The recommendations of the Spanish Ministry of Health on vaccination in risk groups include mesalazine among the treatments with a possible negative effect on its effectiveness. However, this is not the recommendation of most experts. Our objective was to evaluate the effect of mesalazine on the humoral response to the SARS-CoV-2 vaccine in patients with inflammatory bowel disease (IBD).
MethodsVACOVEII is a Spanish, prospective, multicenter study promoted by GETECCU, which evaluates the effectiveness of the SARS-CoV-2 vaccine in patients with IBD. This study includes IBD patients who have recieved the full vaccination schedule and without previous COVID-19 infection. Seroconversion was set at 260 BAU/ml (centralized determination) and was assessed 6 months after full vaccination. In this subanalysis of the study, we compare the effectiveness of the vaccine between patients treated with mesalazine and patients without treatment.
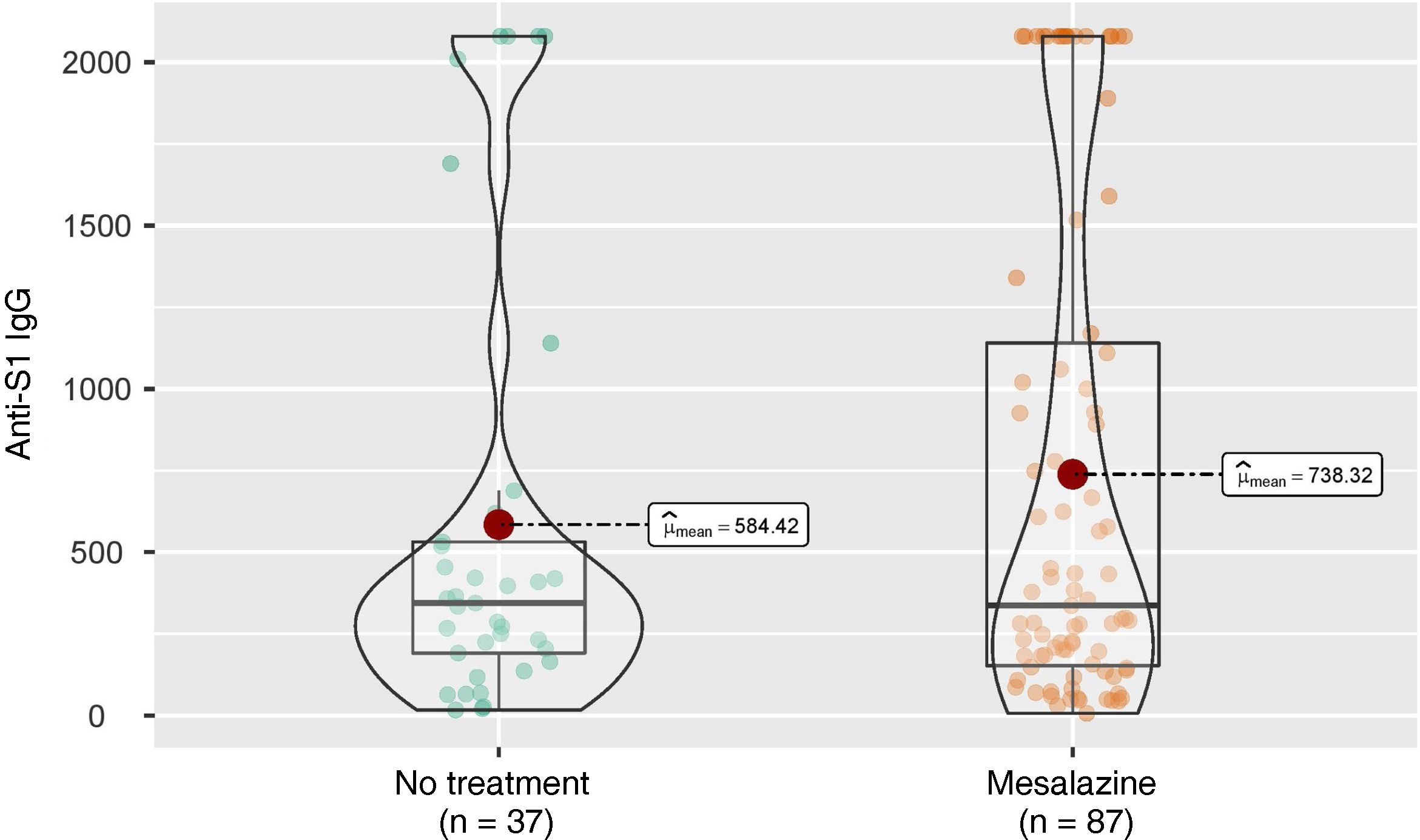
ResultsA total of 124 patients without immunosuppressive therapy were included, of which 32 did not receive any treatment and 92 received only mesalazine. Six months after full vaccination, no significant differences are observed in the mean concentrations of IgG anti-S between both groups. In the multivariate analysis, antibody titers were independently associated with the use of mRNA vaccines and with SARS-CoV-2 infection.
ConclusionMesalazine does not have a negative effect on the response to SARS-CoV-2 vaccines in IBD patients.
Las recomendaciones del Ministerio de Sanidad español sobre vacunación en grupos de riesgo incluyen a la mesalazina entre los tratamientos con un posible efecto negativo en su efectividad. Sin embargo, esta no es la recomendación de la mayoría de los expertos. Nuestro objetivo fue evaluar el efecto de la mesalazina en la respuesta humoral a la vacuna contra el SARS-CoV-2 en los pacientes con enfermedad inflamatoria intestinal (EII).
MétodosVACOVEII es un estudio español, prospectivo y multicéntrico promovido por GETECCU, que evalúa la efectividad de la vacuna contra el SARS-CoV-2 en los pacientes con EII. En él se incluyen pacientes con EII con vacunación completa y sin infección previa por COVID-19. La seroconversión se fijó en 260 BAU/mL (determinación centralizada) y se evaluó a los seis meses después de la vacunación completa. En este subanálisis se comparan los resultados entre pacientes tratados con mesalazina y sin tratamiento.
ResultadosSe incluyeron un total de 124 pacientes sin terapia inmunosupresora, de los cuales 32 no recibían ningún tratamiento y 92 mesalazina únicamente. Seis meses después de la vacunación completa, no se observan diferencias significativas en las concentraciones medias de IgG anti-S entre ambos grupos. En el análisis multivariable los títulos de anticuerpos se asociaron de forma independiente con el uso de vacunas ARNm y con la infección por SARS-CoV-2.
ConclusiónLa mesalazina no tiene un efecto negativo sobre la respuesta a las vacunas contra el SARS-CoV-2 en los pacientes con EII.
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has caused more than seven million deaths worldwide since its beginnings in December 2019. The development of vaccines against the virus has been the best weapon for controlling the disease, having proven to be extremely effective in reducing hospitalisation and mortality rates.1
Inflammatory bowel disease (IBD) is a chronic immune-mediated disease that causes lesions in the digestive tract, and includes Crohn’s disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC). Treatment frequently involves immunosuppressive drugs (thiopurines, methotrexate, steroids, JAK inhibitors and biological agents, such as anti-TNF, vedolizumab and ustekinumab), with vaccination being essential in these patients to prevent possible infectious complications. Both IBD itself and its treatment can have a negative impact on the effectiveness of vaccines, so specific recommendations are often developed in this population.2,3 However, as regards vaccination against SARS-CoV-2, it should be noted that patients with IBD were excluded from most of the vaccine registration clinical trials, as were patients with other immune-mediated diseases, so data on effectiveness in this population are more limited and were obtained in subsequent studies.
All the vaccines against SARS-CoV-2 which have been authorised by the European Medicines Agency to date can be used in patients with IBD, regardless of their immunosuppression and treatment status.4 The first studies already suggested that the response to these vaccines may be adequate in the short term in patients with IBD,3 although with particularities yet to be defined. Their efficacy, measured by the antibody titre response, appears to be lower in patients receiving anti-TNF therapy than in those receiving vedolizumab5–7 or other immunosuppressants.4
However, it is now accepted that an antibody level of 260 BAU/ml (according to World Health Organization standard units8) is the minimum threshold for achieving effective immunisation, according to studies evaluating protection after vaccination in the COV0021414 trial, the COVE trial9 and other cohorts.10–14 The use of this cut-off point may change the interpretation of the results obtained to date. In the VACOVEII study, our primary objective was to evaluate the response after full vaccination against SARS-CoV-2, using the cut-off point of 260 BAU/ml to define the response, as well as to evaluate the impact of different immunosuppressive therapies on it.
Some national recommendations, among them those of the Spanish Ministry of Health, on vaccination in patients in at-risk groups, such as those on immunosuppressive treatment, including patients with IBD, list mesalazine among the treatments with a possible negative effect.15 However, this is neither the opinion nor the recommendation of the majority of experts,16 though the data in this regard are very limited. A secondary objective of the study, the results of which are presented in this article, was to evaluate the effect of mesalazine on the humoral response to the COVID-19 vaccine in patients with IBD.
MethodsStudy designVACOVEII was a non-interventional, prospective, multicentre study whose main objective was to evaluate the humoral effectiveness of vaccination against SARS-CoV-2 in patients with IBD and the potential impact of immunosuppressive therapy on it. This study was carried out under the auspices of the Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) [Spanish Working Group on Crohn’s Disease and Ulcerative Colitis], approved by its Research Committee, and had the participation of thirteen Spanish hospitals.
We included patients diagnosed with IBD according to usual criteria, over 18 years of age, with or without immunosuppressive treatment, and who had received the full vaccination schedule against SARS-CoV-2 with any of the vaccines marketed in Spain. A full regimen was considered according to the technical specifications of the different vaccines (two doses of the ChAdOx1 nCoV-19, BNT162b2 or mRNA-1273 vaccine; and one dose of the JNJ-78436735 vaccine). Patients with known prior SARS-CoV-2 infection, demonstrated by PCR or positive serology prior to vaccination, were excluded, whether symptomatic or not, but for practical reasons, were not excluded from the analyses if the infection was acquired subsequently. Patients were also excluded if they had other causes of immunodeficiency, primary or secondary (active cancer, human immunodeficiency virus infection, solid organ transplant, haematological disease), were being treated with chemotherapy and/or immunotherapy or had received immunosuppressive treatment indicated for a reason other than IBD, and/or who took any concomitant immunosuppressant/immunomodulator drug not used in IBD. Concomitant treatment with topical and oral corticosteroids was not an exclusion criterion for the analysis of the complete study, given its low sample size.
All consecutive patients who met the selection criteria at the 13 participating centres were asked to participate in the study. Those who agreed to participate and signed an informed consent form were included, and a personal interview was conducted to collect all the study variables.
Vaccination efficacy was evaluated 180 days (±28 days) after full vaccination. Patients whose antibody determination was performed outside the protocol time frame were excluded from the analysis. The cut-off point to establish seroconversion was established at anti-S antibody levels of 260 BAU/ml, in accordance with the recommendations of the Spanish Ministry of Health.
VariablesDemographic variables such as gender, age and smoking were collected. Body mass index (BMI) was calculated using weight and height measurements obtained at the consultation. Comorbidity was assessed using the age-adjusted Charlson index.17 IBD-related variables included disease type (Crohn’s disease, ulcerative colitis or indeterminate colitis), its extent, location and behaviour, as well as its duration. Disease activity was assessed using the Harvey-Bradshaw index18 for Crohn’s disease and the Mayo partial score19 for ulcerative colitis.
Regarding the treatment of IBD, for this subanalysis, two groups were established: patients without any specific treatment for IBD or patients on treatment exclusively with mesalazine. None of the patients included in this subanalysis were on treatment with corticosteroids.
All this data was obtained through interviews with the patient and/or according to the electronic records of each participating centre. In terms of vaccination, the type of vaccine (mRNA or non-mRNA), dose and date(s) of administration were collected directly from the electronic medical records of each centre.
Evaluation of the humoral immune responseThe analysis of the antibody response induced by the SARS-CoV-2 vaccine was performed centrally at the Clinical Microbiology Department of the Hospital Universitario Miguel Servet (Zaragoza, Spain). LIAISON® SARS-CoV-2 Trimerics IgG (DiaSorin, Saluggia, Italy), an indirect chemiluminescence immunoassay (CLIA), was used for the detection of SARS-CoV-2 anti-spike IgG (anti-S IgG) antibodies in samples of human serum and plasma. The main components of the test are magnetic particles (solid phase) coated with recombinant trimeric SARS-CoV-2 spike protein and a conjugate reagent containing a mouse anti-human IgG monoclonal antibody linked to an isoluminol derivative. The light signal, therefore the amount of isoluminol-antibody conjugate, is measured by a photomultiplier as relative light units (RLU) and is indicative of antibodies against SARS-CoV-2, present in calibrators, samples or controls. The conversion from RLU units to BAU units and the threshold value (33.8 BAU units) were set according to the manufacturer’s instructions.
Additionally, all patients’ samples were analysed for the presence of past undetected SARS-CoV-2 infections with the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Mannheim, Germany). This electrochemiluminescence immunoassay (ECLIA) uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS-CoV-2. These antibodies are only present in natural infections. The results are automatically determined by the software by comparing the electrochemiluminescence signal obtained from the sample reaction product with the cut-off value signal previously obtained by calibration, with a threshold value >1 (cut-off index). This meant that the impact of SARS-CoV-2 infection during follow-up could be taken into account in the analysis, exclusively evaluating the antibody levels and the seroconversion rate in patients without infection.
Data collectionThe study data was collected and managed using the AEG-REDCap online collaborative research platform (from the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology]). Research Electronic Data Capture (REDCap20) is a secure software platform designed to support data capture for research studies by providing: 1) an intuitive interface for capturing validated data; 2) audit trails to track data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Statistical analysisContinuous variables are expressed as mean and standard deviation or median and interquartile range, and qualitative variables as total number and percentage. The comparison of groups was carried out using χ2 for the qualitative variables and Student’s t test or the Mann–Whitney U test for the qualitative variables, depending on whether or not the sample was normal. When more than two groups were involved, independent groups ANOVA was used. The normality was verified by the Kolmogorov–Smirnov test. Multivariate analysis was performed using linear regression models. Gender, age, vaccine type, treatment group, SARS-CoV-2 infection and smoking status were included as independent variables. Age was treated as a linear variable and its coefficient was expressed per incremental year. All analyses were carried out considering all other potentially confounding variables and limiting the analysis to specific cohorts if necessary.
All tests were two-tailed, with significance set at a p-value of less than 0.05. Analyses were performed with Jamovi software, version 2.3.16 (https://www.jamovi.org, Sydney, Australia).
Given the results shown in this article are those from a sub-analysis carried out on the main VACOVEII study, to determine whether or not the sample size used was adequate, an a posteriori statistical power calculation was carried out, considering a proportion between samples of 2:1, with an alpha precision level of 0.05 to detect a difference of 15 percentage points in the seroconversion rate. With these parameters, the power obtained was 42%.
ResultsCohort baseline characteristicsA total of 124 patients were included, of whom 32 (25.8%) were not receiving any type of treatment for IBD and 92 (74.2%) were taking mesalazine as the only treatment.
The baseline characteristics of the patients can be consulted in Table 1 according to their treatment group.
Baseline characteristics of the patients according to treatment group.
| No treatment (n = 37) | Mesalazine (n = 87) | Total (n = 124) | p Value | |
|---|---|---|---|---|
| Gender | 0.725 | |||
| Male | 20 (54.1%) | 50 (57.5%) | 70 (56.5%) | |
| Female | 17 (45.9%) | 37 (42.5%) | 54 (43.5%) | |
| Age | 0.440 | |||
| Mean (SD) | 48.9 (10.9) | 47.2 (11) | 47.7 (10.9) | |
| Type of IBD | <0.001 | |||
| Ulcerative colitis | 13 (35.1%) | 79 (90.8%) | 92 (74.2%) | |
| Indeterminate colitis | 0 (0%) | 2 (2.3%) | 2 (1.6%) | |
| Crohn’s disease | 24 (64.9%) | 6 (6.9%) | 30 (24.2%) | |
| Active IBD | 0.912 | |||
| No | 33 (89.2%) | 77 (88.5%) | 110 (88.7%) | |
| Yes | 4 (10.8%) | 10 (11.5%) | 14 (11.3%) | |
| Time since onset | 0.634 | |||
| Mean (SD) | 10.2 (11.8) | 11.2 (9.6) | 10.9 (10.3) | |
| Comorbidity | 0.298 | |||
| No comorbidity | 34 (91.9%) | 85 (97.7%) | 119 (96%) | |
| High comorbidity | 1 (2.7%) | 1 (1.1%) | 2 (1.6%) | |
| Low comorbidity | 2 (5.4%) | 1 (1.1%) | 3 (2.4%) | |
| BMI | 0.146 | |||
| Mean (SD) | 24 (4.8) | 25.3 (4.3) | 24.9 (4.4) | |
| Smoker | 0.103 | |||
| Never | 14 (37.8%) | 51 (58.6%) | 65 (52.4%) | |
| Ex-smoker | 18 (48.6%) | 29 (33.3%) | 47 (37.9%) | |
| Active | 5 (13.5%) | 7 (8%) | 12 (9.7%) | |
| Vaccine type | 0.258 | |||
| RNA | 31 (83.8%) | 79 (90.8%) | 110 (88.7%) | |
| Non-RNA | 6 (16.2%) | 8 (9.2%) | 14 (11.3%) |
BMI: body mass index; IBD: inflammatory bowel disease; SD: standard deviation.
Six months after full vaccination, the mean anti-S IgG concentrations were analysed in the group of patients without treatment and in the group of patients treated with mesalazine, with no statistically significant differences found between the two groups (344 [191–531] vs 336 [153–1140]; p = 0.564) (Fig. 1).
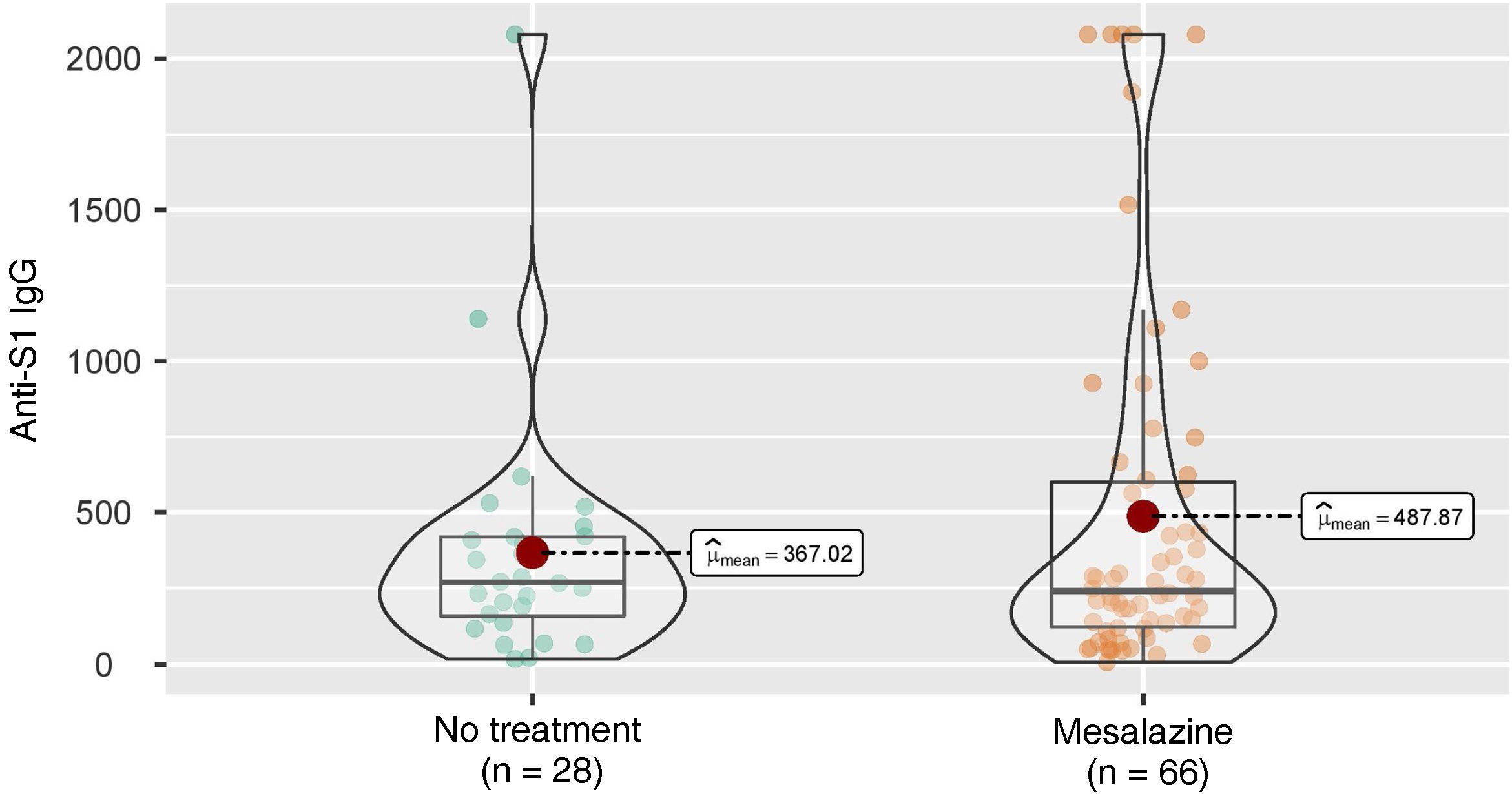
These figures were also analysed in patients who did not develop COVID-19 infection during follow-up (28 in the group of patients without treatment and 66 in the group of patients without mesalazine), with similar results (269 [158–420] vs 241 [123–601]; p = 0.944) (Fig. 2).
Data on the doses of mesalazine received by the patients were collected, with no differences found. Patients with high doses had anti-S IgG values of 564 (151–2080), while patients with low doses had anti-S IgG levels of 378.0 (212.5–1085.0), this difference not being statistically significant (p = 0.525).
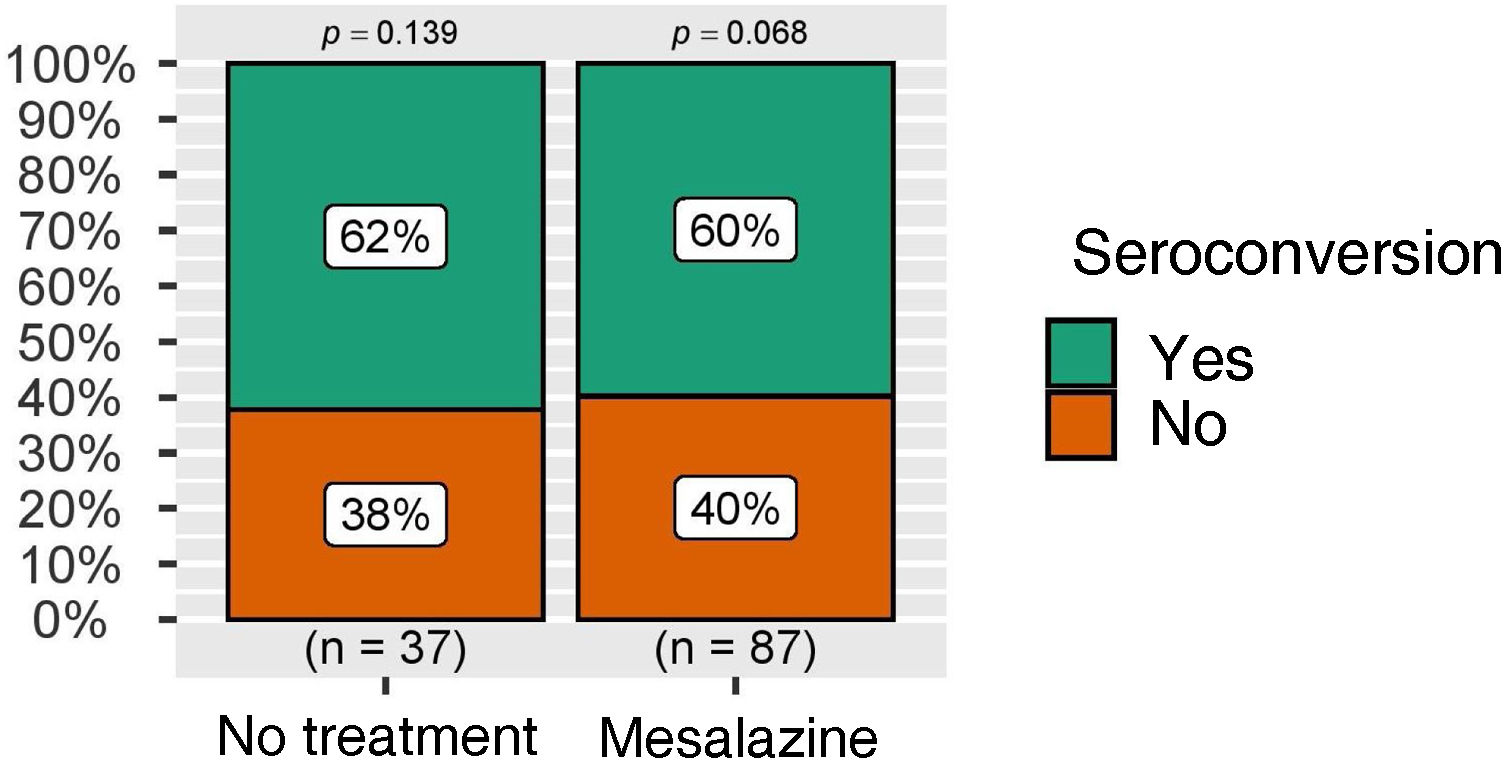
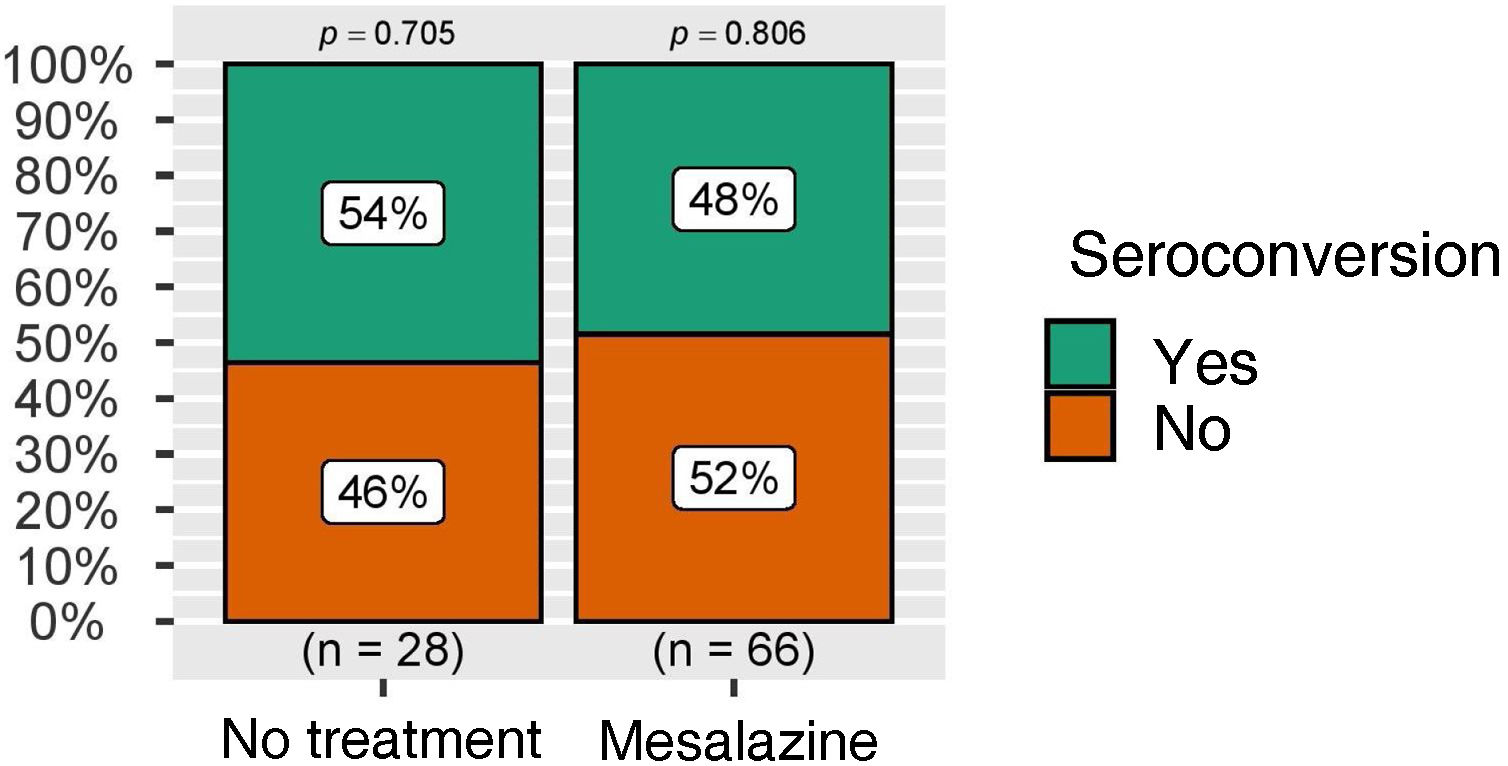
The rate of seroconversion, defined as the presence of anti-S IgG levels greater than 260 BAU/ml, was higher than 50% in both groups, again with no statistically significant differences (Fig. 3). These figures were also analysed in the group of patients without COVID-19 infection during follow-up, with similar results (Fig. 4).
To evaluate the impact of the relevant variables on the seroconversion rate, a multivariate analysis was performed, in which antibody titres were independently associated with the use of mRNA vaccines and with SARS-CoV-2 infection, once again without seeing any impact attributable to mesalazine treatment. This model is shown in Table 2.
Linear regression model for the primary endpoint, anti-S IgG antibody titre.
| Predictor | Estimator | SE | t | p Value |
|---|---|---|---|---|
| Constanta | 136.64 | 343.84 | 0.397 | 0.692 |
| SARS-CoV-2 infection | ||||
| Yes – no | 937.37 | 126.20 | 7.428 | <0.001 |
| Treatment | ||||
| Mesalazine – no treatment | 108.47 | 119.32 | 0.909 | 0.365 |
| Age | −2.74 | 5.39 | −0.508 | 0.612 |
| Vaccine type | ||||
| RNA – non-RNA | 459.24 | 180.47 | 2545 | 0.012 |
| Smoker | ||||
| Former smoker – never | −50.34 | 121.51 | −0.414 | 0.679 |
| Yes – never | −48.84 | 188.72 | −0.259 | 0.796 |
SE: standard error of the estimator; t: tolerance.
The use of immunosuppressive therapies can reduce the response to vaccines in patients with IBD compared to the general population. One of the clearest examples is vaccination against the hepatitis B virus, with various studies showing a lower effectiveness of the vaccine in patients treated with azathioprine and/or infliximab.21 That is why specific vaccination guidelines are established in patients on immunosuppressive treatment, although it is important to establish exactly what we consider as immunosuppressive drugs. In fact, the Spanish Ministry of Health includes oral mesalazine among the drugs with a potential immunosuppressive effect to be taken into account when establishing specific vaccination strategies in these patients.15 However, this is neither the opinion nor the recommendation of IBD experts, who do not consider mesalazine as an immunosuppressive drug.16
The VACOVEII study is one of the first to prospectively evaluate the efficacy of SARS-CoV-2 vaccines in IBD patients in clinical practice using currently recommended seroconversion values. The results of the sub-analysis we have presented show that treatment with mesalazine does not negatively influence the response to vaccination against COVID-19. In the analysis by treatment group, no differences were found in antibody levels or in the seroconversion rate between patients without specific treatment for IBD versus patients treated with mesalazine. Antibody titres were independently and exclusively associated with the use of the mRNA vaccines and with SARS-CoV-2 infection. This is consistent with the results of different studies previously published in this regard, in which both the presence of previous SARS-CoV-2 infection and the type of vaccine used are identified as predictors of a better response to vaccination. Despite limited data on the comparability of the immunogenicity of different types of vaccines, mRNA vaccines have repeatedly been associated with the presence of higher antibody titres.22
Our results are from six months after the full vaccination schedule, an advantage with respect to many previous studies, which analyse the response in much shorter times.6 In addition, we use 260 BAU/ml as a protection measure and seroconversion level, a threshold that is especially relevant for clinical practice, since some public health authorities, as in Spain,23 have adopted it for making decisions about the indication of some medications. This threshold is higher than that established in previous studies, which makes the evaluation of seroconversion much more demanding.
As some already published studies suggest, the response to available vaccines could be affected in relation to the concomitant use of certain immunosuppressive drugs.5–7 However, there are no studies that separately analyse the possible effect regardless of the use of mesalazine.
Seroconversion rates after full vaccination in our entire cohort were low (less than 50%). These rates are clearly lower than in other series in similar populations, where rates above 90% are reached,24 underlining the importance of using the seroconversion point of 260 BAU/ml, an evident strength of the VACOVEII study. Another important strength is the time point at which the response is analysed. Most of the studies mentioned above evaluate response at a maximum of two months after administration. This is particularly relevant because numerous studies have shown that the loss of antibodies is constant between three and six months after vaccine administration.25–27 Therefore, an assessment made too early may provide falsely optimistic data.
Our study has a number of limitations. Firstly, one of the largest waves of infections in Spain occurred during the study period, which makes the analysis difficult since natural immunity plays a key role, although it was taken into account in the statistical analysis. Also, SARS-CoV-2 infection was evaluated as a dichotomous variable, but the presence of different variants during different waves may have caused differential effects on immunity. Furthermore, disease activity was evaluated by clinical indices, but endoscopic or biological activity was not evaluated.
In conclusion, treatment with mesalazine did not show any negative effect on the response to COVID-19 vaccines in IBD patients, with seroconversion rates similar to patients without specific IBD treatment. The Ministry of Health’s recommendation to include this drug within the group of immunosuppressive drugs regarding vaccination in patients with IBD should be reviewed.
Ethical considerationsAll patients included in the study were of legal age on the date of inclusion and gave informed consent for participation. This study was evaluated and approved by the GETECCU Research Committee and by the Comité de Ética de la Investigación de la Comunidad de Aragón (CEICA) [Aragon Region Independent Ethics Committee] with study code EPA21/028.
FundingDCD is partially funded by a Río Hortega grant from the Instituto de Salud Carlos III [Carlos III Health Institute] (Grant number CM21/00067). This study was funded and supported by GETECCU.
Conflicts of interestDr Casas-Deza has been a consultant, speaker, or received funding from MSD, AbbVie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring and Faes Farma. Dr Gisbert has been a consultant, speaker, or received funding from MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma. Dr Barreiro has been a consultant, speaker, or received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma. Dr García-López has worked as a consultant, speaker or has received funding from AbbVie, Janssen, Kern Pharma, MSD, Pfizer, Takeda, Tillots and Vifor Pharma. Dr Calafat has worked as a consultant, speaker or received funding from Takeda, Janssen, Gilead, Falk and Pfizer. Dr Domènech has worked as a consultant, speaker or received funding from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Galapagos, Gilead, GoodGut, Imidomics, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda and Tillots. Dr Ferreiro has worked as a consultant, speaker, or received funding from AbbVie, Adacyte Therapeutics, Janssen, Kern Pharma, MSD, Takeda, Tillots, Casen Recordati, Pfizer, Palex, Shire Pharmaceuticals and Ferring. Dr Gutiérrez has worked as a consultant, speaker or received funding from MSD, AbbVie, Janssen, Kern Pharma, Takeda, Galapagos, Pfizer, Sandoz, Fresenius, Ferring, Faes Farma and Adacyte. Dr Chaparro has served as a consultant, speaker, or received funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead and Lilly.
The other authors declare no conflicts of interest.