Journal Information
Special article
Available online 23 July 2024
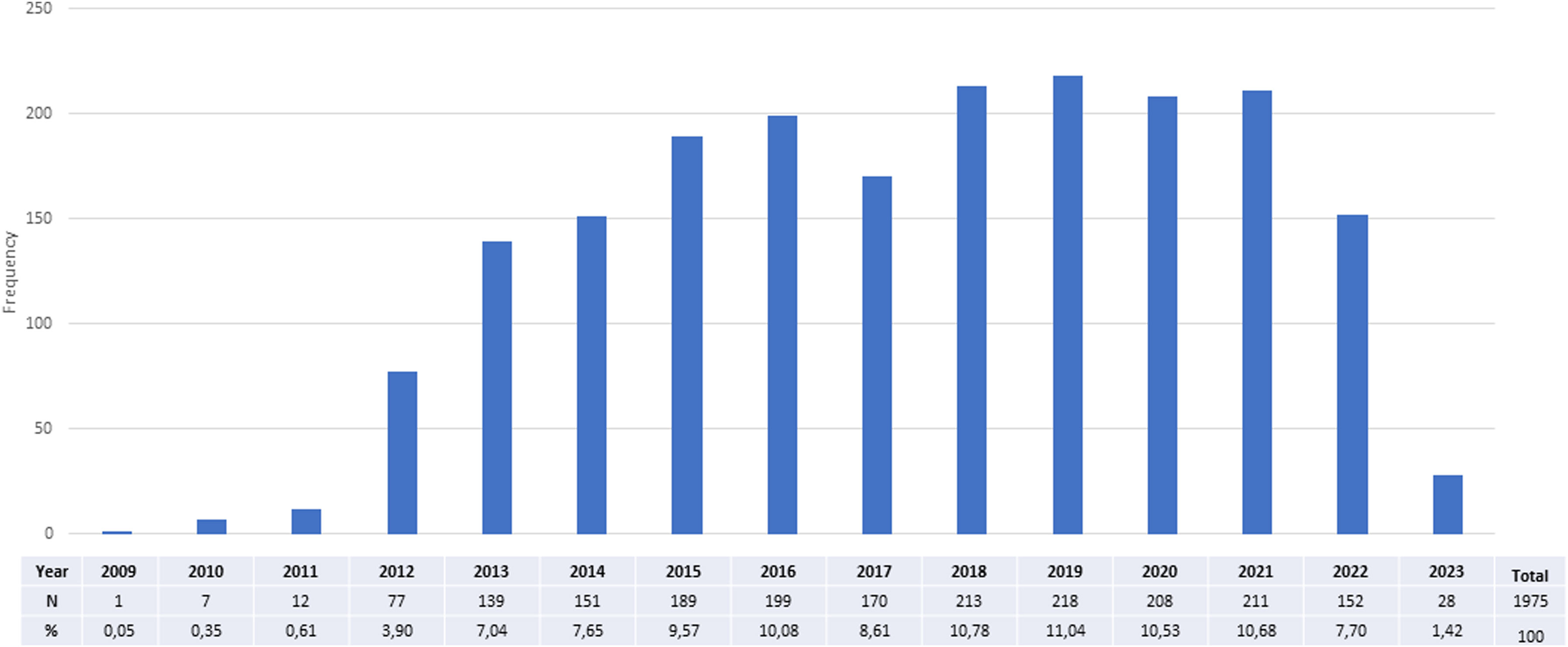
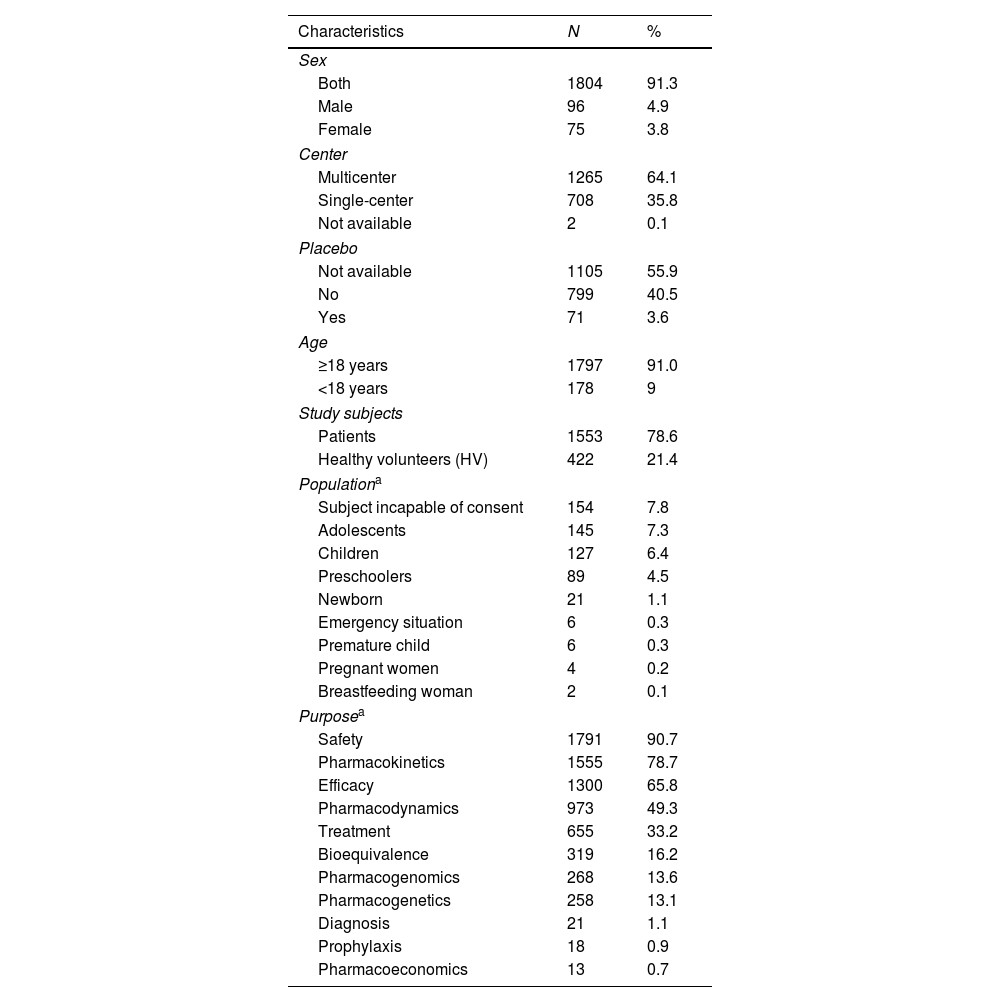
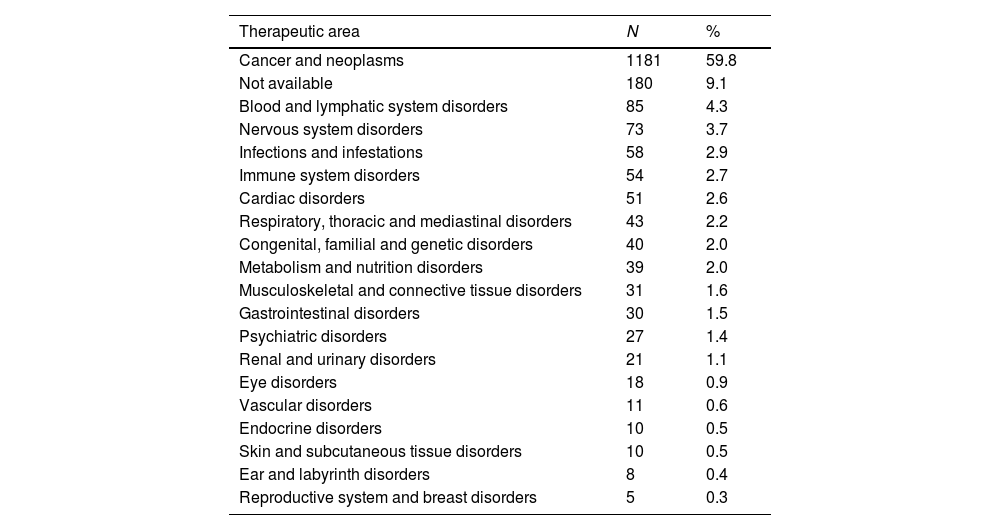
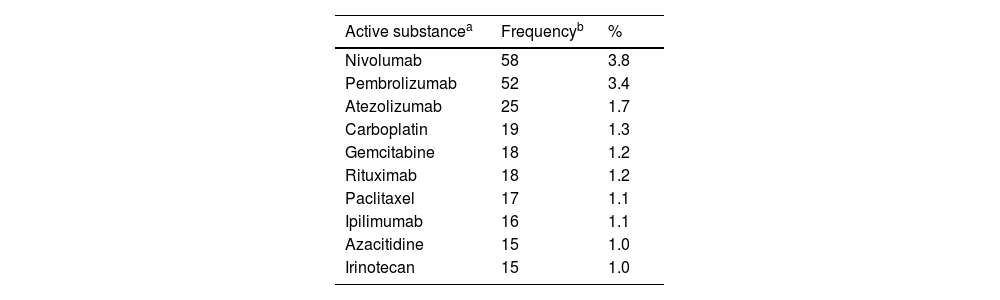
A cross-sectional study of Phase I Clinical Trials authorized in Spain: An analysis of characteristics and times of execution based on experience
Estudio transversal de los ensayos clínicos fase I autorizados en España: un análisis de características y tiempos de ejecución basado en la experiencia
Francesca Filippi-Arriagaa,
, Pol Molinaa, Claudia Erika Delgado-Espinozab,c,d, Rosa Antonijoana,b,c
Corresponding author
a Centre Investigació Medicaments (CIM), Institut de Recerca Sant Pau (IR-Sant Pau), Barcelona, Spain
b Department of Clinical Pharmacology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
c Department of Pharmacology and Therapeutics, Universitat Autònoma de Barcelona (UAB), Barcelona, Spain
d Comitè Ètic per a la Investigació amb medicaments (CEIm) del Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
Article information
These are the options to access the full texts of the publication Medicina Clínica
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail