Data on anti-tumor necrosis factor (anti-TNF) treatment and suboptimal response (SOR) among patients with inflammatory bowel diseases (IBD) in Latin America (LATAM) are scarce. This study evaluated the incidence and indicators of SOR to anti-TNF therapy in patients with ulcerative colitis (UC) and Crohn's disease (CD) from Argentina, Colombia and Mexico.
Patients and methodsWe performed retrospective analysis of data from LATAM patients of the EXPLORE study (NCT03090139) including adult patients with IBD who initiated anti-TNF therapy between March 2010 to March 2015. The cumulative incidence of SOR to first-line anti-TNF therapy was assessed. A physician survey to assess barriers to anti-TNF therapies was also carried out.
ResultsWe included 185 IBD patients (UC/CD: 99/86) treated with first-line anti-TNF from Argentina (38 UC; 40 CD), Colombia (21 UC; 25 CD) and Mexico (40 UC; 21 CD). 36.4% of patients with UC and 46.5% of patients with CD experienced SOR to anti-TNF therapy during the median (interquartile range) observational period: 49.0 months (37.2–60.1) in UC, and 50.0 months (40.9–60.1) in CD. The most common indicator of SOR among patients was augmentation of non-biologic therapy (UC: 41.7%; CD: 35.0%). Affordability and late referral to IBD specialist care centers were the most common barriers to anti-TNF therapies.
ConclusionsSOR to anti-TNF therapy was common in LATAM IBD patients, where augmentation with non-biologic therapy represented the most frequent indicator of SOR across indications. Our findings contribute to the current evidence on the unmet needs associated with anti-TNF in LATAM.
Los datos sobre tratamiento con antagonistas del factor de necrosis tumoral (anti-TNF) y su respuesta subóptima (RSO) en las enfermedades inflamatorias intestinales (EII) en América Latina (LATAM) son escasos. Se evaluaron la incidencia e indicadores de RSO a anti-TNF en pacientes con colitis ulcerosa (CU) y enfermedad de Crohn (EC) de Argentina, Colombia y México.
Pacientes y métodosSe realizó un análisis retrospectivo de datos del estudio EXPLORE LATAM (NCT03090139), incluyendo pacientes adultos con EII que iniciaron anti-TNF entre marzo de 2010 a marzo de 2015. Se evaluó la incidencia acumulada de RSO a los anti-TNF en primera línea. Además, se realizó una encuesta a especialistas sobre las barreras del tratamiento con anti-TNF.
ResultadosSe incluyeron 185 pacientes con EII (CU/EC: 99/86) tratados con anti-TNF en primera línea de Argentina (38 CU; 40 EC), Colombia (21 CU; 25 EC) y México (40 CU; 21 EC); 36,4% de los pacientes con CU y 46,5% de los pacientes con EC experimentaron RSO a anti-TNF durante la mediana (intervalo intercuartílico) de 49 meses (37,2-60,1) en CU y 50 meses (40,9-60,1) en EC. El indicador más común de RSO fue el aumento del tratamiento no biológico (CU: 41,7%; EC: 35,0%). La accesibilidad y la derivación tardía a centros especializados fueron las barreras más comunes para el tratamiento con anti-TNF.
ConclusionesLa RSO a anti-TNF fue frecuente en pacientes con EII de LATAM, el aumento del tratamiento no biológico representó el indicador más frecuente de RSO. Nuestros hallazgos contribuyen a la evidencia actual sobre las necesidades insatisfechas asociadas a los anti-TNF en LATAM.
Inflammatory bowel diseases (IBD) are prevalent in Western countries. In North America and Europe, over 1.5 million and 2 million people suffer from the disease, respectively.1 In newly industrialized countries (NICs), IBD incidence and prevalence is lower; however, recent studies describe increasing rates including Latin America (LATAM).2,3 Main reasons for this epidemiological trend may include socioeconomic (environmental changes, urbanization, lifestyle modifications) as well as health-care related factors (increased IBD awareness).2
Anti-tumor necrosis factor (anti-TNF) agents were introduced during early 2000s for IBD treatment.4 These molecules have been shown to be effective both in inducing and maintaining remission in patients with moderate-to-severe ulcerative colitis (UC) and Crohn's disease (CD). Nevertheless, up to 22% of UC patients and 31% of CD patients are considered primary non-responders (PNR) to anti-TNF induction therapy. Furthermore, it has been estimated that 49%–59% of UC and 23%–64% of CD patients lose response to anti-TNF agents over time (secondary loss of response [SLOR]).5,6 As a consequence, UC and CD patients receiving anti-TNF agents may require dose optimization (escalation of dose and/or frequency of administration), treatment discontinuation or initiation of another biologic agent (switching or class swapping), augmentation of concurrent non-biologic therapy, or surgery. All these strategies are considered as indicators of suboptimal response to anti-TNF therapy.7,8
Since the epidemiology and clinical patterns of IBD differ between different countries and communities, the response to drugs could also be different due to several reasons, including microbiota, genetics and different treatment patterns.9,10 Data on anti-TNF treatment patterns and suboptimal response among IBD patients in LATAM are scarce. The EXPLORE study aimed to describe the incidence and indicators of suboptimal response to anti-TNF therapy in UC and CD patients in the clinical setting in NICs, including Asia-Pacific, LATAM, Russia and the Middle East regions.11 This study showed that suboptimal response to anti-TNF agents is common in IBD patients residing in NICs, but differences were reported across the regions.
We conducted a sub-analysis of the EXPLORE study in LATAM countries, with a focus on real-world suboptimal response of IBD patients to a first anti-TNF agent, diagnosis journey, barriers to anti-TNF prescription, local treatment pathways and other aspects specific to the region, such as the prevalence of opportunistic infections, especially TB.
Patients and methodsStudy design and data collectionThe EXPLORE study design has been described previously.11 Briefly, EXPLORE (NCT03090139) was an international, multicenter, retrospective medical chart review of adult patients with a confirmed diagnosis of UC or CD, treated (or previously treated) in IBD-specialized centers, and who initiated a first anti-TNF therapy (index date [ID]) between March 1st 2010 and March 1st 2015 (eligibility period). The observational period ranged from 2 years (patients who discontinued index therapy within 2 years of the ID) to up to 5 years post-ID (for those who continued therapy beyond 2 years), unless the patient died or was lost to follow-up. IBD-specialist centers from three LATAM countries participated in the EXPLORE study: Argentina, Colombia and Mexico. Comprehensive patient selection criteria have been previously described elsewhere.11 This study was conducted in accordance with local regulatory and ethical committee approval of each country (including patient written informed consent, where required). The study protocol conforms to the ethical guidelines of the Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Demographics, IBD type/description, biochemical and treatment data were extracted from either paper or electronic medical records by site personnel and recorded into a secure electronic data capture form. Biochemical activity (based on the closest assessment within 6 months prior to the ID) was defined as C-reactive protein ≥5mg/L, albumin <3.5g/dL or fecal calprotectin ≥250μg/mg.
Study outcomesSuboptimal response was defined as experiencing at least one of the specific indicators at any time during the observational period: (1) Anti-TNF dose escalation: any increase in dose and/or frequency of anti-TNF therapy occurring more than four months after initiation (to allow for induction period adjustments) for reasons related to non-response; (2) Augmentation with non-biologic therapy: initiating or increasing the dose and/or frequency of a concomitant non-biologic therapy (aminosalicylates, immunosuppressants, corticosteroids) for reasons related to non-response; (3) Discontinuation of anti-TNF therapy: for reasons related to non-response (e.g. discontinuation due to reimbursement issues or adverse events were not considered), including switching to another anti-TNF agent (within two months of discontinuation); (4) IBD-related surgery: colectomy, ileocolectomy, ostomy (colostomy or ileostomy), fistula repair (CD only), abscess repair (CD only), or strictureplasty (CD only); (5) IBD-related hospitalization: for admission reasons related to non-response/disease worsening and with stay ≥3 days.
Additional outcomes included PNR (defined as suboptimal response occurring within four months of index), SLOR (defined as suboptimal response occurring more than four months after index, among patients who did not experience PNR), anti-TNF therapy discontinuation (in general, regardless of the reason for discontinuation), describe main barriers to anti-TNF prescriptions, and in exploratory fashion to assess the prevalence of opportunistic infections in IBD patients on anti-TNF therapy.
Additionally, a physician survey of barriers that IBD specialists and non-IBD gastroenterology (GI) specialists (as perceived by IBD specialists) faced to prescribe anti-TNF therapies was carried out. Participating physicians (n=15) at study sites were asked to complete a single electronic questionnaire at the time of study initiation (between June 2017 and June 2018).
Statistical analysisPatients were stratified by condition (UC/CD) and by country. For all descriptive analysis, categorical variables were summarized as the number and percentages of patients in each category calculated over the number of subjects with available (non-missing) data. Continuous variables were summarized using the mean, standard deviation (SD), median, interquartile range (IQR), minimum, maximum and the eventual number of missing data.
Cumulative incidence of suboptimal response (CISR) to first anti-TNF therapy was analyzed using the Kaplan–Meier method. Patients were censored at the end of the observation period or when presenting an indicator of suboptimal response, and a log-rank test was used for group comparison. Treatment discontinuation due to reasons unrelated to response such as adverse event or reimbursement were excluded from this analysis. CISR to first anti-TNF therapy was assessed within 2 years of the ID and over the complete treatment period. Given the small number of patients (and thus, event rate), post hoc univariate and multivariate analyses were not conducted for the UC and CD cohorts.
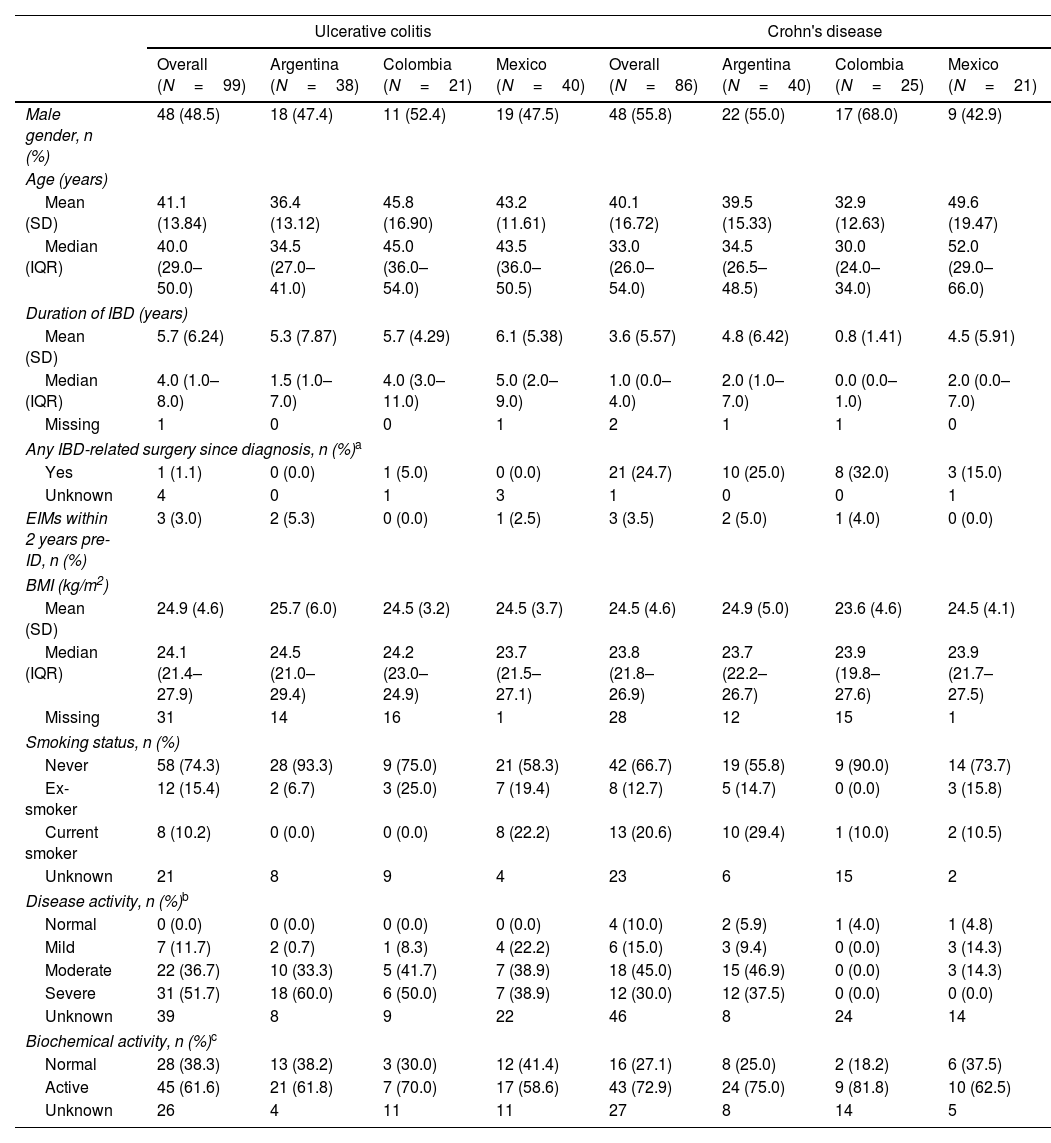
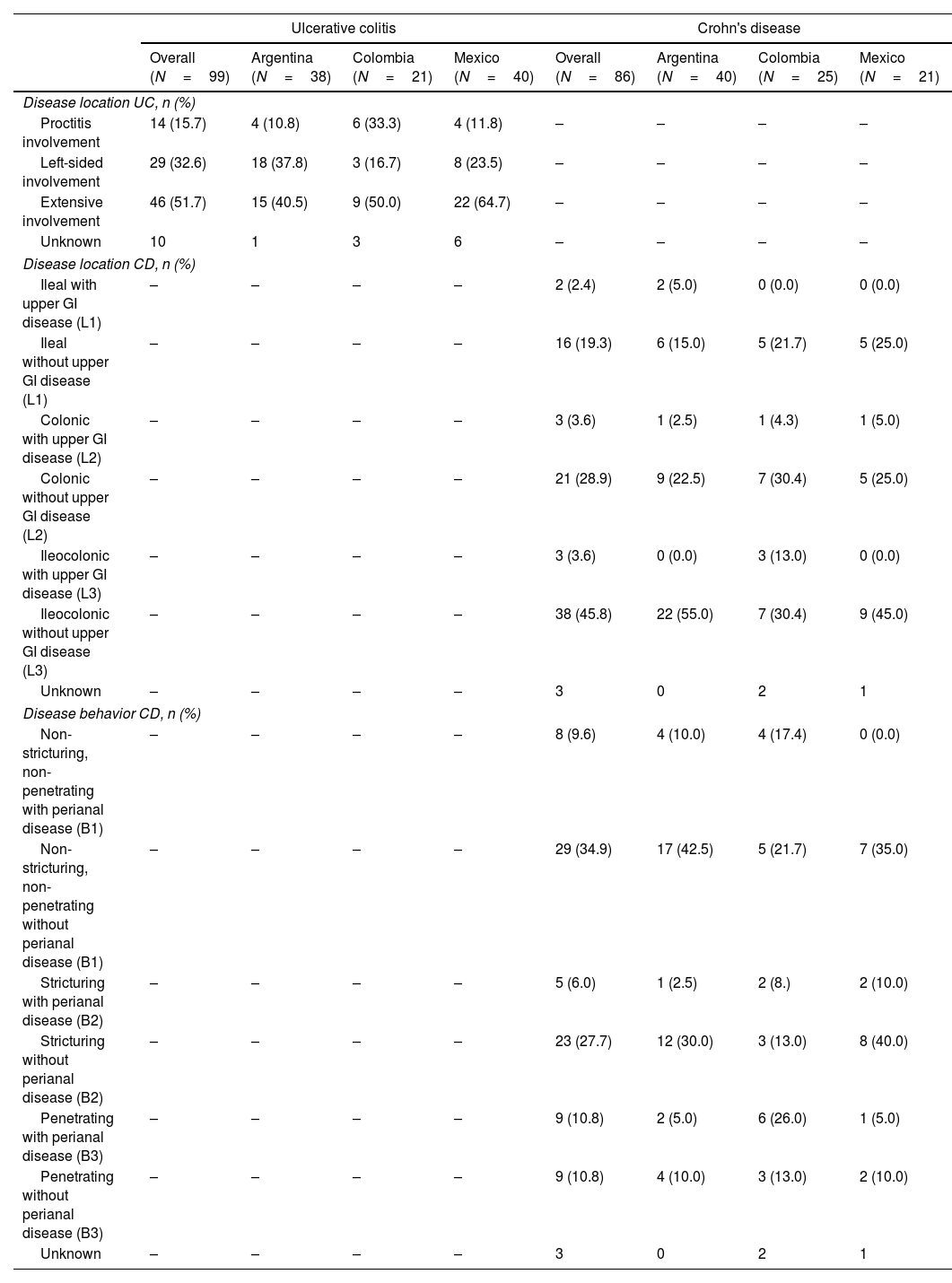
ResultsDemographic and clinical characteristicsThis study included 185 IBD LATAM patients. The UC LATAM population consisted of 99 subjects, including 38 patients from Argentina (38.4%), 21 from Colombia (21.2%) and 40 from Mexico (40.4%). The CD LATAM population consisted of 86 subjects, including 40 patients from Argentina (46.5%), 25 from Colombia (29.1%) and 21 from Mexico (24.4%). Median (IQR) observational period was 49.0 months (37.2–60.1) in the UC and 50.0 (40.9–60.1) months in the CD population. Mean (±SD) age at diagnosis was 34.3±13.6 years for UC and 35.8±16.1 for CD patients. Median time (IQR) since first IBD-related symptoms to the first anti-TNF therapy was 5.0 years (2.0–10.0) and 4.0 years (2.0–9.0) for UC and CD, respectively.
At index, median (IQR) duration of IBD was 4.0 years (1.0–8.0) for UC and 1.0 year (0.0–4.0) for CD and mean (±SD) age was 41.1±13.8 years for UC patients and 40.1±16.7 years for CD patients. Within 6 months prior to index, most UC patients had severe disease activity (51.7%), while moderate disease was the most frequent presentation among CD patients (45%). Further clinical characteristics are shown in Tables 1 and 2.
Demographic and clinical characteristics of UC and CD patients at index date.
| Ulcerative colitis | Crohn's disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N=99) | Argentina (N=38) | Colombia (N=21) | Mexico (N=40) | Overall (N=86) | Argentina (N=40) | Colombia (N=25) | Mexico (N=21) | |
| Male gender, n (%) | 48 (48.5) | 18 (47.4) | 11 (52.4) | 19 (47.5) | 48 (55.8) | 22 (55.0) | 17 (68.0) | 9 (42.9) |
| Age (years) | ||||||||
| Mean (SD) | 41.1 (13.84) | 36.4 (13.12) | 45.8 (16.90) | 43.2 (11.61) | 40.1 (16.72) | 39.5 (15.33) | 32.9 (12.63) | 49.6 (19.47) |
| Median (IQR) | 40.0 (29.0–50.0) | 34.5 (27.0–41.0) | 45.0 (36.0–54.0) | 43.5 (36.0–50.5) | 33.0 (26.0–54.0) | 34.5 (26.5–48.5) | 30.0 (24.0–34.0) | 52.0 (29.0–66.0) |
| Duration of IBD (years) | ||||||||
| Mean (SD) | 5.7 (6.24) | 5.3 (7.87) | 5.7 (4.29) | 6.1 (5.38) | 3.6 (5.57) | 4.8 (6.42) | 0.8 (1.41) | 4.5 (5.91) |
| Median (IQR) | 4.0 (1.0–8.0) | 1.5 (1.0–7.0) | 4.0 (3.0–11.0) | 5.0 (2.0–9.0) | 1.0 (0.0–4.0) | 2.0 (1.0–7.0) | 0.0 (0.0–1.0) | 2.0 (0.0–7.0) |
| Missing | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 |
| Any IBD-related surgery since diagnosis, n (%)a | ||||||||
| Yes | 1 (1.1) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 21 (24.7) | 10 (25.0) | 8 (32.0) | 3 (15.0) |
| Unknown | 4 | 0 | 1 | 3 | 1 | 0 | 0 | 1 |
| EIMs within 2 years pre-ID, n (%) | 3 (3.0) | 2 (5.3) | 0 (0.0) | 1 (2.5) | 3 (3.5) | 2 (5.0) | 1 (4.0) | 0 (0.0) |
| BMI (kg/m2) | ||||||||
| Mean (SD) | 24.9 (4.6) | 25.7 (6.0) | 24.5 (3.2) | 24.5 (3.7) | 24.5 (4.6) | 24.9 (5.0) | 23.6 (4.6) | 24.5 (4.1) |
| Median (IQR) | 24.1 (21.4–27.9) | 24.5 (21.0–29.4) | 24.2 (23.0–24.9) | 23.7 (21.5–27.1) | 23.8 (21.8–26.9) | 23.7 (22.2–26.7) | 23.9 (19.8–27.6) | 23.9 (21.7–27.5) |
| Missing | 31 | 14 | 16 | 1 | 28 | 12 | 15 | 1 |
| Smoking status, n (%) | ||||||||
| Never | 58 (74.3) | 28 (93.3) | 9 (75.0) | 21 (58.3) | 42 (66.7) | 19 (55.8) | 9 (90.0) | 14 (73.7) |
| Ex-smoker | 12 (15.4) | 2 (6.7) | 3 (25.0) | 7 (19.4) | 8 (12.7) | 5 (14.7) | 0 (0.0) | 3 (15.8) |
| Current smoker | 8 (10.2) | 0 (0.0) | 0 (0.0) | 8 (22.2) | 13 (20.6) | 10 (29.4) | 1 (10.0) | 2 (10.5) |
| Unknown | 21 | 8 | 9 | 4 | 23 | 6 | 15 | 2 |
| Disease activity, n (%)b | ||||||||
| Normal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (10.0) | 2 (5.9) | 1 (4.0) | 1 (4.8) |
| Mild | 7 (11.7) | 2 (0.7) | 1 (8.3) | 4 (22.2) | 6 (15.0) | 3 (9.4) | 0 (0.0) | 3 (14.3) |
| Moderate | 22 (36.7) | 10 (33.3) | 5 (41.7) | 7 (38.9) | 18 (45.0) | 15 (46.9) | 0 (0.0) | 3 (14.3) |
| Severe | 31 (51.7) | 18 (60.0) | 6 (50.0) | 7 (38.9) | 12 (30.0) | 12 (37.5) | 0 (0.0) | 0 (0.0) |
| Unknown | 39 | 8 | 9 | 22 | 46 | 8 | 24 | 14 |
| Biochemical activity, n (%)c | ||||||||
| Normal | 28 (38.3) | 13 (38.2) | 3 (30.0) | 12 (41.4) | 16 (27.1) | 8 (25.0) | 2 (18.2) | 6 (37.5) |
| Active | 45 (61.6) | 21 (61.8) | 7 (70.0) | 17 (58.6) | 43 (72.9) | 24 (75.0) | 9 (81.8) | 10 (62.5) |
| Unknown | 26 | 4 | 11 | 11 | 27 | 8 | 14 | 5 |
IBD: inflammatory bowel disease; IQR: interquartile range; SD: standard deviation. ID: index date. Percentage among the total number of patients with available data.
IBD-related surgeries including total proctocolectomy, total and partial colectomy, ileocolonic bowel resection, small bowel resection, strictureplasty, perianal surgery, ileostomy reversal.
Disease activity primarily based on the closest assessment within 6 months prior to the index date of any endoscopic measurement if available, or of any documented measurement of full Mayo (UC; 0–2 Normal, 3–5 Mild, 6–10 Moderate, 11–12 Severe), partial Mayo (UC; 0–1 Normal, 2–4 Mild, 5–7 Moderate, >7 Severe), CDAI (CD; <150 Normal, 150–219 Mild, 220–450 Moderate, >450 Severe), HBI (CD; 0–4 Normal, 5–7 Mild, 8–16 Moderate, ≥16 Severe) or PGA (0 Normal, 1 Mild, 2 Moderate, 3 Severe).
Disease location and behavior in UC and CD patients.
| Ulcerative colitis | Crohn's disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N=99) | Argentina (N=38) | Colombia (N=21) | Mexico (N=40) | Overall (N=86) | Argentina (N=40) | Colombia (N=25) | Mexico (N=21) | |
| Disease location UC, n (%) | ||||||||
| Proctitis involvement | 14 (15.7) | 4 (10.8) | 6 (33.3) | 4 (11.8) | – | – | – | – |
| Left-sided involvement | 29 (32.6) | 18 (37.8) | 3 (16.7) | 8 (23.5) | – | – | – | – |
| Extensive involvement | 46 (51.7) | 15 (40.5) | 9 (50.0) | 22 (64.7) | – | – | – | – |
| Unknown | 10 | 1 | 3 | 6 | – | – | – | – |
| Disease location CD, n (%) | ||||||||
| Ileal with upper GI disease (L1) | – | – | – | – | 2 (2.4) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Ileal without upper GI disease (L1) | – | – | – | – | 16 (19.3) | 6 (15.0) | 5 (21.7) | 5 (25.0) |
| Colonic with upper GI disease (L2) | – | – | – | – | 3 (3.6) | 1 (2.5) | 1 (4.3) | 1 (5.0) |
| Colonic without upper GI disease (L2) | – | – | – | – | 21 (28.9) | 9 (22.5) | 7 (30.4) | 5 (25.0) |
| Ileocolonic with upper GI disease (L3) | – | – | – | – | 3 (3.6) | 0 (0.0) | 3 (13.0) | 0 (0.0) |
| Ileocolonic without upper GI disease (L3) | – | – | – | – | 38 (45.8) | 22 (55.0) | 7 (30.4) | 9 (45.0) |
| Unknown | – | – | – | – | 3 | 0 | 2 | 1 |
| Disease behavior CD, n (%) | ||||||||
| Non-stricturing, non-penetrating with perianal disease (B1) | – | – | – | – | 8 (9.6) | 4 (10.0) | 4 (17.4) | 0 (0.0) |
| Non-stricturing, non-penetrating without perianal disease (B1) | – | – | – | – | 29 (34.9) | 17 (42.5) | 5 (21.7) | 7 (35.0) |
| Stricturing with perianal disease (B2) | – | – | – | – | 5 (6.0) | 1 (2.5) | 2 (8.) | 2 (10.0) |
| Stricturing without perianal disease (B2) | – | – | – | – | 23 (27.7) | 12 (30.0) | 3 (13.0) | 8 (40.0) |
| Penetrating with perianal disease (B3) | – | – | – | – | 9 (10.8) | 2 (5.0) | 6 (26.0) | 1 (5.0) |
| Penetrating without perianal disease (B3) | – | – | – | – | 9 (10.8) | 4 (10.0) | 3 (13.0) | 2 (10.0) |
| Unknown | – | – | – | – | 3 | 0 | 2 | 1 |
CD: Crohn's disease; IBD: inflammatory bowel disease; GI: gastrointestinal; UC: ulcerative colitis. Percentage among the total number of patients with available data.
More than half of UC patients presented with extensive disease (n=46, 51.7%). In CD, half of the patients (n=41, 49.4%) presented with ileocolonic involvement, non-stricturing and non-penetrating disease behavior was present in 37 patients (44.5%); and perianal disease was present in 22 patients (26.5%). Detailed information on UC and CD location and behavior is described in Table 2.
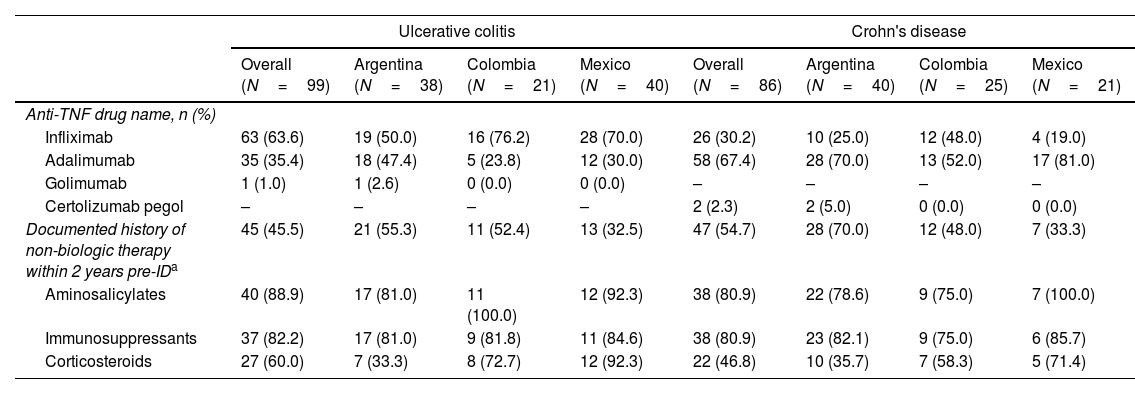
Anti-TNF and non-biologic treatment history at indexThe most frequent first-line anti-TNF treatment for UC patients was infliximab (63.6%), followed by adalimumab (35.4%); and only one patient received golimumab. In all countries, infliximab was the most prescribed first anti-TNF; however, in Argentina, the prescription of infliximab barely exceeded that of adalimumab (50% and 47.4%, respectively) (Table 3).
Anti-TNF therapy and non-biologic treatment history at index date.
| Ulcerative colitis | Crohn's disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N=99) | Argentina (N=38) | Colombia (N=21) | Mexico (N=40) | Overall (N=86) | Argentina (N=40) | Colombia (N=25) | Mexico (N=21) | |
| Anti-TNF drug name, n (%) | ||||||||
| Infliximab | 63 (63.6) | 19 (50.0) | 16 (76.2) | 28 (70.0) | 26 (30.2) | 10 (25.0) | 12 (48.0) | 4 (19.0) |
| Adalimumab | 35 (35.4) | 18 (47.4) | 5 (23.8) | 12 (30.0) | 58 (67.4) | 28 (70.0) | 13 (52.0) | 17 (81.0) |
| Golimumab | 1 (1.0) | 1 (2.6) | 0 (0.0) | 0 (0.0) | – | – | – | – |
| Certolizumab pegol | – | – | – | – | 2 (2.3) | 2 (5.0) | 0 (0.0) | 0 (0.0) |
| Documented history of non-biologic therapy within 2 years pre-IDa | 45 (45.5) | 21 (55.3) | 11 (52.4) | 13 (32.5) | 47 (54.7) | 28 (70.0) | 12 (48.0) | 7 (33.3) |
| Aminosalicylates | 40 (88.9) | 17 (81.0) | 11 (100.0) | 12 (92.3) | 38 (80.9) | 22 (78.6) | 9 (75.0) | 7 (100.0) |
| Immunosuppressants | 37 (82.2) | 17 (81.0) | 9 (81.8) | 11 (84.6) | 38 (80.9) | 23 (82.1) | 9 (75.0) | 6 (85.7) |
| Corticosteroids | 27 (60.0) | 7 (33.3) | 8 (72.7) | 12 (92.3) | 22 (46.8) | 10 (35.7) | 7 (58.3) | 5 (71.4) |
TNF: tumor necrosis factor; ID: index date.
Among CD subjects, adalimumab was the most prescribed first-line anti-TNF (67.4%), followed by infliximab (30.2%) and certolizumab pegol (2.3%). No patient received biosimilars. Adalimumab was highly prescribed in Argentina (70%) and Mexico (81%). Nevertheless, prescription rates of adalimumab and infliximab were similar in Colombia (52% and 48%, respectively) (Table 3).
About 45% of UC patients reported a history of non-biologic therapy within two years of ID while half of CD patients (54.7%) had a documented history of such treatment strategies. Detailed information on non-biologic treatment history is described in Table 3.
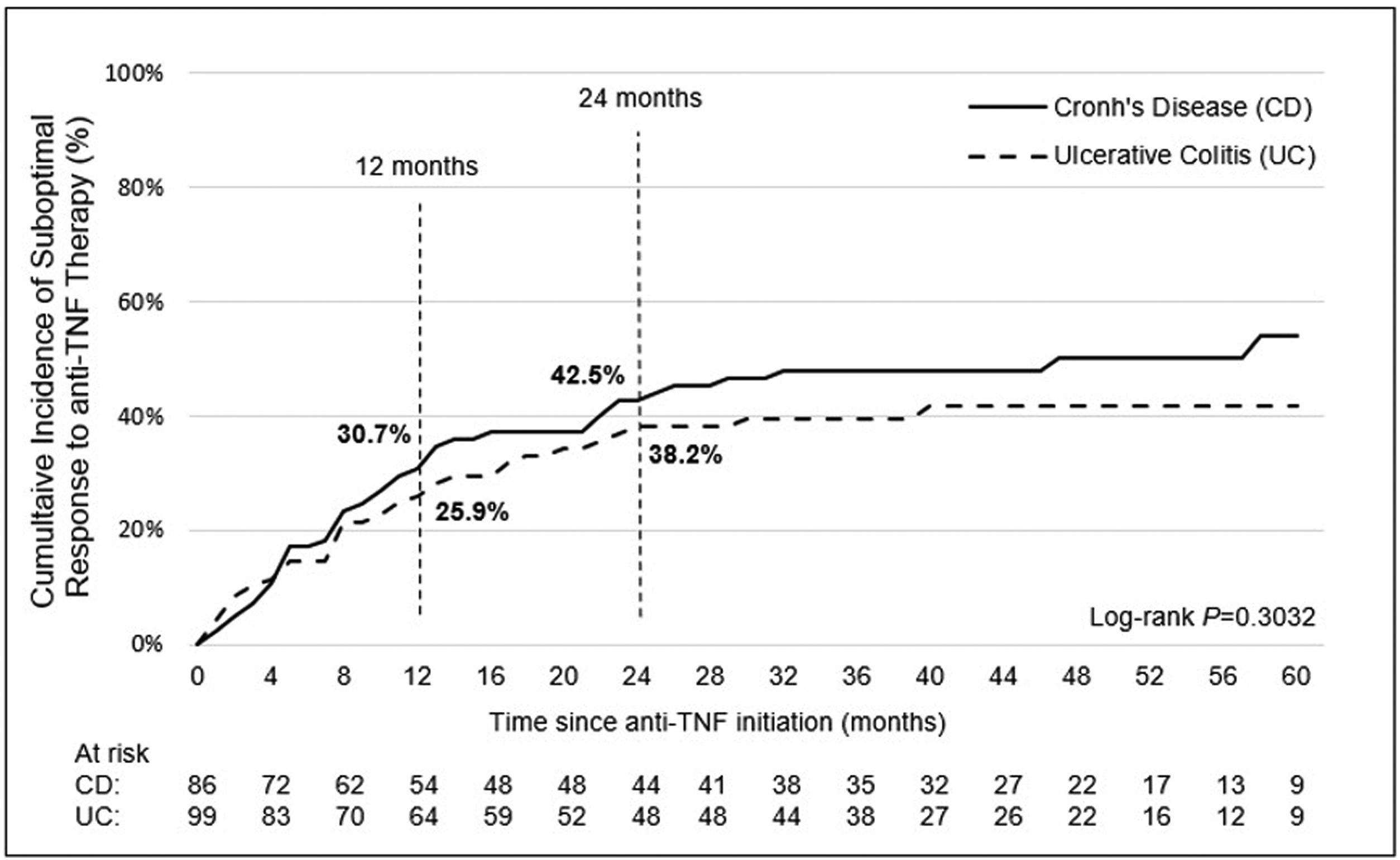
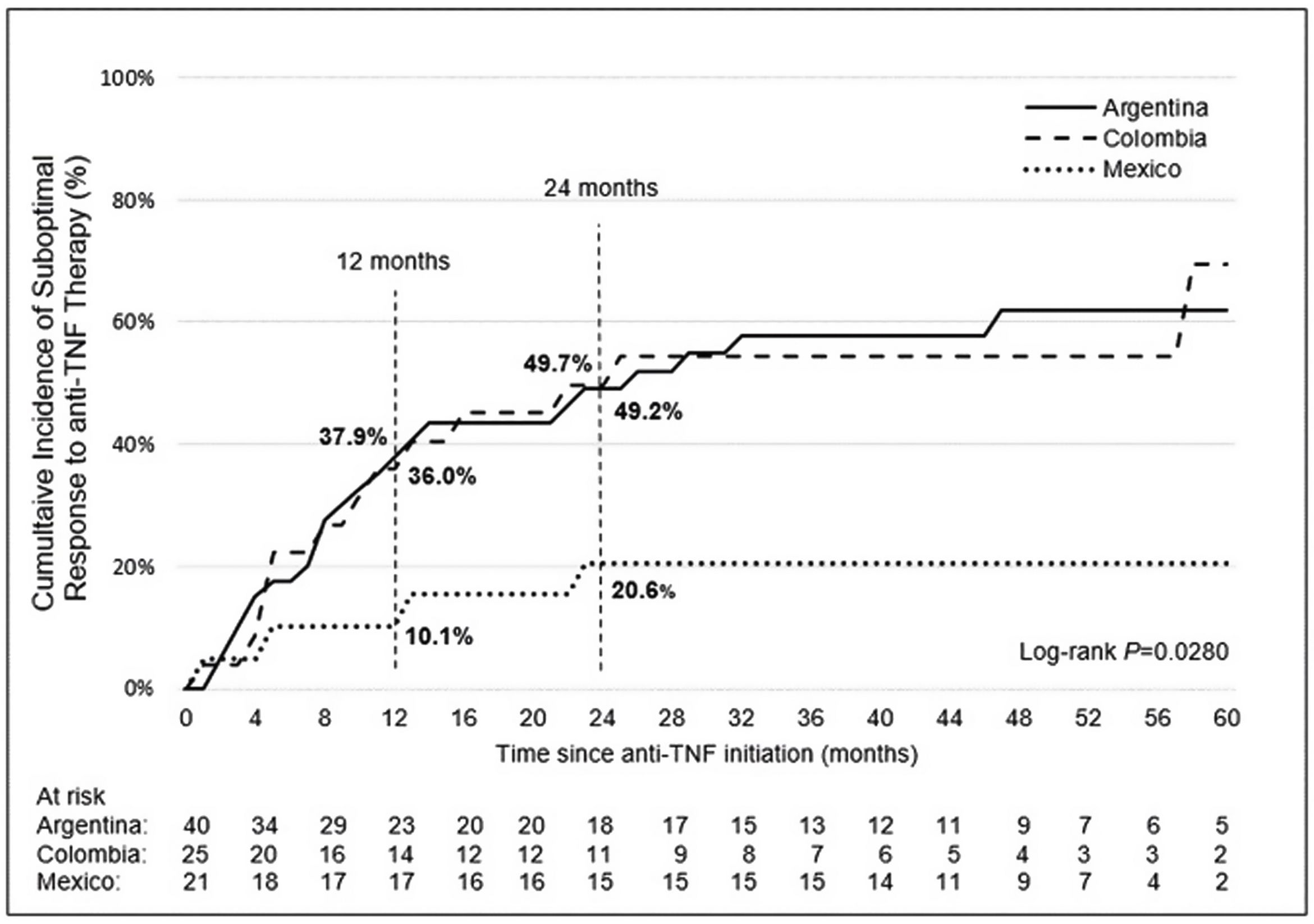
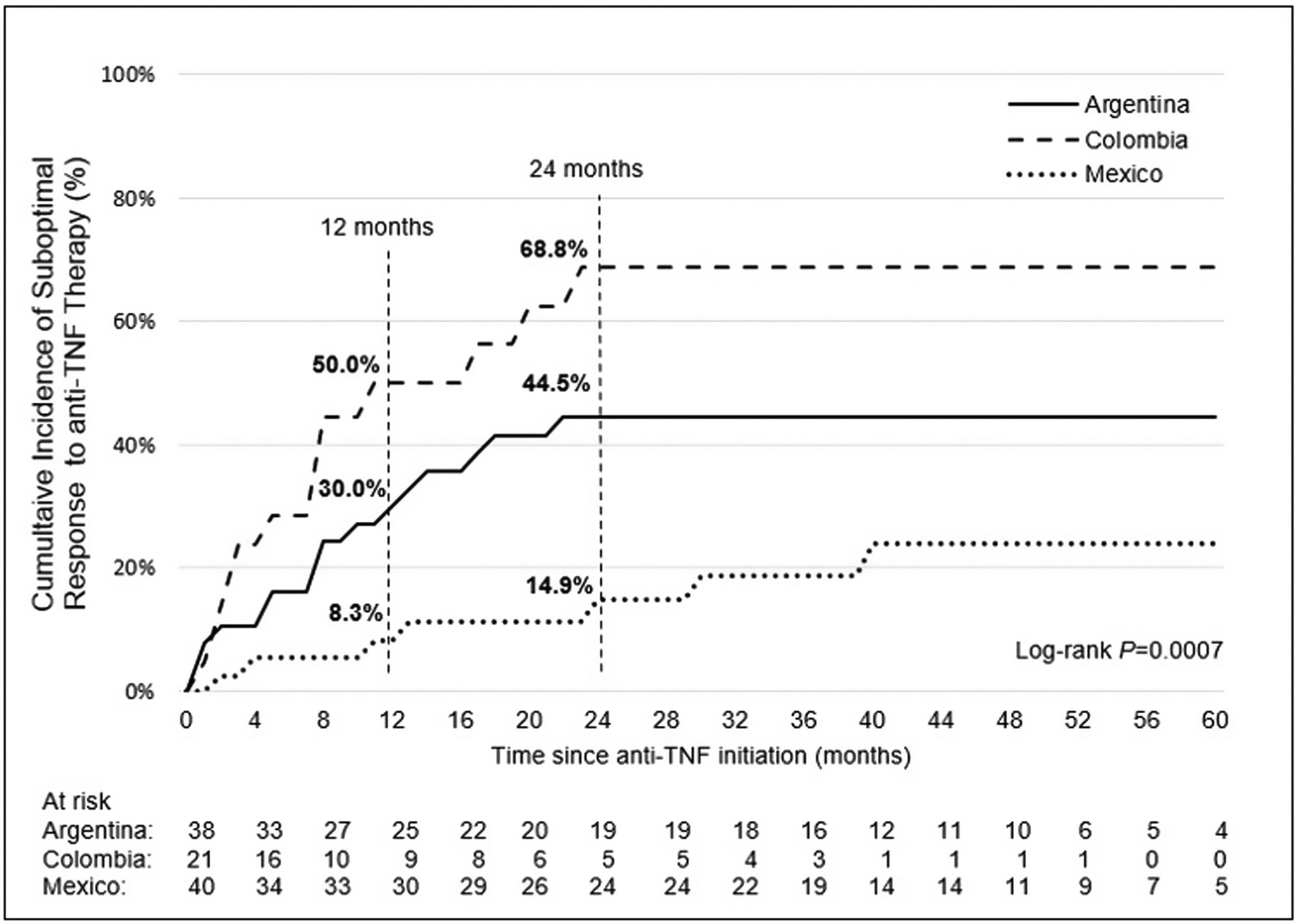
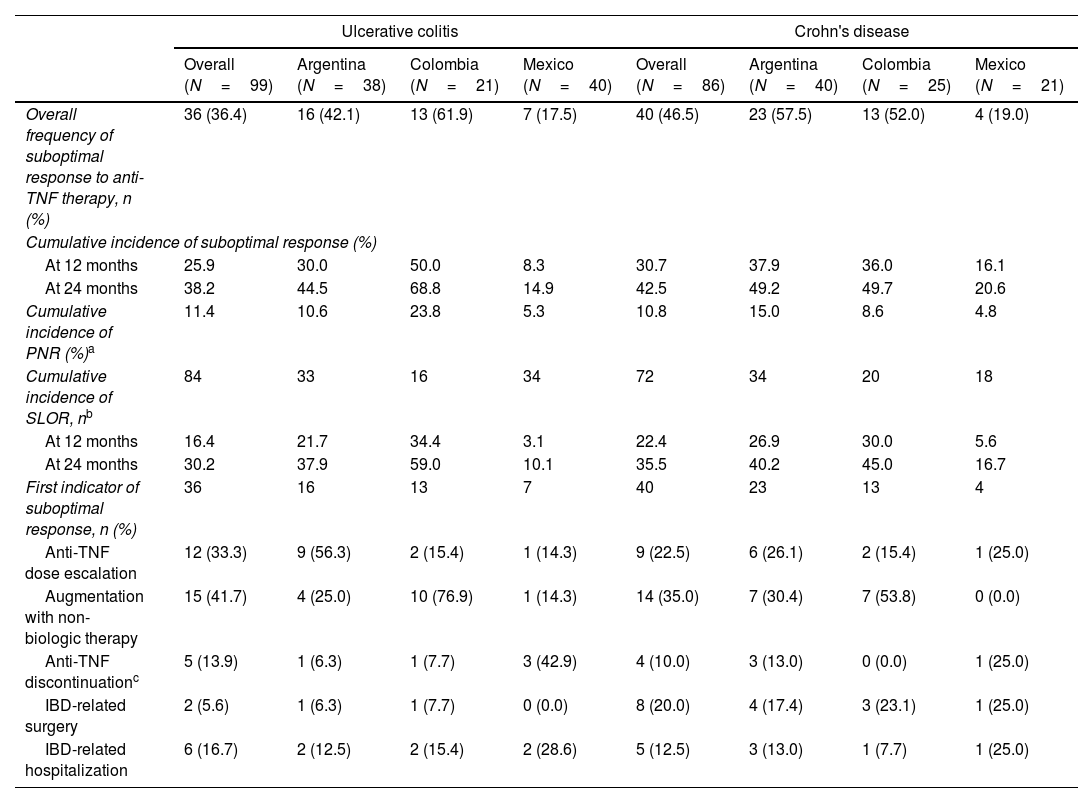
Incidence and indicators of suboptimal response to anti-TNF therapyOne-third of UC patients (36.4%) and nearly one-half of CD (46.5%) patients in LATAM experienced suboptimal response to their first anti-TNF therapy during the observational period. Overall, CISR in LATAM was similar between UC and CD. CISR was 25.9% at 12 months and 38.2% at 24 months in UC patients. Corresponding values for CD patients were 30.7% and 42.5%, respectively (Fig. 1; Table 4). Differences in CISR were observed between countries, especially in CD; Mexico had the lowest rates for both UC (12 months: 8.3%; 24 months: 14.9%) and CD (12 months: 16.1%; 24 months: 20.6%) compared to Argentina and Colombia (UC: log-rank p-value <0.05; CD: log-rank p-value <0.0001) (Figs. 2 and 3; Table 4). Cumulative incidence of PNR was 11.4% for UC and 10.8% for CD patients, and cumulative incidence of SLOR at 12 and 24 months was 16.4% and 30.2% in UC patients, respectively, and 22.4% and 35.5% in CD patients (Table 4). The most common first indicator of suboptimal response was augmentation of non-biologic therapy (UC: 41.7%, CD: 35%). Nevertheless, the most frequent indicator of suboptimal response to anti-TNF varied across the region (Table 4).
Overall frequency and cumulative incidence of suboptimal response to first-line anti-TNF therapy in UC and CD patients.
| Ulcerative colitis | Crohn's disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N=99) | Argentina (N=38) | Colombia (N=21) | Mexico (N=40) | Overall (N=86) | Argentina (N=40) | Colombia (N=25) | Mexico (N=21) | |
| Overall frequency of suboptimal response to anti-TNF therapy, n (%) | 36 (36.4) | 16 (42.1) | 13 (61.9) | 7 (17.5) | 40 (46.5) | 23 (57.5) | 13 (52.0) | 4 (19.0) |
| Cumulative incidence of suboptimal response (%) | ||||||||
| At 12 months | 25.9 | 30.0 | 50.0 | 8.3 | 30.7 | 37.9 | 36.0 | 16.1 |
| At 24 months | 38.2 | 44.5 | 68.8 | 14.9 | 42.5 | 49.2 | 49.7 | 20.6 |
| Cumulative incidence of PNR (%)a | 11.4 | 10.6 | 23.8 | 5.3 | 10.8 | 15.0 | 8.6 | 4.8 |
| Cumulative incidence of SLOR, nb | 84 | 33 | 16 | 34 | 72 | 34 | 20 | 18 |
| At 12 months | 16.4 | 21.7 | 34.4 | 3.1 | 22.4 | 26.9 | 30.0 | 5.6 |
| At 24 months | 30.2 | 37.9 | 59.0 | 10.1 | 35.5 | 40.2 | 45.0 | 16.7 |
| First indicator of suboptimal response, n (%) | 36 | 16 | 13 | 7 | 40 | 23 | 13 | 4 |
| Anti-TNF dose escalation | 12 (33.3) | 9 (56.3) | 2 (15.4) | 1 (14.3) | 9 (22.5) | 6 (26.1) | 2 (15.4) | 1 (25.0) |
| Augmentation with non-biologic therapy | 15 (41.7) | 4 (25.0) | 10 (76.9) | 1 (14.3) | 14 (35.0) | 7 (30.4) | 7 (53.8) | 0 (0.0) |
| Anti-TNF discontinuationc | 5 (13.9) | 1 (6.3) | 1 (7.7) | 3 (42.9) | 4 (10.0) | 3 (13.0) | 0 (0.0) | 1 (25.0) |
| IBD-related surgery | 2 (5.6) | 1 (6.3) | 1 (7.7) | 0 (0.0) | 8 (20.0) | 4 (17.4) | 3 (23.1) | 1 (25.0) |
| IBD-related hospitalization | 6 (16.7) | 2 (12.5) | 2 (15.4) | 2 (28.6) | 5 (12.5) | 3 (13.0) | 1 (7.7) | 1 (25.0) |
IBD: inflammatory bowel disease; PNR: primary non-response; SLOR: secondary loss of response; TNF: tumor necrosis factor.
The survey was completed by 15 physicians (6 from Mexico, 5 from Argentina and 4 from Colombia), who treated IBD patients for a median (range) of 13 years (5–30) and prescribed anti-TNF agents for a median (range) of 10 years (3–13). The median rate of UC and CD patients managed at each institution was 15% and 8%, respectively, with a wide variation among countries. The estimated median (range) number of biologic-naïve UC and CD patients being referred to these centers were 9 (1–100) and 20 (4–100) patients, respectively. A median proportion of 3% of UC and 1% of CD patients who were medically indicated for anti-TNF therapy did not receive such treatments during 2016. Barriers for prescribing anti-TNF therapy were also analyzed. Among IBD specialists, patient affordability (27%) and late referral to IBD specialist care centers (27%) were the most commonly reported barriers. According to IBD specialists, late diagnosis (40%) was the primary barrier to prescription for non-IBD GI specialists. Data are summarized in Supplementary Tables 1 and 2.
Prevalence of TB history at first dose of anti-TNF therapyIn patients with UC, TB screening status at ID was documented for 91.9% (n=91) of patients. Among patients with documented TB screening status at index date, 96.7% (n=88) were screened for TB prior to anti-TNF initiation. Rate of positive screening result was 4.6% (90.5% latent TB [LTB]), with wide variations among countries (0% for Argentina, 5.7% for Mexico and 11.8% for Colombia). The prevalence of TB history among patients screened at ID or with a known history of TB prior to ID was 4.4% (n=4).
In patients with CD, TB screening status at ID was documented for 97.7% (n=84). Among patients with documented TB screening status at ID, 97.6% (n=82) were screened for TB prior to anti-TNF initiation. Rate of positive screening result was 6.1% (85.7% LTB), also with important variations among countries (0% for Argentina, 10% for Mexico and 13% for Colombia).
DiscussionThe present study describes the largest cohort of LATAM IBD patients with anti-TNFs utilization to date. Our main goal was to establish the CISR to anti-TNF agents in UC and CD patients in LATAM participants of the EXPLORE study.11 Our results showed that suboptimal response to the first anti-TNF for both conditions is common in LATAM, occurring in more than one-third of UC patients (36.4%) and nearly half of CD patients (46.5%) at 24 months, respectively. A multinational retrospective chart review study from Europe and Canada (n=1195 subjects) showed that more than half of IBD patients (CU: 64.1%; CD: 58.1%) had at least one indicator of suboptimal response to anti-TNFs at 24 months.12 In the latter study, infliximab users represented 92.2% and 55.6% of UC and CD patient cohorts, respectively. A nationwide population study in South Korea showed a higher CISR in UC patients at 24 months (80.5%).13 Similarly, a large commercial U.S. claims database study found that 86% of both, UC and CD patients experience at least one indicator of suboptimal response at 24 months.14 In the present study, PNR CI rate was 11.4% for UC and 10.8% for CD patients. Both percentages were slightly lower compared with the main study (13.6% and 16.9%, respectively). In contrast, SLOR rates in LATAM for both diseases were higher compared to the main EXPLORE study.11
Shin et al. found that UC patients receiving adalimumab were significantly more likely to experience at least one suboptimal response than those patients on infliximab (representing 56.2% of the overall UC patient population), driven by a higher risk of drug discontinuation and dose escalation.13 Thus, variations in the proportion of the different anti-TNF agents are expected to impact the rate of suboptimal response to these therapies. In the present study, the most frequent first-line anti-TNF agent for UC patients was infliximab, while adalimumab was the most prescribed agent for CD subjects. Nearly 45% of adalimumab prescriptions were related to the preferred route of administration. Of note, adalimumab was frequently prescribed for both UC and CD in Argentina. Several country-specific differences were reported in prescription rates, availability, accessibility, and local experience may partly explain such differences. Overall, most patients received anti-TNFs at infusion centers; however, self-administration or home infusion was common in Argentina and Mexico, but infrequent in Colombia.
Differences in the incidence of suboptimal response to anti-TNFs, and the most frequent suboptimal indicator were observed across countries and indications, probably reflecting different IBD management practices. The most common indicator of suboptimal response among LATAM patients was augmentation of non-biologic therapies (UC: 41.7%; CD: 35%), while IBD-related hospitalization was the leading indicator in the main study (UC: 33%; CD: 36.1%), driven by Asia-Pacific and Russia/Middle East cohorts.11 In fact, IBD-related hospitalizations were the main indicator of suboptimal response in the Chinese cohort of the EXPLORE study, where patients presented with a more complicated disease at index (early disease onset, perianal involvement).15 Nevertheless, augmentation with non-biologic therapies was the second indicator of suboptimal response in the main EXPLORE study and in the Chinese cohort.11,15 Shin et al. also identified augmentation of conventional therapy as the most frequent indicator of suboptimal response in UC (47.6%) in South Korea, while Lindsay et al. showed anti-TNFs discontinuation (UC: 34.2%; CD: 31.1%) and dose escalation (UC: 29.7%; CD: 21.3%) as leading indicators in Europe and Canada, probably reflecting better access to advanced therapy in the latter study.12,13 It should be noted that IBD-related surgery rate (20%) was also an important indicator of suboptimal response among CD LATAM patients. Augmentation of concomitant non-biological therapies is more feasible than increasing anti-TNF doses or switching biologics due to several barriers. The lack of therapeutic drug monitoring strategies in LATAM to determine anti-drug antibodies and trough drug levels in real clinical practice might have played a role in these findings. The presence of anti-drug antibodies and circumstances that favor immunogenicity, such as lack of regular drug supply, which is frequent in our region, may be related to loss of response. The latter may lead to consideration of both augmentation with non-biologic therapies and anti-TNF drug dose escalation as frequent strategies.2
We found a noticeably lower incidence of suboptimal response in Mexico that can be related to a lower-than-expected frequency of non-biologic therapy prior to anti-TNF therapy initiation. A possible explanation may be a suspected gap in documentation, which might have led to an underestimation of the incidence of suboptimal response in the current analysis. This is supported by the higher CISR observed in patients with a documented history of non-biological therapy within two years prior to anti-TNF initiation versus those with no history of non-biologic use within 2 years prior to anti-TNF initiation.8,11
New publications highlighted the complexity of implementing updated international guidelines in LATAM countries due to limitations in treatment availability, access, reimbursement, and the presence of endemic diseases contraindicating the use of some drugs.3,11,16 The proportions of UC and CD LATAM patients who were medically indicated for anti-TNF therapy and did not receive such treatments were lower than reported in the overall EXPLORE cohort. LATAM patients who were treated with anti-TNF agents had more severe and prolonged disease; these characteristics may explain such difference, and possibly, a lower response rate to these drugs could be inferred. Local practice preferences and drug accessibility should also be taken into consideration as other potential causes of differences.
Data on TB reported in this study showed that TB remains endemic in LATAM and our detection rates are consistent with local literature.17,18 Of note, wide variations were reported among LATAM countries, even though our small sample, as aforementioned. In accordance with our data, Fortes et al. found a positive rate of 10.9% in a series of 184 Brazilian patients with IBD screened for LTB before starting immunosuppressive or immunobiological therapy.19 Despite recommended LTB screening, patients treated with anti-TNFs in endemic areas remain at risk of TB infections. According to a meta-analysis including more than 130,000 patients from several regions worldwide, there is a definite risk of TB related to anti-TNFs use in patients with IBD, primarily depending on the local TB burden and being independent of disease or treatment type.20 Thus, given the high incidence of active TB in LATAM countries and the concern of reactivation of LTB with anti-TNF therapies, the need for safer treatment strategies with lower risk of reactivation is recognized.19,21 Anti-TNF may only be preferred for specific patient subgroups in LATAM.22 Updated American and European guidelines endorse the indication of newer drugs to be used also as first-line treatments.23–25 Furthermore, vedolizumab and ustekinumab may be chosen over anti-TNF in elderly patients, patients with a history of neoplasia, and TB endemic areas.21,26
Despite the considerable incidence of suboptimal response to anti-TNFs, we found a low rate of anti-TNF therapy discontinuation (UC: 13.9%; CD: 10.0%) that could partly be due to the lack of other advanced therapies available during the study period. The therapeutic management of IBD patients experiencing loss of response to anti-TNF therapy has become a critical issue. The emergence of several promising agents (biologics and small molecules) suggests they may replace or be used in combination with anti-TNF agents in the near future, which in the latter case will likely improve treatment persistence with anti-TNFs. According to recent data, anti-TNF drugs continue to be the biological therapies with the highest prescription rates in LATAM.27,28
In LATAM, there is little information about physicians’ decisions to prescribe anti-TNF agents to IBD patients. We found that patient affordability and late referral to IBD specialist care centers (27% for both variables) were the most commonly reported barriers, while late diagnosis (40%) was perceived as the main barrier to prescription for non-IBD GI specialists. These results highlight the delay in CD diagnosis for a significant proportion of LATAM patients that has also been reported in a recent multicenter study including 9 LATAM countries.28
Limitations of the present study include its retrospective design and possible incomplete information in the medical records, as evidenced by the lower suboptimal response rate compared to other studies which used similar clinical parameters for anti-TNF loss of response.12–14 Also, the present study did not evaluate predictors of suboptimal response in this patient cohort. However, it should be emphasized that the present study describes useful insights into the IBD LATAM population, usually underrepresented in clinical trials of anti-TNF agents.
ConclusionsWe observed that suboptimal response to anti-TNF therapy was common among IBD patients in LATAM, where augmentation with non-biologic therapy represented the most frequent indicator of suboptimal response. Overall, our findings contribute to the current body of evidence on the unmet needs associated with anti-TNF agents, especially in the era of newer IBD therapies with novel mechanisms of action, where a personalized approach may improve long-term outcomes in IBD patients in LATAM.
Data availabilityThe datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
Authors’ contributionsAll authors participated in the acquisition, analysis, and interpretation of the data, drafted the initial manuscript and revised the article critically for relevant intellectual content.
FundingThis retrospective analysis was sponsored by Takeda Argentina S.A.
Conflicts of interestBalderramo, DC has received travel support from Takeda, Abbvie, Janssen and Ferring, received lecture fees from Takeda, Abbvie and Janssen, and participated at advisory boards from Takeda. Yamamoto-Furusho, JK is an advisory committee/board member for Takeda Pharmaceuticals Company Ltd; has received honoraria from AbbVie, Takeda, Janssen, Farmasa, Ferring, Alfasigma, Hospira, UCB, Danone, Almirall and Pfizer as a speaker, key opinion leader, and member of national and international advisory boards; has received research funds from Bristol, Shire, Pfizer and Takeda and former President of the Pan American Crohn's and Colitis Organization (PANCCO). Ponce de Leon, E is a consultant or advisory/board member for AbbVie, Janssen and Takeda Pharmaceuticals Company Ltd. De Maria, J has received travel support from Takeda, Abbvie and Janssen. Zubiaurre, has received travel support from Takeda and Abbvie, received lecture fees from Takeda, Abbvie, Janssen and Ferring, participated at advisory boards from Takeda and Abbvie, and is a consultant for Takeda and Abbvie. Pedreira, SC has nothing to declare. Brion, L; Lis, C are Takeda Argentina employees. De Paula, JA has received travel support from Takeda, Abbvie, Janssen and Ferring, received lecture fees from Takeda, Abbvie, Janssen, Ferring and Shire (now part of Takeda), received financial support for research from Danone, participated at advisory boards from Takeda, Abbvie and Janssen and held preceptorship programs funded by Takeda, Abbvie and Janssen.
The authors would like to thank all the site investigators and their research teams for their participation and contribution in the EXPLORE study. In addition, the authors would like to thanks Patricia Guimaraens for the critical review of the manuscript.