worsen the other. Therefore, a close collaboration between primary care physician, endocrinologists and hepatologists is essential to optimize the management of patients with NAFLD and T2D. Here, we summarize relevant aspects about NAFLD and T2D that all clinician managing these patients should know as well as current therapeutic options for the treatment of T2D associated with NAFLD.
La esteatosis hepática metabólica (EHmet) se está convirtiendo en una de las causas más importantes de morbimortalidad relacionada con las enfermedades hepáticas. Es importante destacar que la EHmet se considera un mediador de enfermedades sistémicas, incluidas las enfermedades cardiovasculares. Se espera que su prevalencia aumente, principalmente debido a su estrecha relación con la obesidad y la diabetes mellitus tipo 2 (DM2). Además, la DM2 y la EHmet comparten mecanismos fisiopatológicos comunes y una puede provocar o empeorar la otra. Por lo tanto, una estrecha colaboración entre el médico de atención primaria, endocrinólogos y hepatólogos es fundamental para optimizar el manejo de los pacientes con EHmet y DM2. En esta guía resumimos aspectos relevantes sobre EHmet y DM2 que todo médico que maneja a estos pacientes debe conocer, así como las opciones terapéuticas actuales para el tratamiento de DM2 asociada a EHmet.
Non-alcoholic fatty liver disease (NAFLD) is defined as the accumulation of intrahepatic fat which cannot be attributed to secondary causes such as alcohol or drugs. It encompasses a spectrum of disease from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis. NASH is characterized by the presence of hepatic inflammation and hepatocyte injury (ballooning) with or without fibrosis, and it is the progressive subtype that can lead to cirrhosis and hepatocellular carcinoma (HCC).1 NAFLD and type 2 diabetes (T2D) are very related diseases and frequently co-exist; in fact, the prevalence of NAFLD is increasing in parallel to obesity and T2D epidemics. Early detection of NAFLD patients at high-risk of liver disease progression, as well as management of T2D and other metabolic comorbidities have the potential to reduce the burden of cardiovascular and liver-related mortality. Therefore, a close collaboration between primary care physician, endocrinologists and hepatologists is essential to optimize the management of NAFLD patients. Here, we summarize relevant aspects about NAFLD and T2D that all clinician managing these patients should know as well as current therapeutic options for the treatment of T2D associated with NAFLD.
Prevalence of NAFLD in patients with type 2 diabetesNAFLD is the leading cause of chronic liver disease globally, with an estimated prevalence in general population of 25%.2 The overall prevalence of NASH is between 12 and 14%. However, in subjects with T2D the prevalence of NAFLD and NASH are much higher reaching 55–70% and 30–40%, respectively.2,3
Development of fibrosis is a key predictor of liver-related outcomes. Although the exact prevalence of fibrosis in the general population is not known, a very recent study combining data from a large set of subjects from the general population and a cohort of patients diagnosed with NAFLD by liver biopsy estimates that the estimated prevalence of NAFLD with significant fibrosis (stages≥F2) and cirrhosis in the Spanish adult population is 1.33% (95% CI: 0.29–5.98) and 0.70% (95% CI: 0.10–4.95), respectively.4 In persons with T2D approximately 15% have clinically significant liver fibrosis.5 In fact, T2D is a major driver of liver disease progression. There is also growing evidence that suggests an increased risk of HCC in NAFLD patients with T2D regardless of other well-established risk factors for HCC.6 Therefore, due to the increasing incidence of T2D, the prevalence of NAFLD-HCC is on the rise, being NASH the second most common cause of liver transplantation for HCC currently in the USA.7 However, NAFLD is not a simple consequence of T2D or metabolic syndrome. Clinical evidence highlights that NAFLD could be a precursor for the future development of T2D.8,9 Furthermore, the presence of NAFLD in patients with T2D is associated with many adverse metabolic alterations, worse cardiovascular risk and increased microvascular complications.10 Despite its high prevalence and the fact that the presence of NAFLD identifies a subgroup of T2D patients that is metabolically more complex and at higher risk of cardiovascular events and of developing liver cirrhosis and HCC, NAFLD is under-diagnosed and under-estimated in diabetes clinics. In a survey conducted to 133 diabetes specialists in the UK,11 the prevalence of NAFLD and advanced fibrosis (stages≥3) were underestimated. 47% of responders were not aware of any non-invasive scoring systems to help stage NAFLD and more than a half had not used them in the past 12 months. So, there is no doubt that the first step should be to raise awareness of the disease, especially between clinicians attending high risk patients.
Links between NAFLD and type 2 diabetesThere is a clear association between T2D and NAFLD. Although the pathogenesis of T2DM and NAFLD is complex and not fully understood, the presence of many common elements in the development of both diseases has been demonstrated. Genetic predisposition, epigenetic factors, unhealthy lifestyle (high-calorie diets, excessive consumption of fructose, sedentary lifestyle), and dysregulated gut microbiome have been related to the development of both disease.12 As mentioned above, several studies have shown that NAFLD predicts the development of T2D and vice versa, and that each condition serves as a progression factor for the other.
The liver has a crucial role in the carbohydrate and lipid metabolism, contributing substantially to the development of insulin resistance and T2D. In addition, chronic hyperinsulinemia from systemic insulin resistance results in lipolysis and, hence, increased circulating free fatty acids (FFA), which may be accumulated in the liver promoting hepatic insulin resistance. The complex pathophysiological interactions between NAFLD and T2DM reaffirm the need for a comprehensive diagnostic and therapeutic approach to these diseases.
On the other hand, the mortality rate among NAFLD patients is substantially higher than in the general population, and cardiovascular disease (CVD) represents the leading cause of morbidity and mortality in them, followed by extrahepatic malignancies and liver-related complications.2
Current clinical evidence highlights that NAFLD could be a precursor for the future development of metabolic syndrome (MetS) components,8,9,13 linked to an increased cardiovascular risk (CVR) independently of MetS risk factors.13 Several studies show a higher risk of cardiovascular mortality in patients with NASH and fibrosis compared to those with NAFL.14–16 NAFLD has been associated with a higher prevalence of subclinical atherosclerosis, coronary artery disease, cardiomyopathy, valvular heart disease, cardiac arrhythmias, and cerebrovascular and peripheral vascular disease.17,18 Therefore, an adequate assessment and treatment, if applicable, of vascular risk in NAFLD patients (with or without T2D) is essential. Additionally, the association between NAFLD and T2D brings an additional risk of both hepatic and cardiovascular adverse clinical outcomes, thus, hepatologists should incorporate routinely screening for T2D and CVR work-up periodically. Both international and Spanish national practice guidelines for the management of NAFLD recommend a comprehensive CVR assessment in all NAFLD patients,1,19,20 but without detailing how; thus, this is not always considered by the clinicians in charge of these patients.21 We recommend following the main guidelines for the evaluation and treatment of CVR.22,23
In addition to the well-known cardiovascular effects of these two diseases, their renal effect has recently been added, establishing the novel concept of “cardio-renal-hepatic axis”.24 T2D and NAFLD close the triangle of this axis as they are, on the one hand, established CVR factors and, on the other, T2D is the main cause of terminal chronic renal failure in developed countries.
Globally, both pathologies interact in a bidirectional way, increasing the prevalence and the risk of severe disease and poor outcomes of both pathologies. This association is clinically relevant as it is necessary to identify patients at high-risk of poor outcomes in order to develop screening algorithms to detect them in early stages of liver diseases, target treatments and eventually reduce the increased cardiovascular and liver morbi-mortality.
Diagnosis and characterization of liver disease severity in subjects with T2DUnlike other micro- and macrovascular complications of T2D, systematic screening has not been widely adopted in routine diabetes care to assess for presence and severity of NAFLD. Considering the high prevalence of NAFLD with significant fibrosis (12–20%) in patients with T2D, the risk of progression to cirrhosis and even HCC in these patients, and knowing that fibrosis is the most important predictor of liver and overall outcomes, patients with T2D should be screened for NAFLD and liver fibrosis,25 in order to make an early intervention, stop de disease progression and improve outcomes. Importantly, among patients with T2D, some characteristics would increase the risk of fibrosis and cirrhosis such as age (>50 years), features associated with insulin resistance (metabolic syndrome, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome), and probably long-standing T2D (>10 years). Besides, the existence of NAFLD identifies a subgroup of T2D with different prognostic and therapeutic implications. These patients usually present other metabolic alterations, such as atherogenic dyslipidemia and arterial hypertension, which are especially pronounced and make the treatment of these patients especially difficult, and with a higher risk of developing cardiovascular and renal disease.26 According, the American Association of Clinical Endocrinology (AACE) recommend screening for NAFLD and significant liver fibrosis in patients with prediabetes or T2D, even if they have normal liver enzyme levels.25 Liver disease is frequently clinically and biochemically silent until there is end-stage liver disease.
Since liver biopsy is invasive, expensive and prone to sampling error, various non-invasive screening tools are available such as clinic-biological tests and vibration-controlled transient elastography (VCTE) to detect steatosis and more importantly fibrosis to facilitate appropriate surveillance.
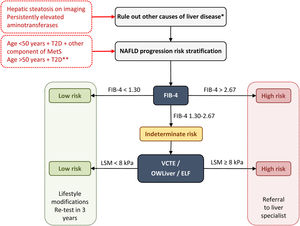
Diagnostic algorithm for NAFLD in T2DPrimary care physicians and endocrinologists play a fundamental role in the prevention and diagnosis of NAFLD in T2D patients. The identification and grading of NAFLD are an essential component to a comprehensive assessment of this liver disease. Indeed, defining the degree of NAFLD will become even more important as effective treatment options become available. Screening for liver fibrosis should be performed in patients with T2D at high risk of significant fibrosis, those with hepatic steatosis on imaging, and/or persistently elevated aminotransferases (over 6 months). Other causes of liver disease must be excluded (e.g., significant alcohol consumption, drugs, viral hepatitis, autoimmune hepatitis, hemochromatosis and Wilson's disease) in the presence of steatosis or elevated aminotransferases.27
It is generally accepted that non-invasive methods should be used to stratify the risk of advanced fibrosis. While the best screening approach remains unclear, most noncommercial panels use a combination of clinical features with routine chemistries to obtain very simple scores that are easy to transfer to the patient's bedside, such as the fibrosis-4 index (FIB-4), the nonalcoholic fatty liver disease fibrosis score (NFS) or the HEPAmet fibrosis score.28 The two most used markers are the FIB-4 and NFS, although FIB-4 seems to be taking advantage, given that the latest consensus conferences and recommendations of scientific societies favor the use of FIB-4 in primary and endocrinology clinical settings for the diagnosis of significant fibrosis (≥F2),25 probably due to its greater ease of use and less influence by metabolic comorbidities. However, any of the non-invasive test for the diagnosis of advanced fibrosis have limitations. Indeed, both FIB-4 and NFS present a wide range of indeterminate values between the areas of low and high probability of advanced fibrosis. A combination of the FIB-4 or NFS followed by VCTE seems to be the best approach to reduce the “intermediate zone”.29 These tools present excellent AUROC for the detection of advanced fibrosis (0.80 for FIB4 and NFS and greater than 0.9 for VCTE) and high negative predictive value (NPV) (>90%) for ruling out advanced fibrosis.30 Nevertheless, both AUROC and NPV are lower for ruling out significant fibrosis.31
Of interest, patented serum biomarker such as Enhanced Liver Fibrosis (ELF) and lipidomics test (OWLiver tests) have also been evaluated for diagnosing of advanced and significant fibrosis respectively. ELF has showed improvement in detecting of advanced fibrosis in patients with indeterminate FIB-4 results reducing unnecessary referrals by 88%.32 OWLiver test were developed and validated using more than 700 biopsy-proven NAFLD patients showing a NPV more than 75% for NASH and significant fibrosis.33 Mayo R et al. evaluated 140 patients with NAFLD and T2D and determined that the cutoff FIB-4 of 2.67 had a suboptimal sensitivity and positive predictive value (29.2% and 73.7%, respectively) for advanced fibrosis. However, they found that the sequential application of FIB-4 and OWLiver tests identified 88.3% of diabetic patients with significant fibrosis.34 The great advantage of this lipidomics test is that with a minimal serum sample it is possible to characterize NAFLD, determining the existence not only of significant fibrosis but also of steatohepatitis. In addition, this test is sufficiently sensitive to changes, whether induced by medication or spontaneous (progression/regression), which would allow an adequate evaluation of the treatments.
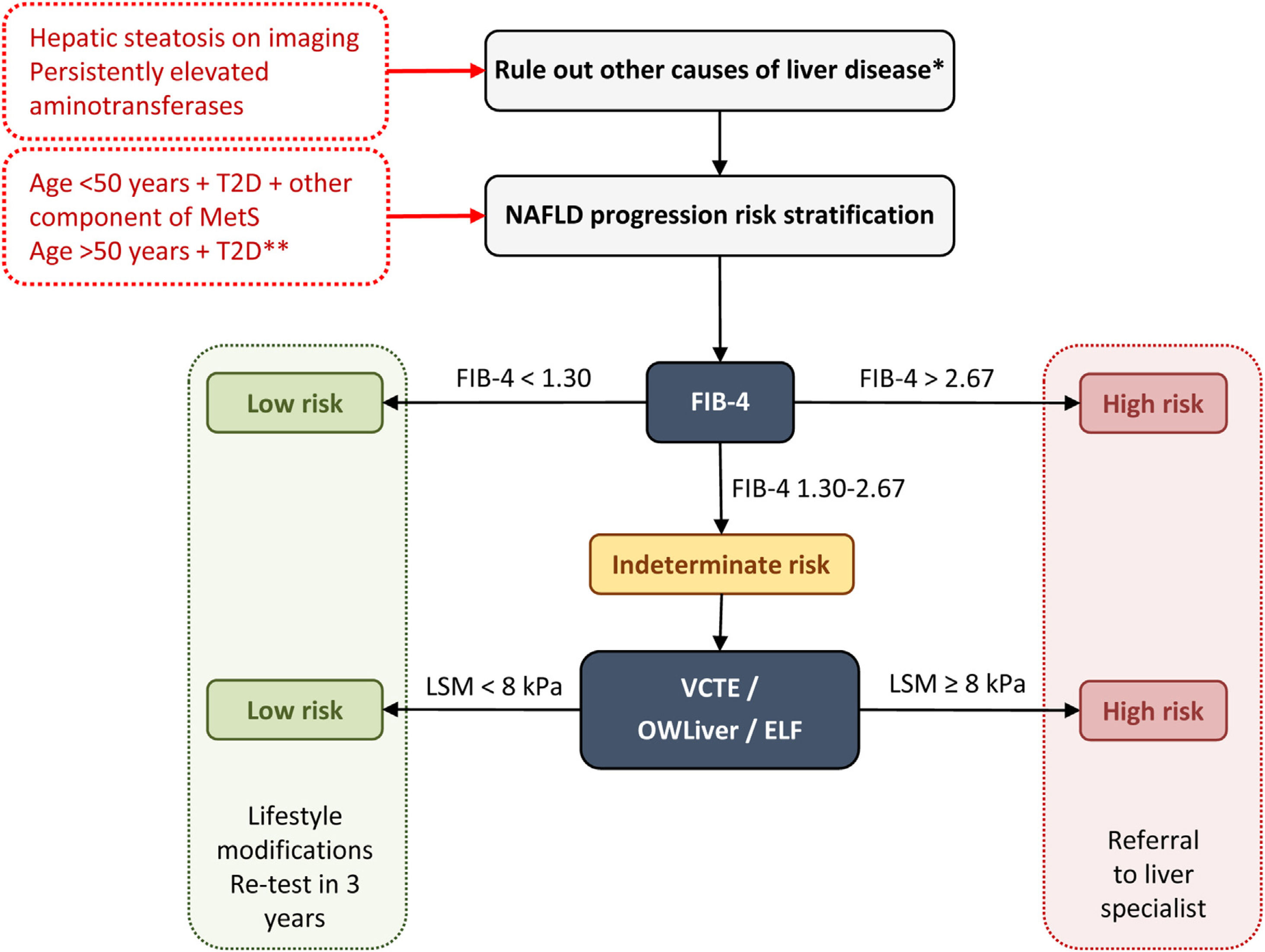
The main international entities (AACE, American Association for the Study of Liver Disease – AASLD –, European Association for the Study of the Liver – EASL) support a two-step approach for screening for advanced liver fibrosis in individuals at risk. On this note, we propose the following diagnostic algorithm (Fig. 1):
- •
First step: any non-invasive clinic-biological test can be used. The FIB-4 is widely available and easily used because it is based on common clinical parameters (age) and biological parameters (aminotransferases and platelet count).
Diagnostic algorithm for NAFLD in patients with T2D. Two-step approach for screening for advanced liver fibrosis in individuals at risk. *Significant alcohol consumption, drugs, viral hepatitis, autoimmune hepatitis, hemochromatosis, and Wilson's disease. **The diabetic patients with the highest risk of fibrosis are: >50 years old, MetS, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome and, long-standing diabetes (>10 years). ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes; VCTE, vibration-controlled transient elastography.
- -
A FIB-4 score of less than 1.30 (less than 2.0 in subjects older than 65 years) identifies patients with low risk and they do not need further assessment. They should receive recommendations for lifestyle modifications and retesting in 3 years.
- -
A FIB-4 score of more than 2.67 identifies patients with high risk of advanced diseases. They should be sent to a specialized hepatology consultation for further assessment.
- -
A FIB-4 score between 1.3 and 2.67 (between 2.0 and 2.67 for those aged ≥65 years) identifies patients with intermediate risk who would benefit from the second step.
- •
Second step: Patented serum biomarkers (ELF or OWLiver tests) could be considered in patients with intermediate risk according to local availability. Otherwise VCTE using FibroScan, as the most widely available and validated point-of-care technique, appears to be the tool of choice. FibroScan provides a liver stiffness measurement that can be combined with the controlled attenuation parameters (CAP) to detect steatosis.
- -
At a value lower than 8kPa, the patient is considered at a low risk for advanced fibrosis. They should receive recommendations for lifestyle modifications and retesting in 3 years.
- -
At a value higher than 8kPa, the patient is considered at an intermediate-high risk for advanced fibrosis. They should be sent to a specialized hepatology consultation.
Regarding OWLiver tests and ELF: (a) OWLiver tests: if the result rules out significant fibrosis, patients can be managed by primary care, while a OWLiver test that suggests significant fibrosis, patients should be referred to a specialized hepatology clinic; (b) ELF: If the ELF value is lower than 7.7, the existence of advanced fibrosis is safely ruled out; otherwise, they should be referred to a specialized hepatology clinic.
Treatment of patients with NAFLD and T2DAt present, there are no approved drugs for the treatment of NAFLD. The treatment of NAFLD, especially when it coexists with T2DM, consists mainly in lifestyle modification. It is also suggested that some drugs, including hypoglycemic agents, may be used to treat NAFLD.
Given the complexity of care posed by patients with T2D and NAFLD, successful intervention requires a cohesive multidisciplinary team including the primary care physician, an endocrinologist and hepatologist. Most patients with T2D are followed-up in primary care; those patients with sub-optimal response to the treatment, with high complexity, when other etiology for the hyperglycemia is suspected or when bariatric surgery is considered, an endocrinologist should also manage the patient.
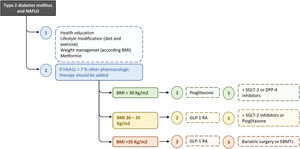
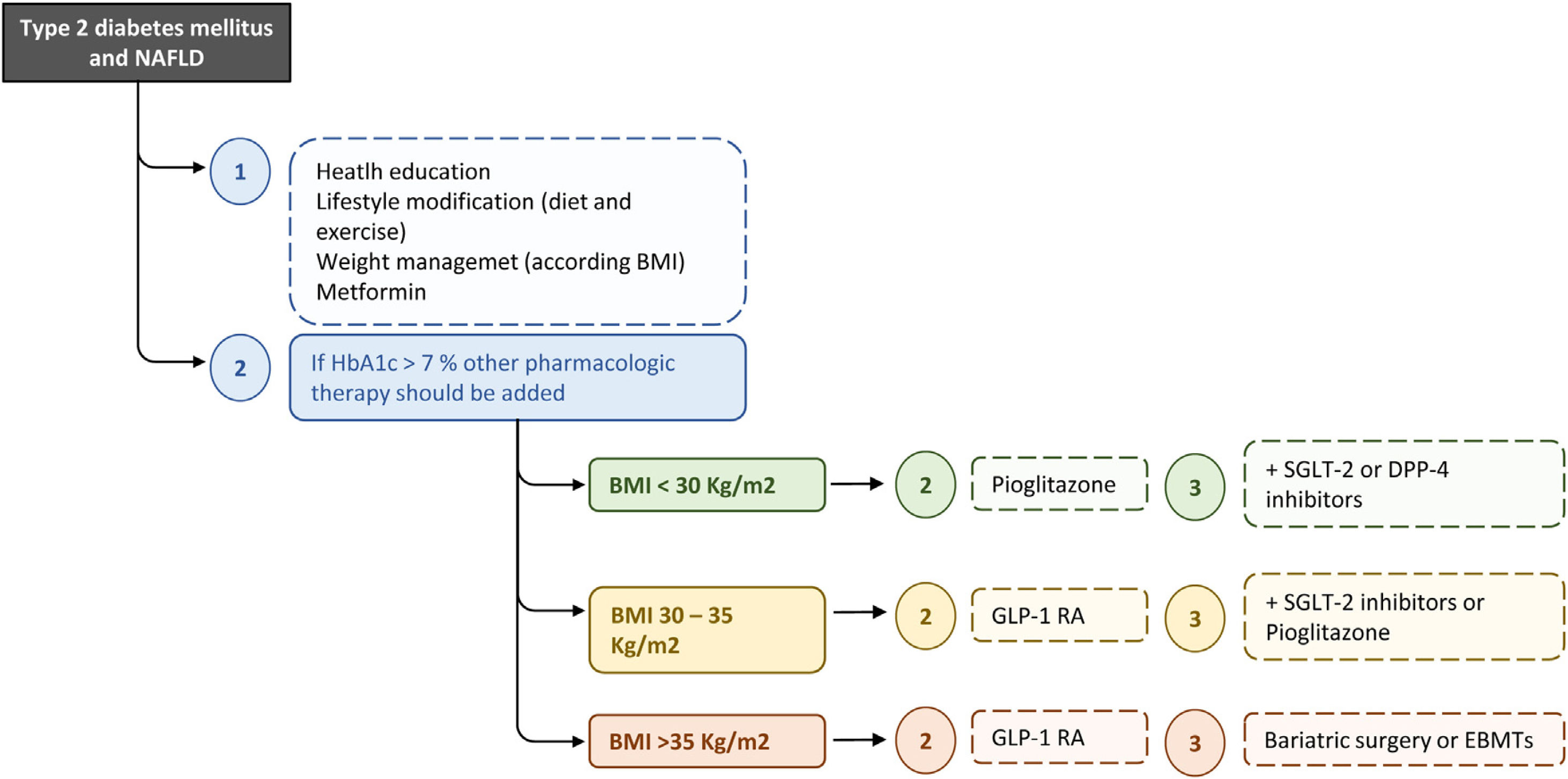
The ideal therapy for patients with T2D and NAFLD will be that which achieves glycemic control, reverses the liver injury and fibrosis, and improves other metabolic factors as well as cardiovascular risk. Hence, this treatment should be multifactorial and progressive/additive to adapt it to the evolutionary phase of the disease (Fig. 2). In this context, probably the most appropriate strategy to establish the different therapeutic measures is the staggered use of these, but prioritizing the treatments that act on the causal factors (unhealthy diet, sedentarism, obesity) and/or improve a greater number of pathophysiological elements and components of the MetS or, at least, have no negative effects on other metabolic factors.
Recommended treatment algorithm for T2D and NAFLD. The ideal therapy for patients with T2D and NAFLD will be on that achieves glycaemic control, reverses the liver injury and fibrosis, and improves other metabolic factors as well as cardiovascular risk. Hence, this treatment should be multifactorial and progressive/additive to adapt it to the evolutionary phase of the disease. BMI, body mass index; DPP-4, dipeptidyl peptidase-4; EBMTs, endoscopic bariatric and metabolic therapies; GLP-1 RA, glucagon-like peptide-1 agonist receptor; SGLT-2, sodium-glucose linked transporter 2.
According to this proposed scheme, the primary treatment for T2D and NAFLD is lifestyle modification through diet and exercise. The weight loss is the major therapeutic target in NAFLD and T2D. A reduction of 5% of body weight has demonstrated beneficial effects in insulin resistance, glycemic control, CVR and ectopic adiposity, including hepatic fat, whereas a weight loss of 7–10% can improve hepatic inflammation. Importantly, a >10% reduction of the body weight is associated with fibrosis regression,35 and modify the course of diabetes and even its remission.36 The joint EASL, European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice guidelines recommend at least a 7–10% weight loss target in NAFLD patients with overweight/obesity.1
The Mediterranean diet has been associated with an improvement in NAFLD, mortality and risk of T2D and cardiovascular events in follow-up.37 Accordingly, this type of diet has been recommended for the NAFLD treatment by the European guidelines.1 It is noteworthy that there is a subgroup of subjects who do not respond to the diet even when it is carried out adequately (the so-called “diet-resistant”), probably due to decreased skeletal muscle mitochondrial function. The addition of physical exercise in diet-resistant subject has been shown to enhance skeletal muscle metabolism and improve body composition.38
Exercise, even in the absence of weight loss, has shown to reduce hepatic steatosis. Regarding the duration and intensity of the exercise program, further data are required. However, the different forms of exercise (aerobic and resistance exercise) appear to have similar effects on hepatic steatosis, with a beneficial effect observed following 40–45min/session, 3 times/week of either one.39
If objectives are not achieved with lifestyle modification, pharmacologic therapy is warranted. Managing CVR factors, such as arterial hypertension and dyslipidemia, should follow recommended standards of care. Statin and non-statin lipid lowering therapies (fibrates, ezetimibe, Omega-3 and bile acid sequestrants) are safe for patients with liver disease and should be prescribed for all high CVR patients based on current guidelines.22
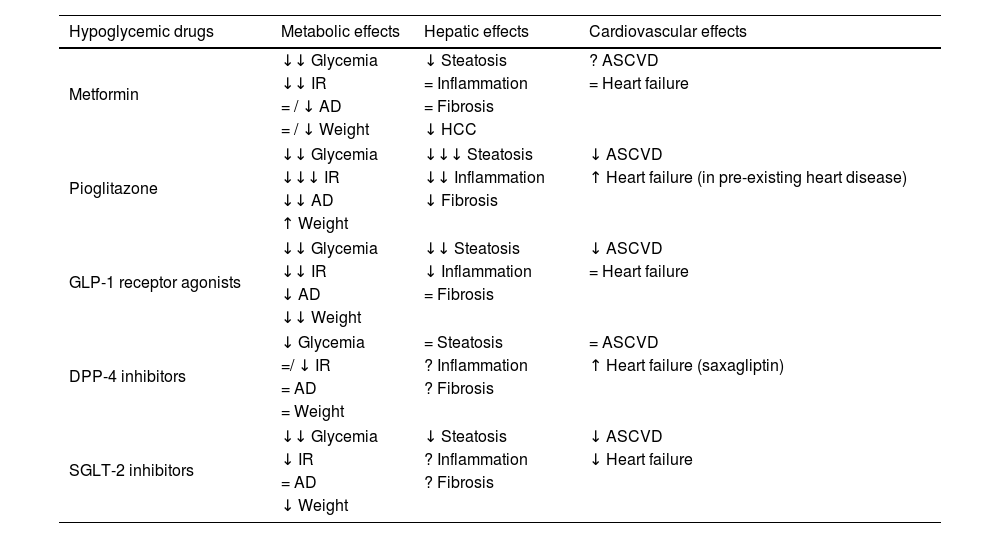
Hypoglycemic drugs in patients with T2D and NAFLDGlucose-lowering medications should be used to optimize glycemic control at the time of T2D diagnosis (with lifestyle modification) or after lifestyle modification, considering HbA1c level and other primary targets, as is the case in most patients with NAFLD. In patients with NAFLD the ideal treatment, apart from improving hyperglycemia, would improve/revert NASH and liver fibrosis and should offer cardiovascular protection and favoring effects on weight and other T2D related complications. Primary targets can be addressed concurrently and not necessarily sequentially. The main effects of hypoglycemic drugs at metabolic, cardiovascular and hepatic level are summarized in Table 1.
Effects of noninsulin glucose-lowering agents.
| Hypoglycemic drugs | Metabolic effects | Hepatic effects | Cardiovascular effects |
|---|---|---|---|
| Metformin | ↓↓ Glycemia | ↓ Steatosis | ? ASCVD |
| ↓↓ IR | = Inflammation | = Heart failure | |
| = / ↓ AD | = Fibrosis | ||
| = / ↓ Weight | ↓ HCC | ||
| Pioglitazone | ↓↓ Glycemia | ↓↓↓ Steatosis | ↓ ASCVD |
| ↓↓↓ IR | ↓↓ Inflammation | ↑ Heart failure (in pre-existing heart disease) | |
| ↓↓ AD | ↓ Fibrosis | ||
| ↑ Weight | |||
| GLP-1 receptor agonists | ↓↓ Glycemia | ↓↓ Steatosis | ↓ ASCVD |
| ↓↓ IR | ↓ Inflammation | = Heart failure | |
| ↓ AD | = Fibrosis | ||
| ↓↓ Weight | |||
| DPP-4 inhibitors | ↓ Glycemia | = Steatosis | = ASCVD |
| =/ ↓ IR | ? Inflammation | ↑ Heart failure (saxagliptin) | |
| = AD | ? Fibrosis | ||
| = Weight | |||
| SGLT-2 inhibitors | ↓↓ Glycemia | ↓ Steatosis | ↓ ASCVD |
| ↓ IR | ? Inflammation | ↓ Heart failure | |
| = AD | ? Fibrosis | ||
| ↓ Weight | |||
AD, atherogenic dyslipidaemia; ASCDV, atherosclerotic cardiovascular disease; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; HCC, hepatocellular carcinoma; IR, insulin resistance; SGLT-2, sodium-glucose linked transporter 2.
Glycemic targets in patients with NAFLD should be those established by national and international guidelines for T2D treatment. A general HbA1c <7% goal seems appropriate; however, more or less stringent glycemic goals may be established based on individualized criteria. A HbA1c <8–8.5% can be the treatment goal in patients with limited life expectancy, fragility, high risk of hypoglycemia or advanced comorbidities. The determination of HbA1c should be performed at least twice a year in all patients, and three or four times (every 3–4 months) in those whose treatment is modified or who have not reached the proposed levels.22
Metformin is a first line treatment of T2D with an important effect in reducing insulin resistance. A systematic review about the effects of metformin in NAFLD showed an improvement of the liver enzymes and a decrease in insulin resistance and body mass index (BMI). However, it showed no histological response.40
Pioglitazone is a tiazolidinadione which reduces insulin resistance. It acts by agonism at the preoxisome proliferator-activated receptor-gamma (PPARγ) which leads to an increase insulin sensitivity in hepatic cells, adipose tissue, and skeletal muscle. Pioglitazone has demonstrated resolution of NASH and amelioration of fibrosis of any stage, benefits seen even in non-diabetic patients.41 Additionally, Pioglitazone has CV benefits with reduction of major adverse CV events.42 Although the benefit of pioglitazone on NAFLD is well-established, it usage is limited manly because of its side effects, especially weight gain, increased fracture risk, and heart failure if used in persons with preexisting heart disease.42 We consider that adverse effects of pioglitazone are outweighed by the multiple benefits and it is an attractive option for the treatment of selected patients with T2D.
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) stimulates the production and release of insulin, inhibits glucagon secretion, delays gastric emptying, reduces appetite and food intake and have demonstrated efficacy in hyperglycemia treatment, weight reduction and CV protection.43 Thus, regardless of HbA1c level or the presence of other glucose-lowering agents, GLP-1 RA with proven cardiovascular benefit should be prescribed at all individuals with diabetes and established CVD or multiple CVR factors.43 They also have numerous beneficial metabolic effects relevant to the pathophysiology of NAFLD. Liraglutide showed resolution of NASH without worsening of fibrosis (39%) compared with placebo (9%) in a small (52 patients) randomized phase 2 trial.44 Another agent is semaglutide which also showed significant beneficial effects on NASH resolution but not on fibrosis improvement in a placebo controlled, phase 2 trial.45 The most common side effects of GLP-1 RA are gastrointestinal (nausea, vomiting and diarrhea) and tend to occur during initiation and dose escalation and diminish over time.
Dipeptidyl peptidase 4 (DPP-4) inhibitors work by blocking the enzymatic inactivation of endogenous incretin hormones, resulting in glucose-dependent insulin release and a decrease in glucagon secretion. They have a neutral effect on weight and CVR. To date, no DPP-4 inhibitor has demonstrated improvement in NAFLD's histological aspect.46
Tirzepatide, a novel once-weekly injectable dual glucose-dependent insulinotropic polypeptide (GIP) receptor and GLP-1 RA combination drug, has been recently approved by the FDA and EMA for T2D treatment. They improve blood glucose levels and results in weight loss to a greater extent than other diabetes medications. In the SURPASS-3 MRI sub-study,47 patients with Tirzepatide had significantly greater reductions in liver fat content, visceral adipose tissue and abdominal subcutaneous adipose tissue compared with insulin. These results support further evaluation of tirzepatide in patients with NASH.
Another important family in the treatment of T2D are the SGLT-2 inhibitors. They promote urinary excretion of glucose by inhibiting its renal proximal tubular reabsorption. Their use for T2D treatment is very extended as, apart from glycemic benefits, they have demonstrated weight loss and cardio-renal benefits. NAFLD patients with T2D treated with either canagliflozin, dapagliflozin or empagliflozin showed a decrease in steatosis and in some liver enzymes. However, there are not data yet on its possible benefit in NASH and fibrosis.48,49
Other drugs with cardiovascular effectsAs we mentioned before, we recommend following the main guidelines for the treatment of CVR. Atherogenic dyslipidemia and arterial hypertension are comorbidities commons in patients with NAFLD and T2D and contribute to the increased CVR associated with these conditions; thus, clinician must manage them based on the current standards of care.22,23
It should be noted that current evidence suggests that statin use is overall safe in patients with chronic liver disease or cirrhosis.50 In fact, statins have been demonstrated to reduce steatosis in those with NASH and prevent liver events in patients with metabolic syndrome with advanced NASH.51,52 Besides, a possible protective role of statin treatment against NAFLD progression to HCC was also demonstrated in observational studies.53,54 Given the efficacy of statins in preventing CV events and reducing CV mortality in patients with T2D, they are the drug of first choice in patients at increased risk of CVD and/or dyslipidemia. Interestingly, it has been shown that statins reduce cardiovascular risk more in NAFLD vs. non-NAFLD high-risk individuals.55 These evidences highlight the importance of effective lipid modulation in NAFLD.
Bariatric surgeryIn the specific setting of patients with severe obesity (BMI ≥40 or BMI between 35 and 40 with comorbidities), bariatric surgery induces long-term maintenance of weight loss. According to the ADA 2022 guidelines,56 metabolic surgery should be a recommended option to treat T2D in screened surgical candidates with BMI ≥40kg/m2 and in adults with BMI 35.0–39.9kg/m2 who do not achieve durable weight loss and improvement in comorbidities with nonsurgical methods, and can also be considered as an option to treat T2D in those with BMI 30.0–34.9 who do not reach glycemic targets and achieve durable weight loss with nonsurgical methods.
Whatever the surgical procedure, a 14%–25% weight loss is observed 10 years after surgery, along with improvement in insulin resistance, the remission of diabetes, and fewer cardiovascular events.57 Regarding liver injury, numerous studies have pointed to an improvement in steatosis and other features of NASH after bariatric surgery,58,59 but also a lower risk of incident major adverse liver outcomes.60 Lassailly et al. assessed the long-term effects of bariatric surgery in 180 obese patients with biopsy-proven NASH. They observed resolution of NASH in liver samples from 84% of patients 5 years later.58 Although there are no guidelines of bariatric surgery indications for the management of NASH, their effect on liver histology may prompt consideration for bariatric surgery in those patients with NAFLD and a BMI>35.
Metabolic endoscopyAs mentioned above, a total body weight loss (TBWL) of 10% guaranteed NASH resolution in 90% of participants, fibrosis regression in 80% and steatosis disappearance in 100%. However, only 10% of subject reached enough body weight reduction with lifestyle modifications.35 Therapies such as bariatric surgery and endoscopic bariatric and metabolic therapies (EBMTs) can be useful alternatives. In recent years EBMTs have emerged as safe and effective for the treatment of obesity and T2D. Advantages of EBMTs include their reversibility, short procedure time, technical ease and lower adverse event rates. EBMT include gastric and duodenal devices and techniques such as intragastric balloons and endoscopic sleeve gastroplasty (ESG). Intragastric balloons have shown a TBWL of 11% at 12 months in obese subjects and NASH resolution in 50% of NAFLD patients.61 ESG has gained recognition for being an effective weight loss procedure with an adequate safety profile.62 This technique involves an endoscopic suturing device to reduce gastric volume. A meta-analysis of studies with ESG and 1859 patients showed a TBWL of 15–16% at 6–12 months.63 A NASH randomized, controlled trial with ESG is currently underway, showing significant improvement in NASH activity score (NAS) at 18 months in interim results (NCT03426111). Likewise, its efficacy is being evaluated in comparison with the surgical sleeve (NCT04060368).64 Future research in EBMTs should focus on defining its role in NAFLD treatment and its place in the NAFLD management algorithm.
Recommended treatment algorithmA treatment algorithm for T2D in patients with NAFLD is showed in Fig. 2. Although does not improve liver histology, in the absence of specific contraindications, we suggest metformin as initial therapy for patients with newly diagnosed T2D with NAFLD. It is supported by glycemic efficacy, absence of weight gain and hypoglycemia, favorable tolerability and cost, and the reduction of cardiovascular and microvascular risk, and possibly that of hepatocellular carcinoma. When lifestyle changes and metformin are insufficient, other pharmacologic therapy should be added to prevent progression of liver and cardiovascular disease. Pioglitazone and GLP-1 RAs have proven to be safe and effective to reverse NASH in people with obesity, prediabetes or T2D, while SGLT2 inhibitors have been considered potentially beneficial for NAFLD.
We suggest that pioglitazone can be used as first-line agent for the treatment of patients with T2D and NAFLD, especially in individuals with severe insulin-resistant diabetes (SIRD) cluster or with conditions suggestive of marked insulin resistance (larger waist circumference, higher triglycerides and lower HDL cholesterol). In this subgroup improving insulin sensitivity and glucose and lipid metabolism with pioglitazone can exert greater benefits to prevent complications. Adverse effects of pioglitazone may be minimized by using 15–30mg rather than 45mg dose and weight gain, fluid retention and heart failure can be prevented by combining with SGLT2 inhibitors.65 In patients with NAFLD and obesity, we should give preference to GLP-1 RA as first-line glucose-lowering medications because of clinical benefits, including weight loss, glycemic control, and cardiometabolic improvements. When the individual treatment targets are not achieved, pioglitazone or SGLT2 inhibitors may be considered as adjunctive therapy to GLP-1 RA considering patient characteristics. Finally, for selected patients with BMI ≥35 bariatric surgery should be considered.
ConclusionsNAFLD and T2D are two highly prevalent diseases that are increasing due to the fact that many of their shared risk factors are also in constant progression today. These diseases also interact with each other through multiple interrelated metabolic pathways, which justifies the high prevalence of both and their mutual aggravation when they coexist.
To achieve an adequate diagnosis of such a prevalent disease, we propose a diagnostic algorithm to rule out NAFLD in all T2D patients. This algorithm is based on two stages, first a non-invasive analytical index (FIB-4 or NFS), discriminating between those that will require follow-up by a hepatologist from those that will not, and the application of a second test (transitional elastography, OWLiver or ELF) in those with intermediate risk to decide on subsequent management. It should also be noted that NAFLD is associated, independently of other traditional CVR factors, with higher cardiovascular morbidity and mortality, and in the case of NAFLD with significant fibrosis, with a higher risk of developing T2D.
Given the complexity of the treatment of these two diseases, their management must be multidisciplinary and stratified according to the type of patient, taking into account the associated risk factors (T2D, obesity, sedentary lifestyle), since they will require a different approach. Both screening for advanced NAFLD in patients with diabetes and initial therapeutic management should be carried out by primary care and endocrinologists. The main element of treatment for T2D and NAFLD is lifestyle modification through diet and exercise to achieve weight loss. Due to the frequent difficulty that patients present in achieving significant weight loss, pharmacologic therapy is warranted. There is currently no approved pharmacological treatment for NAFLD, but there are very efficient drugs for the treatment of T2D that improve liver disease, reduce CVR and improve kidney function. The most aggressive bariatric surgery treatments have classically been reserved for patients with obesity who do not respond to previous therapeutic measures, due to their high morbidity, although new bariatric and metabolic endoscopy techniques offer a hopeful alternative with comparable efficacy and lower risk of complications.
FundingSpanish Carlos III Health Institute (ISCIII) [J. Crespo (FIS PI18/01304)].
Conflict of interestThe authors report no relevant conflict of interest or disclosures relevant to this manuscript.






 50 years old, MetS, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome and, long-standing diabetes (>10 years). ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes; VCTE, vibration-controlled transient elastography.' title='Diagnostic algorithm for NAFLD in patients with T2D. Two-step approach for screening for advanced liver fibrosis in individuals at risk. *Significant alcohol consumption, drugs, viral hepatitis, autoimmune hepatitis, hemochromatosis, and Wilson's disease. **The diabetic patients with the highest risk of fibrosis are: >50 years old, MetS, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome and, long-standing diabetes (>10 years). ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes; VCTE, vibration-controlled transient elastography.'/>
50 years old, MetS, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome and, long-standing diabetes (>10 years). ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes; VCTE, vibration-controlled transient elastography.' title='Diagnostic algorithm for NAFLD in patients with T2D. Two-step approach for screening for advanced liver fibrosis in individuals at risk. *Significant alcohol consumption, drugs, viral hepatitis, autoimmune hepatitis, hemochromatosis, and Wilson's disease. **The diabetic patients with the highest risk of fibrosis are: >50 years old, MetS, atherogenic dyslipidemia, hypertriglyceridemic waist phenotype, polycystic ovary syndrome and, long-standing diabetes (>10 years). ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; T2D, type 2 diabetes; VCTE, vibration-controlled transient elastography.'/>