Despite the availability of new, powerful drugs for Crohn's disease, a significant proportion of patients will undergo an intestinal resection to control the disease as it develops. In the absence of an effective preventative treatment, the appearance of new intestinal lesions after surgery for Crohn's disease is the norm; this is known as post-operative recurrence and may appear very early on, even a few weeks after the surgical resection. Furthermore, the drugs that are currently available for the prevention of post-operative recurrence have a limited effect; up to 50% of cases present recurrent Crohn's disease activity despite the preventative treatment, which may require further surgery with the consequent loss of intestinal function, leading some patients to suffer from short bowel syndrome as an irreversible complication. The management of Crohn's disease patients who undergo an intestinal resection should thus be geared towards prevention, early detection and, in the worst case scenario, the treatment of post-operative recurrence. This article reviews the natural history, diagnostic measures, monitoring, prevention and treatment of post-operative recurrence, and proposes recommendations based on existing knowledge.
A pesar de la disponibilidad de nuevos y potentes fármacos para la enfermedad de Crohn, una relevante proporción de pacientes se someterá a una resección intestinal para el control de la enfermedad a lo largo de su evolución. En ausencia de un tratamiento preventivo eficaz, la aparición de nuevas lesiones intestinales tras la cirugía por la enfermedad de Crohn es la norma; a este hecho se le conoce como recurrencia posquirúrgica y puede aparecer muy precozmente, incluso pocas semanas después de la resección quirúrgica. Además, los fármacos actualmente disponibles para la prevención de la recurrencia posquirúrgica tienen una eficacia limitada; hasta el 50% de los casos vuelven a presentar actividad de la enfermedad de Crohn a pesar del tratamiento preventivo, lo que puede conducir a una nueva cirugía con la consiguiente pérdida de función intestinal que finalmente puede derivar a la aparición de síndrome de intestino corto, como complicación irreversible, en algunos pacientes. Por tanto, el manejo de los pacientes con enfermedad de Crohn sometidos a resección intestinal debe estar orientado a la prevención, detección precoz y, en el peor de los casos, al tratamiento de la recurrencia posquirúrgica. En este artículo se revisan la historia natural, las medidas de diagnóstico, monitorización, prevención y el tratamiento de la recurrencia posquirúrgica y se proponen recomendaciones en base al conocimiento actual.
Crohn's disease (CD) is characterised by chronic inflammation of the digestive tract, which usually affects the distal segment of the small intestine, various segments of the large intestine, or both. This inflammatory process, the origin of which has not been clearly established, often leads to irreversible tissue damage in the form of intestinal stenosis or fistulas, inflammatory masses or intra-abdominal abscesses in 20–40% of newly diagnosed patients, and up to 55% of patients after an average of 5 years of history, according to population studies.1 Although some of these complications can initially be managed with medical treatment, most of them will require resection of the affected intestinal segment. Further, a small number of patients with CD will develop a resistance to medical treatment, and despite not suffering from stenosis or penetrating complications, they will also require bowel resection to control the symptoms. A recently published population study showed that the number of patients with CD undergoing bowel resection had significantly decreased in the past decades; however, in the most recent diagnostic cohort (1998–2003), 19% of patients required bowel resection at the onset of the disease and up to 25% in the first 5 years of history.2 Thus, bowel resection is still needed today in a significant number of patients with CD, despite new drugs.
Unfortunately, after surgery a high percentage of patients will get new lesions in intestinal segments previously unaffected by the disease, a phenomenon known as postoperative recurrence (POR). POR entails a greater risk of needing new bowel resections, and this means there is morbidity and mortality associated with the operation itself and the risk of loss of intestinal function. Patients with CD who have had a bowel resection should therefore be evaluated, monitored and treated appropriately, to prevent irreversible intestinal loss. In the past few years, results from different studies have provided relevant data regarding monitoring with new tools, such as calprotectin, the need for early monitoring after surgery or the use of new drugs to prevent POR, which may change our clinical practice. The aims of this article are to provide an updated view of the natural course of CD after bowel resection with ileocolic anastomosis; to review the strategies and treatments that have been evaluated and shown to be effective in the prevention and treatment of POR; and to propose a set of rules for the long-term postoperative management of patients with CD. For this purpose, several bibliographic searches were carried out on PubMed by combining the MeSH terms [Crohn's disease] [recurrence] [postoperative] [natural history] [ileostomy] [outcome] [prevention] [treatment] [infliximab] [adalimumab] [azathioprine] [thiopurines] [mesalamine] [diagnosis] [timing].
Natural course of Crohn's disease after bowel resectionThe term POR is used to define the appearance of new lesions in CD after a theoretically curative surgical bowel resection, unlike the term recurrence, which refers to the reappearance of clinical symptoms during a flare-up of inflammation in a patient who was previously in clinical remission.
The most relevant study for our understanding of the natural course of POR was done in 1984.3 Rutgeerts et al. prospectively followed 114 patients who, after ileal or ileocolic resection with anastomosis, and with no treatment, were monitored annually with a colonoscopy with intubation and view of 40cm of the neoileum. This study described a POR rate of 72% per year of resection and showed that the lesions are located more often in or near the area of anastomosis, usually reproducing the same initial pattern of the disease. The normal macroscopic and histological state of this area, demonstrated by using perioperative endoscopy4 and intraoperative biopsies,3 does not prevent a POR from occurring later on. Though it has been suggested that post-resection lesions should be considered “new”, the theory that CD is a diffuse disease of the gastrointestinal tract seems more plausible, and the idea of a “cure” should not be assumed even when there is no apparent evidence of the disease after surgery. The presence of morphological changes detected by electron microscopy in histologically normal areas of ileal and colonic mucosa, including resection margins, supports the idea that the disease involves a diffuse and generalised pattern.4 In addition, the fact that the POR rate assessed by endoscopy one year after surgery is related to the presence of microscopic lesions reinforces their role as precursors of posterior lesions.4
The causes of why POR occurs preferentially in the neoterminal ileum are not known, but this anatomical preference may have the same cause as the initial ileocecal location that is so common in CD. It can be speculated that based on an intestine predisposed to diffuse chronic inflammation, the action of environmental agents might activate a greater macroscopic inflammatory response in a particular area. In fact, it has been suggested that POR does not occur (at least not so rapidly) if the anastomosis of the faecal flow is protected by a proximal ileostomy.5 However, the possibility of other factors related to POR, in addition to the endoluminal ones, is supported by the possibility discussed below that POR may appear after an end ileostomy.
Recently, different microbiota-related profiles (assessed during resection) have been described in relation to the subsequent clinical course, and these profiles are different in patients with and without subsequent POR.6 Moreover, the effectiveness of antibiotics in preventing POR is well known, and recently the possible benefit of probiotics has also been suggested.7
Several events have been described that play a role in mucosal changes in the neoterminal ileum, including localised ischaemia-related events,8 but their actual impact is not known. In a study of 28 patients who underwent right hemicolectomy or ileocecal resection with ileocolic anastomosis due to adenocarcinoma of the colon or villous adenoma, no lesions were detected in the anastomosis.9
Classification of postoperative recurrenceIn 1990 the Belgian group that most contributed to our understanding of POR published the well-known Rutgeerts index,10 now considered the gold standard for scoring recurrent lesions (Table 1). The relevance of this study is that it showed that the severity of endoscopic lesions viewed in the first 30cm of the neoileum correlates with the probability of a patient's developing symptoms typical of the disease. The symptoms appear when there are already advanced endoscopic lesions, and it is fairly common to see patients with advanced lesions who nevertheless remain asymptomatic. The sore or ulceration in the anastomosis may be the initial lesion, preceded by inflammatory changes in the submucosa, and it tends to progress to ulcers. These later converge, become deeper and may cause fistulas, fibrosis and stenosis.
Rutgeerts’ endoscopic index for the endoscopic grading of postoperative recurrence.
| i0 | Normal mucosa |
| i1 | Presence of fewer than 5 aphthous lesions |
| i2 | >5 aphthous lesions with normal mucosa between them or more relevant discontinuous lesions or lesions confined to <1cm of the anastomosis, generally ulcers |
| i3 | Diffuse aphthous ileitis of affected mucosa |
| i4 | Ileitis with deep or extensive ulcers, stone formation or stenosis |
The fact that mucosal lesions appear relatively early after surgery and always precede (often by years) the appearance of CD symptoms led to the definition of what is known as endoscopic POR (ePOR) when there are lesions seen in the ileoscopy (regardless of the presence of symptoms), and clinical POR (cPOR) when there are symptoms attributable to CD in the presence of lesions in the neoileum. This differentiation and the fact that ePOR occurs early and is much more common than cPOR have caused most clinical trials to use ePOR prevention as the main objective in order to shorten follow-up time. Some authors have used other concepts such as morphological POR (defined by any imaging test: ileoscopy, magnetic resonance enterocolonography, barium X-ray, capsule endoscopy); histological POR (inflammatory changes in the pathology study with no macroscopic lesions); or surgical POR (need for a new bowel resection due to the onset of POR), though these terms are not used often in clinical practice.
Although the concept of ePOR is clear, the criteria for defining it are arbitrary. The authors who described the endoscopic index consider that lesions greater than i1 should be defined as ePOR. However, there are some arguments that could encourage one to consider only more advanced lesions as ePOR. First, the initial study of Rutgeerts10 showed a high probability of cPOR in patients with i3–i4 lesions and a very low one in those with i0–i1 lesions. Patients with i2 lesions presented with an “intermediate” risk. Therefore, it has been suggested that the primary objective of studies evaluating drugs used to prevent POR should be the prevention of i3–i4 lesions.11 Second, it is possible that not all lesions able to be classified as i2 have the same prognostic significance, and it has even been suggested that the prognosis for lesions confined exclusively to the anastomosis may have better prognosis.12 In fact, the designation of subgroup i2a for this type of lesion has been proposed,12 excluding this group from the definition of an ePOR in the most recently conducted POR-prevention clinical trials. Third, it should be kept in mind that the difference between i1 and i2 may be only an aphthous lesion, which would not make a difference in prognosis.
A relevant aspect when evaluating ePOR is the neoterminal ileum segment to be examined. In the initial study by Rutgeerts,10 patients with advanced lesions (i3–i4) had a mean bowel length of 22cm (10–55cm), whereas this was only 8cm (2–15cm) in patients with less advanced lesions (i1–i2). From this it can be extrapolated that the examination of 15cm of the neoterminal ileum is enough to be able to classify the lesions, especially if there are none or they are minor. There is no consensus on whether isolated lesions beyond the vicinity of the surgical anastomosis should be considered as POR. In fact, lesions are detected in the small intestine proximal to the segment affected in up to 65% of patients if an intraoperative enteroscopy is performed,13 and the presence of these lesions does not seem to influence the risk of early POR during follow-up.14
The incidence of POR varies according to the studies published and by type of POR considered (endoscopic, clinical, radiological or surgical), and in the absence of treatment, it tends to be as high as in the initial study by Rutgeerts.10 Thus, more than 60% of patients undergoing surgery have ePOR one year after surgery, whereas cPOR rates with no treatment are 20–30% one year after resection, with an increase of 10% in each subsequent year.15
Risk factors for postoperative recurrenceNumerous risk factors have been analysed to assess their relationship to POR. The absence of uniform criteria makes it very difficult to draw definitive conclusions.16,17 The factors that have been most frequently evaluated may be related to the characteristics of the patient, the disease or factors related to the surgery performed (Table 2).
Risk factors potentially related to postoperative recurrence.
| Patient-related |
| Smoker |
| Younger age at onset of disease |
| Family history of inflammatory bowel disease |
| Previous or existing extraintestinal symptoms |
| Genetic factors: NOD2/CARD15 mutations |
| Disease-related |
| Ileal or ileocolic location |
| Less time from diagnosis to resection |
| Previous bowel resection |
| Penetrating complications |
| Perianal disease |
| Extensive involvement (or extensive resection) |
| Elevated anti-Saccharomyces cerevisiae antibodies (ASCA) |
| Presence of granulomas in the resected part |
| Severity of myenteric plexitis in the resected part |
| Surgery-related |
| None definitely related |
The only risk factor universally accepted and definitely related to the occurrence of POR is smoking. Patients with CD who smoke after surgery are 2.5 times more likely to have POR and twice as likely to have cPOR compared with non-smokers.18 As discussed above, it is possible that there are endoluminal factors related to the characteristics of the intestinal microbiota that increase the chances of developing POR. Very recently it has been suggested that patients with an increase in the genus Proteus and a reduction in Faecalibacterium have greater chances of developing POR, and specifically, people who smoke have a higher proportion of Proteus.19 Other potential factors that predispose people to POR have been evaluated in numerous studies, but the results are inconclusive and should be interpreted on a case-by-case basis. Some patients, such as those with a previous history of resection, aggressive onset with fistulising CD, perianal disease, resection greater than 50cm or extensive small bowel disease,17 have a more aggressive form of the disease requiring intensive early treatment or are at a greater risk of intestinal failure in case of repeated resection.
Regarding the surgical factors that could have an impact on the later development of POR, and after previous contradictory results and controversies about the type of intervention and anastomosis, it has been shown that there are no differences in POR rates in the short or long term between open and laparoscopic surgery,20 or between the type of anastomosis performed (end-to-end versus latero-lateral).21 Nor are there differences in the length of the resection margins between the healthy area and the affected area,22 and so the rule is that the macroscopically affected area alone must be resected with a minimum safety margin (approximately 2cm).
Myenteric plexitis, which involves submucosal mast cells, is the most clearly related pathological factor to the onset of early POR. Of the three published studies that have shown this association, only one is prospective23 and evaluates the presence of endoscopic lesions 3 months and one year after surgery. Early POR is more common with a greater severity of the plexitis in the margins of the resected area. However, the presence of plexitis is related to an enteric neuromodulation that increases paracellular permeability and may be an epiphenomenon related to inflammation.
Risk of postoperative recurrence in patients with end ileostomyThere is very little evidence on the actual incidence and the factors associated with POR after an end ileostomy has been performed. Traditionally it has been considered to be a situation in which the risk of new lesions is low, and so no preventive or monitoring measures were recommended. But the most recent data have described a higher-than-expected POR frequency. In this regard, retrospective studies have described that POR may occur in 35% of cases.24 In our country, a 31% of cPOR has been reported in patients who have undergone an end ileostomy, without identifying the associated clinical or epidemiological risk factors.25 The results of a recent meta-analysis have now been published, which included 18 studies with a total of more than 1000 patients. Here the risk of cPOR after proctocolectomy with end ileostomy was 28%, with accumulated mean rates of 23% and 40% after 5 and 10 years, respectively. Patients with a history of ileal involvement were at a 3.2-fold higher risk (RR 3.2; 95% CI; 1.8–5.6), while penetrating complications and being of a young age when CD was diagnosed were also associated with an increased risk.26 Therefore, although the POR rate in patients who have had end ileostomy is lower than when there is ileocolic anastomosis, it seems advisable to perform endoscopic monitoring even if the frequency, interpretation of the lesions and therapeutic approach have not been defined.
Therapeutic goals after bowel resection in Crohn's diseaseAs mentioned above, bowel resection in CD is usually indicated when CD-related complications occur or when there is no response to medical treatment. This fact, together with the high probability of POR if preventive measures are not taken, justifies considering these patients as an at-risk group or to have poor prognosis. The therapeutic goals after bowel resection are therefore more demanding and should be focused on preventing de novo lesions, or reversing or slowing the progression towards POR from minimal morphological lesions to new structural damage, with the ultimate aim of preserving maximum intestinal integrity.27
Preventing postoperative recurrenceSmoking cessationActive smoking is the most commonly associated factor with an increased risk of POR,18 a finding recently corroborated by prospective studies and large case-series.28,29 Active smoking is fairly common in these patients. In two recent Italian and Spanish multicentre cohorts, the proportion of patients who were active smokers at the time of surgery was about one-third.30,31 Although there is no (nor will there be) any controlled study that has evaluated this, the recommendation to stop smoking is considered to be the only measure that should be indicated to all patients with CD undergoing bowel resection. In this regard, several studies have shown that the fact of merely telling patients about the harmful effect of smoking may be useful for helping patients with CD to quit.32,33 In addition, smoking cessation entails benefits beyond the context of CD, because quitting reduces the risk of cardiovascular disease and of developing certain cancers, among others.
Pharmacological preventionNumerous controlled clinical trials have evaluated the efficacy of different drugs in the prevention of POR. The main objective of most of these trials has been the prevention of ePOR (defined as the existence of i2 lesions or higher as detected by ileocolonoscopy) as an indirect indicator of the risk of cPOR. As mentioned above, the risk of cPOR is high in the medium term in patients with i3–i4 lesions but clearly lower in cases of i2 lesions. In this sense, only some studies provide data related to the prevention of what has come to be called advanced or significant POR (defined by the existence of i3–i4 lesions), which seems to be of greater clinical relevance, at least in the short to medium term.
Some drugs, such as budesonide, oral interleukin-10 or some probiotics, have not been shown to be superior to placebo in preventing ePOR.17
Despite being the most studied drug for this indication, Mesalazine has only demonstrated a minor preventive effect in some of the different meta-analyses conducted in this regard.34,35 For this reason, together with its excellent safety profile and low cost, it could only be considered in patients at a low risk of POR or those with an absolute or relative contraindication for thiopurines or anti-TNF.
Nitroimidazole antibiotics (metronidazole, ornidazole) have been shown to be superior to placebo in the prevention of ePOR and cPOR in the short term (12 months).36,37 However, their use is associated not only with a high rate of adverse effects that compromise its short-term use (especially in relation to digestive intolerance) but also with the risk of potentially irreversible neurotoxicity if administered for prolonged periods. Its applicability in long-term prevention is therefore practically null, but its administration during the first 3 months after surgery concomitantly with thiopurines seems to slightly increase the short-term efficacy of thiopurines, probably due to its delayed therapeutic effect.38 For this reason, several controlled clinical trials have used them either in low-risk patients or together with other drugs in high-risk patients.29,39
The preventive administration of thiopurines has been evaluated in several clinical trials, though there are significant methodological differences among them, thus making it difficult to reach solid conclusions in this regard. In spite of this fact, the most recently published meta-analysis40 supports their effectiveness in preventing ePOR and cPOR. The two most suitably designed studies are consistent in showing that the 12-month ePOR rate is around 44% when using conventional doses of azathioprine (2–2.5mg/kg/day) combined with metronidazole during the first 3 months after surgery.38,41 The main limitation of thiopurines is the associated rate of treatment intolerance; in a controlled clinical trial of POR prevention strategies, 30% of patients meeting the criteria for a high risk of POR had a previous or current intolerance to thiopurines.29
Anti-TNF antibodies led to high expectations after the spectacular results obtained in a pilot study.42 The publication of the results of a controlled clinical trial with sufficient statistical power has shown that infliximab (at a dose of 5mg/kg every 8 weeks, with no induction regimen) is clearly superior to placebo in preventing medium-term ePOR (with 18-month ePOR rates of 22% in the infliximab group versus 51% in the placebo group; p<0.001). However, in this study there were no differences in the prevention of cPOR (13% vs 20%, respectively; p=0.97)43. So far, only one study has compared an anti-TNF agent with thiopurines in a controlled study. In a GETECCU-sponsored study, López-Sanromán et al. observed no differences in the 54-week ePOR rate between adalimumab (induction with 160mg and 80mg, and maintenance with 40mg every 2 weeks) and azathioprine (2–2.5mg/kg/day) combined with a course of metronidazole in the first 3 months after surgery (29% vs 33% according to the per protocol analysis; p=0.76), though the study may have had an insufficient statistical power.39
Prevention strategiesAs mentioned above, the risk of developing POR is associated with certain risk factors, which means that there is a population in which the risk/benefit ratio of starting preventive treatment with thiopurines or anti-TNF agents may not be justified. Most controlled studies of POR prevention have not stratified patients according to their risk factors, or have only included patients considered to be at a high risk (usually defined by the existence of penetrating complications, active smoking or previous surgery). The results of the first clinical trial in which the preventive treatment differed, depending on whether the patients were at a high or low risk of POR, so that high-risk patients received thiopurines (or adalimumab in cases of intolerance) and low-risk patients received only one course of metronidazole for 3 months, were recently published.29 Although there was some difference in the ePOR rate to the advantage of the low-risk group at the 18-month follow-up (50% vs 70%), this was not statistically significant (p=0.4), and half of the “low-risk” patients presented with ePOR after 18 months. Therefore, while waiting for new data in this respect, the decision to give preventive treatment based on the factors associated with POR available to date does not seem justified.
Some authors question the need to start pharmacological prevention systematically in all patients with CD who undergo ileocecal resection. A prospective, controlled and randomised study, sponsored by the International Organization for the Study of Inflammatory Bowel Diseases, evaluated the initiation of thiopurines after surgery or their introduction when ePOR was seen in an ileoscopy performed after 6 or 12 months of follow-up in “high-risk” CD patients (penetrating complications in the 2 months before resection, previous surgery, smoking, elevated C-reactive protein or use of antibiotics at the time of surgery and/or under 30 years of age).44 In the group of patients who at first did not start preventive treatment, 58% had ePOR (80% of them in the first endoscopic follow-up after 6 months), compared with 50% in the group treated systematically with azathioprine. Although there were no differences in the 18-month ePOR rates between the groups, the study included only 63 of the 200 patients initially planned to reach the necessary statistical power. We know that between 60% and 75% of patients who have ePOR within 12 months of surgery already have endoscopic lesions after 6 months.38,44 Last, in none of the studies—in which the start or intensification of treatment in patients with ePOR (either with mesalazine, azathioprine or adalimumab) was evaluated prospectively—did the disappearance of the lesions occur in more than 50% of the cases.29,45 For this reason, waiting to treat when lesions are present may be a risky strategy if we consider that these are patients who have already had a CD-related complication and have undergone bowel resection.
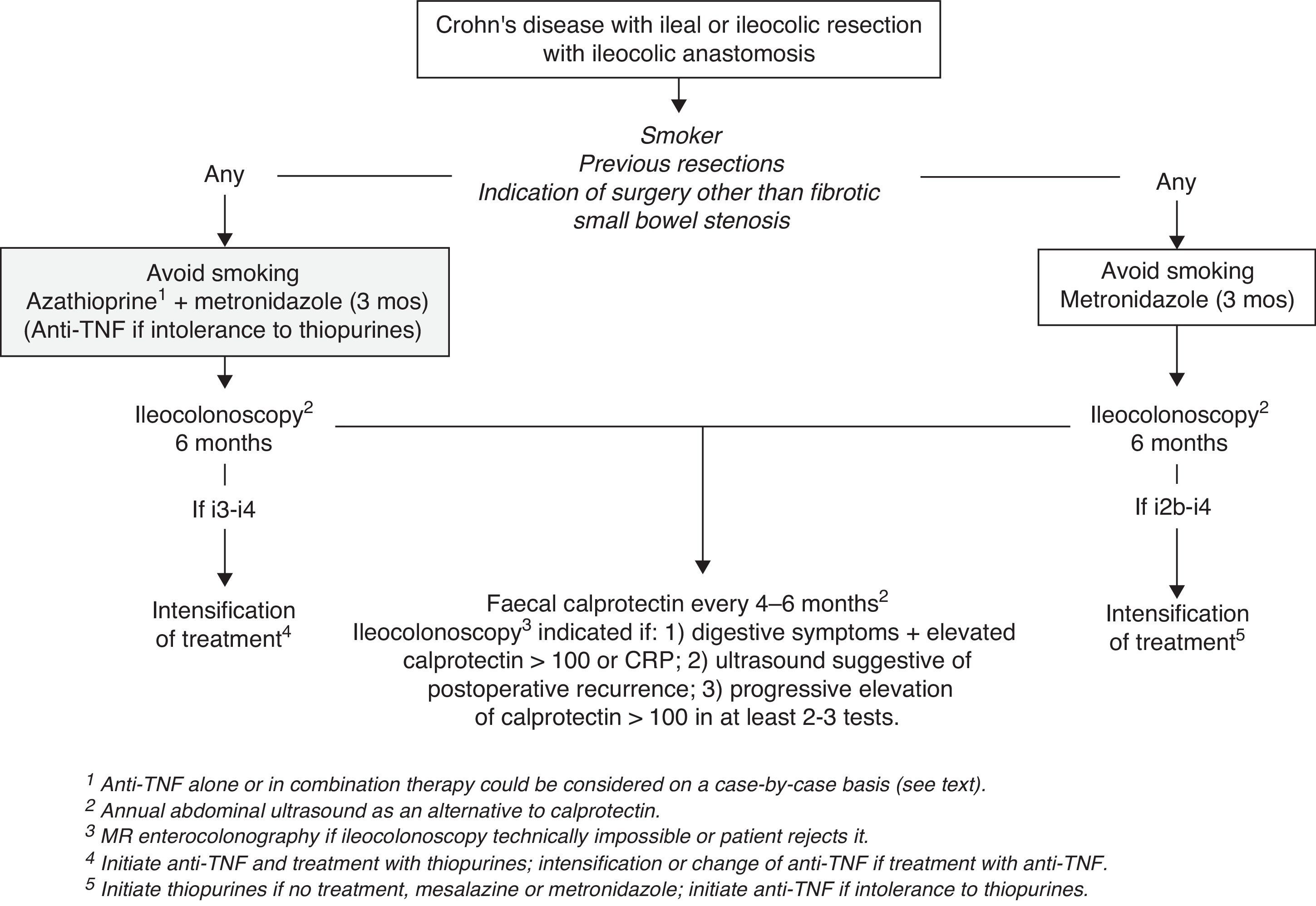
It is therefore recommended to insist on and facilitate smoking cessation and to start a 3-month course of metronidazole at doses of 15–20mg/kg/day in all patients with CD who are undergoing bowel resection. Also, except for patients with resections indicated due to fibrotic small bowel stenosis and with no previous resections, preventive treatment with thiopurines or anti-TNF agents should be started after surgery as soon as possible (Fig. 1). Since no differences between thiopurines and anti-TNF have been shown for the time being, and because of their reduced cost, thiopurines should be the drug of choice. However, treatment with an anti-TNF agent alone or as part of combined treatment should be considered individually and according to the patient's previous history of the disease and characteristics (age, comorbidities). A history of multiple bowel resections or extensive resections might justify the indication of anti-TNF (alone or in combination therapy), given the greater risk of short bowel syndrome if POR has occurred. Further, anti-TNF alone may be considered to avoid the use of thiopurines in cases of advanced age or history of lymphoma.
Follow-up of Crohn's disease after bowel resectionThe postoperative follow-up of CD should aim at detecting and assessing the severity of POR at a time when it is treatable. As mentioned above, POR is usually detectable in the first 6–12 months after surgery, and therefore, it is possible to separate the initial follow-up and the long-term follow-up. In fact, between 60% and 75% of patients presenting with ePOR 12 months after surgery have already had endoscopic lesions after 6 months,38,44 whereas after 12 months’ time the percentage increases only slightly, after 3 and 10 years, with a maximum of 79%.3 For this reason—and because of the better results regarding medium-term ePOR if an early endoscopic evaluation is performed (after 6 months) with intensive therapy in cases of endoscopic lesions29—it is currently considered that these patients should be monitored early after surgery (6 months).
In this section we will briefly examine the tools available for monitoring POR, and then we will propose a set of rules for follow-up that can be adjusted to most patients’ situations.
Endoscopic monitoring of postoperative recurrenceBecause the Rutgeerts index has been the gold standard for diagnosis, scoring and evaluation of morphological POR in both clinical trials and clinical practice, ileocolonoscopy remains the method of choice for initially evaluating POR. It is important to note that ileocolonoscopy should only be performed if there is a therapeutic plan defined in case ePOR is found. However, the use of ileocolonoscopy should depend neither on whether preventive treatment has been stared nor on which drug has been chosen (whether thiopurines or an anti-TNF agent), because we know that none is completely effective.
Endoscopy should attempt to examine at least 15–20cm of the neoterminal ileum and should allow for calculating the Rutgeerts index. The subsequent modification of this index by Domènech, i.e., dividing the recurrence score of i2 into i2a (lesions confined to anastomosis) and i2b (lesions outside anastomosis), allows us to clear up a controversial point, since i2a lesions may behave more benignly; however, this does not rule out their progression into anastomotic stenosis.12 Colonoscopy should be performed by a gastroenterologist with experience in the endoscopic evaluation of this type of lesion, as interobserver variability is high (especially in grade i2), and this may lead to incorrect therapeutic decisions.46 The correct interpretation and scoring of lesions is not trivial, given the risks and costs of the treatments used for POR; so whenever possible it would be advisable to record or photograph the images. It is necessary to take into account that ileoscopy may be technically impossible in up to 10% of cases,47 in which case the use of a segment-based imaging technique will be required as a starting test.
Less advanced lesions (i0 and i1) often do not progress to cPOR, but the more serious lesions (i3 and i4) almost always progress to it. However, this refers to a short- to medium-term follow-up, and there is no certainty that minor lesions will not result in a symptomatic recurrence if the follow-up is prolonged.10,48 For this reason, there are no guidelines or agreement on the duration of postoperative follow-up after the initial ileocolonoscopy, but the general opinion is that it should continue.48,49 The frequency of new ileocolonoscopies after a first examination (even if it has been normal) has not been established. However, there are some circumstances in which repeated endoscopy seems clearly indicated, such as when POR is suspected, whether based on clinical (symptoms) or biological (biomarkers) criteria or other non-invasive imaging tests.
Monitoring of postoperative recurrence with non-invasive methodsAt present, non-invasive methods are usually limited to the follow-up of POR after an initial endoscopic evaluation and are therefore part of a long-term follow-up strategy. As discussed below, some of these methods have an excellent correlation with POR evaluated by ileocolonoscopy, so that in the future they could be used (particularly those that are lower in cost, such as faecal calprotectin or abdominal ultrasound) as screening to decide what method to perform even at the first endoscopic examination. Non-invasive methods can be grouped into three types: clinical follow-up, inflammatory markers (mainly C-reactive protein and calprotectin) and imaging techniques.48
Clinical follow-upSymptom-based follow-up is not a reliable way to diagnose ePOR.48 The symptoms usually occur only when the endoscopic lesions are advanced. Thus, in the classic study by Rutgeerts, only 20% of patients had cPOR, compared with 73% with ePOR.3 Postoperative patients often experience clinical conditions, the symptoms of which may mimic a recurrence without being one, such as bile acid malabsorption, bacterial overgrowth or bridles.
C-reactive proteinC-reactive protein (CRP) is the serum marker of inflammation that best correlates with CD activity.50 It is widely available and its cost is reasonable, and so it is often requested during the postoperative follow-up of patients with CD. However, CRP does not show an adequate correlation with the appearance of endoscopic changes,51,52 and so it should be used as an additional piece of information without an absolute value. Still, persistently high values in the absence of another suspicion or in the presence of digestive symptoms would justify performing other examinations to rule out POR.
Faecal calprotectinFaecal calprotectin (FC) is currently the most promising biomarker of activity, and particularly remission, in inflammatory bowel disease. Its resistance to degradation, which makes it stable at room temperature up to 72h after obtaining the sample, and its low cost make it a strong candidate for use in monitoring POR. Nevertheless, it shows some intra-individual variability, and so it has been recommended that relevant decisions (such as starting immunosuppressive or biological treatment) be made based on more than one test.53,54
Several prospective studies that have evaluated the correlation of FC in the diagnosis of ePOR (defined in all of them by >i1 lesions on Rutgeerts’ index) conclude that an elevated FC correlates well with the presence of recurrent lesions. The usefulness of FC lies in its high negative predictive value; so values below 100mcg/g are highly indicative of the absence of ePOR.55–57 The joint use of PCR and FC is of special interest. The concurrence of respective values of <5mg/l and <100mcg/g has a negative predictive value of 81% for the diagnosis of ePOR.48,57 It is important to remember that FC values may remain high in the first 3 months after surgery, and its determination during this period is therefore not useful.53 Despite its promising potential in monitoring POR, further studies are needed to establish the frequency of testing and how many high values are cost-effective to indicate a diagnostic ileocolonoscopy.
Imaging techniquesThe use of imaging techniques to study POR is important in the follow-up after the initial ileocolonoscopy and in cases (fairly common) where ileocolonoscopy was not technically possible or because the patient refused to undergo colonoscopy. Each of these options has its advantages and disadvantages, but magnetic resonance enterocolonography is probably the most appropriate in this context. However, choosing the imaging test will generally depend on five factors: availability, experience, diagnostic certainty, safety and cost. The routine use of computed tomography (CT), even though the equipment has been modernised to result in shorter exposure times and smaller radiation doses, cannot be recommended for studying POR. Bowel transit time testing, in spite of being a widely available technique, has fallen into disuse because it is weighed down by the need for radiation and its low sensitivity in the diagnosis of POR.58
The difference between mucosal recurrence, detectable by endoscopy or video-capsule endoscopy, and the changes in the wall and vessels that are often observed in segment-based imaging techniques, is an aspect that has not been sufficiently investigated. The correlation between them is not perfect,59 and their relevance is not known, nor how to include it in sets of rules.
Abdominal ultrasound (simple or with contrast). Abdominal ultrasound is a technique available in most centres; it is cheap, involves no radiation and is well accepted by patients. On the other hand, obtaining adequate images is greatly dependent on a patient's anatomy (less satisfactory in obese patients and more satisfactory in the ascending colon, descending colon and terminal ileum) and the technician's experience. There is some controversy about the parameters that should be evaluated in studying POR, but there is unanimity in considering that the wall thickness of the neoileum is the most important factor. If there is a thickness greater than 3.5mm, the positive predictive value is 94% and the negative predictive value 71%.58 These results can be improved with the use of intraluminal contrast (macrogol), commonly called small intestine contrast ultrasonography (SICUS).60 Other data to be evaluated may include neoileum stiffness, retrograde dilatation (>25mm), ileal stenosis (<10mm) and the hyperechogenicity of mesenteric fat, fistulas or abscesses.61 To a certain extent the study of intestinal flow by Doppler ultrasound allows for the inflammation to be determined. Observing inflammation, together with any wall thickening, is probably the most reliable set of diagnostic criteria in the ultrasound study of POR.62
Magnetic resonance enterocolonography. Magnetic resonance enterocolonography combines some of the advantages of ultrasound (no radiation) with others of CT scans (reproducible images, assessment of extraluminal complications).58 A significant amount of oral contrast (polyethylene glycol, with or without mannitol) is usually given, and IV contrast is used to assess inflammation. The diagnostic capacity of magnetic resonance enterocolonography is at least similar to that of CT and even superior in the diagnosis of stenosis and inflammation. It has been evaluated repeatedly in checks performed for POR,63–65 but care must be taken in extrapolating data from studies that use enteroclysis, a more invasive, complex and bothersome technique, which cannot be recommended for routine use. The most characteristic signs of recurrence are thickening of the intestinal wall, hypervascularisation (the comb sign) and contrast uptake, while transmural images of complications, such as ulcers or fistulas, might indicate a more serious recurrence.63 A sensitivity of 75–90% has been reported, with a specificity of 80–100%.66 It is also possible to establish a score with magnetic resonance enterocolonography,63 the importance of which has not been validated. Unlike other radiological examinations, this is a very uncomfortable examination for the patient, difficult to interpret, and requires a dedicated and trained imaging specialist.
Video-capsule endoscopy. Its role in the diagnosis of POR is interesting, especially as it is the technique that most closely resembles ileocolonoscopy. In addition, it exceeds ileocolonoscopy in the capacity to evaluate the presence of proximal disease, which may be present in two out of every three patients (though this will not necessarily change the therapeutic approach). Its potential role in the diagnosis of POR has been evaluated several times,47,67,68 showing sensitivity-related figures lower than those of colonoscopy (around 75–80%) and a specificity close to 100%. However, its cost and the risk of impaction must be taken into account, which limit its use in cases of incomplete ileocolonoscopy and suspicion of stenotic recurrence.
Set of rules for the follow-up of Crohn's disease after bowel resectionWith the evidence available it seems advisable to perform an ileocolonoscopy 6 months after surgery (or the restoration of intestinal continuity in cases of temporary ileostomy) as the first way to evaluate POR, regardless of the preventive treatment the patient is on. At the same time it seems reasonable to carry out the examinations (as a baseline reference) that have been chosen as a method for long-term monitoring, with FC and transabdominal ultrasound being the most cost-effective in this indication (Fig. 1). With the data available, FC should be considered the most suitable test for long-term follow-up, with tests done every 4 to 6 months. Since this is a technique that depends on the technician and is not used often in intestinal evaluations in our setting, ultrasound should be considered if there is no FC available and it should probably be performed annually. Performing a new ileocolonoscopy would not be indicated in cases where FC is below 100mcg/mg, given the high negative predictive value that all studies have shown. However, repeating an ileocolonoscopy should be considered in the following cases: (1) appearance of digestive symptoms together with elevated biological markers, such as FC or CRP; (2) suspicion of POR by ultrasound; and (3) progressive elevation of FC in at least 2 or 3 consecutive tests. In patients who have previously had ileocolonoscopy and who present with mild-to-moderate lesions (i1–i2) and an FC value >100mcg/mg, periodic ileocolonoscopies may be considered, since FC has not been shown to determine the severity of recurrent lesions and the risk that mucosal lesions will progress lasts over time.
In patients who cannot have ileocolonoscopy or who refuse to have colonoscopy, magnetic resonance enterocolonography should be the technique of choice, performed at the same frequency and with the same criteria as for ileocolonoscopy.
Treatment of established postoperative recurrenceIn all, 25–50% of patients who start preventive treatment for POR with thiopurines or anti-TNF agents will develop endoscopic lesions in the first 6–12 months after surgery. In these patients the therapeutic goal should be geared towards eliminating or improving the lesions to prevent symptoms from developing. A large number of patients will therefore need their treatments changed or optimised if ePOR is detected. The results of recent studies on therapeutic management after bowel resection in patients with CD indicate that treatment intensification based on the 6-month endoscopic findings helps to better control the disease and is a cost-effective strategy.29,69,70 The POCER study compared two strategies for the follow-up and management of patients with CD and bowel resection.29 Patients on preventive treatment that was intensified based on endoscopic findings 6 months after surgery had a significantly lower ePOR rate—as well as a proportion of patients with normal ileoscopy (i0) that was significantly higher after 18 months—than the group of patients on preventive treatment who had not been monitored and had not had any change in treatment until the end of the study. However, it is also true that there were no differences in the rate of patients with advanced ePOR.
Salicylates, thiopurines and anti-TNF agents are the only drugs to have been evaluated for the treatment of POR. In a prospective, randomised controlled study, Reinisch et al.45 compared the efficacy of mesalazine and azathioprine for preventing cPOR in patients who had advanced ePOR (i3–i4) in the first 24 months after surgery and who were not on preventive treatment. The 78 patients included were randomised to receive azathioprine 2–2.5mg/kg/day or mesalazine 4g/day. Azathioprine led to a lower rate of cPOR and a higher rate of endoscopic improvement compared with those treated with mesalazine. We also have data from patients in the POCER study29 who presented with ePOR (defined by i2 lesions or higher) in the endoscopic follow-up performed 6 months after surgery and in whom treatment was “intensified”. After 12 months, treatment with thiopurines (in patients with no previous preventive treatment) showed endoscopic remission in 25%; adalimumab (in patients who had had previous preventive treatment with thiopurines) led to endoscopic remission in 39%; and the intensification of adalimumab from biweekly to weekly showed remission in 50% of cases.
Data from two open-label, prospective studies comparing different treatments for POR demonstrated that patients treated with anti-TNF agents have significantly higher percentages of improvement on the Rutgeerts index (69–75%) or a complete mucosal cure (38–54%) compared with patients treated with mesalazine (0%) or azathioprine (38%),69,70 as well as a lower rate of cPOR. Regueiro et al. published the results obtained in a controlled pilot study from patients who were treated initially with placebo and who developed ePOR by the end of the follow-up. Of the 12 patients who started infliximab, 58% showed endoscopic improvement, but about half (those with a more severe Rutgeerts score) required surgery after a mean follow-up of 3 years.71 One open-label study evaluated infliximab at a dose of 3mg/kg every 8 weeks for the treatment of ePOR (≥i2), showing that mucosal cure had been achieved in all treated patients after one year.72 Adalimumab has also been evaluated in this context in a prospective, open-label study in patients on preventive treatment with azathioprine, salicylates or infliximab and with ePOR 6 months after surgery; endoscopic improvement was seen in 60% after two years of treatment (with i0 lesions disappearing in 15%).73 Last, in a retrospective study of patients on preventive treatment with azathioprine, with ePOR (i2b or i3) but with no cPOR, the clinical course was compared after adding mesalazine (mean dose of 3g/day) or making no change in treatment. After almost two years of follow-up, improvement in the lesions was noted in 49%; there was no change in 22%; and 30% progressed, with no differences between the study groups.74
Therefore, we can conclude that aminosalicylates are not indicated in the treatment of established POR. For patients with ePOR with no previous preventive treatment, it is recommended to start treatment with thiopurines and/or anti-TNF agents if i2b lesions or higher appear, using the same criteria discussed in the prevention of POR. For patients on preventive treatment with thiopurines or anti-TNF agents, dose escalation seems more reasonable only in cases of advanced ePOR (i3–i4) (Fig. 1). In all cases, close monitoring should be done to assess the response. Although no data are available, it also seems advisable to insist on quitting smoking in patients with ePOR who smoke.
Conflicts of interestEugeni Domènech: scientific advice, research support and/or training activities of MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Takeda, Celgene, Ferring, Shire Pharmaceuticals, Tillotts Pharma, Gebro Pharma, Otsuka Pharmaceutical.
Antonio López San Román: scientific advice, research support and/or training activities of MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Takeda, Celgene, Ferring, Shire Pharmaceuticals, Tillotts Pharma, Gebro Pharma, FAES.
Pilar Nos: scientific advice, research support and/or training activities of Abbvie, Hospira, Pfizer, Kern Pharma. Takeda, Ferring, Otsuka Pharmaceutica.
Maribel Vera: scientific advice, research support and/or training activities of MSD, Abbvie, Pfizer, Ferring, Shire Pharmaceuticals, Takeda.
María Chaparro: scientific advice, research support and/or training activities of Tillotts Pharma, Shire Pharmaceuticals, Abbvie, MSD and Takeda.
Maria Esteve: scientific advice, research support and/or training activities of Tillotts Pharma, Abbie, MSD, Gebro Pharma, Takeda, MSD, Abbie and Faes Farma.
Javier P. Gisbert: scientific advice, research support and/or training activities of MSD, Abbvie, Hospira, Kern Pharma, Biogen, Takeda, Janssen, Pfizer, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma.
Míriam Mañosa: scientific advice, research support and/or training activities of MSD, Abbvie, Ferring, FAES, Otsuka Pharmaceutica, Shire Pharmaceuticals.
Please cite this article as: Domènech E, López-Sanromán A, Nos P, Vera M, Chaparro M, Esteve M, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre la monitorización, prevención y tratamiento de la recurrencia posquirúrgica en la enfermedad de Crohn. Gastroenterol Hepatol. 2017;40:472–483.