COVID-19 represents a worldwide pandemic and vaccination remains the most effective preventive strategy. Among hematological patients, COVID-19 has been associated with a high mortality rate. Vaccination against SARS-CoV-2 has shown high efficacy in reducing community transmission, hospitalization and deaths related to severe COVID-19 disease. However, patients with impaired immunity may have lower sero-responsiveness to vaccination.
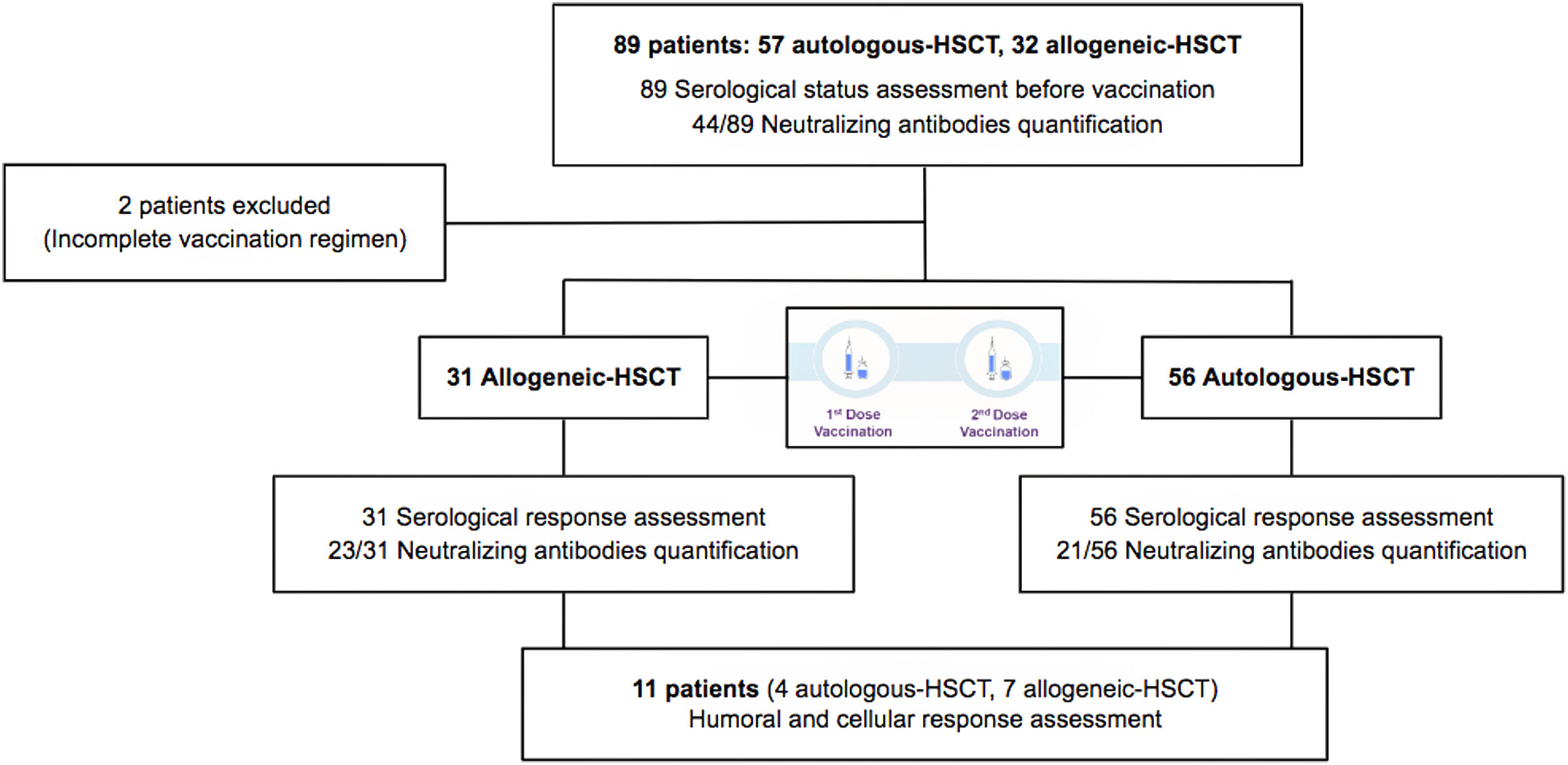
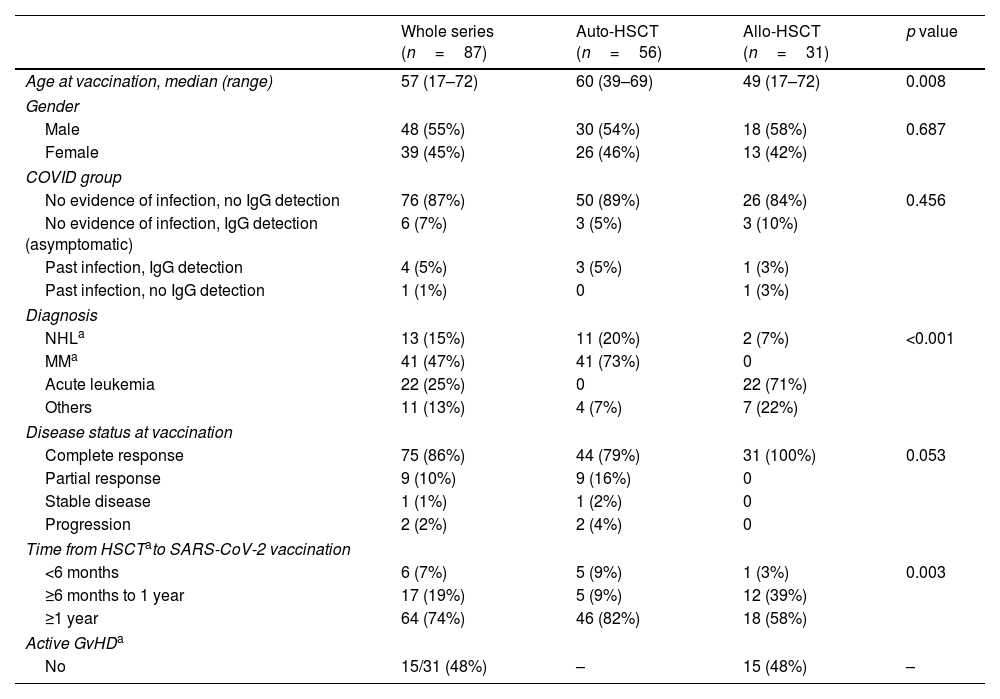
MethodsThis study focuses on hematopoietic stem cell transplantation (HSCT) recipients. We performed a unicenter, prospective, observational study of a cohort of 31 allogeneic and 56 autologous-HSCT recipients monitored between March 2021 and May 2021 for serological response after COVID-19 vaccination with two doses of mRNA1273 vaccine (Moderna). In order to determine seroconversion, serological status before vaccination was studied.
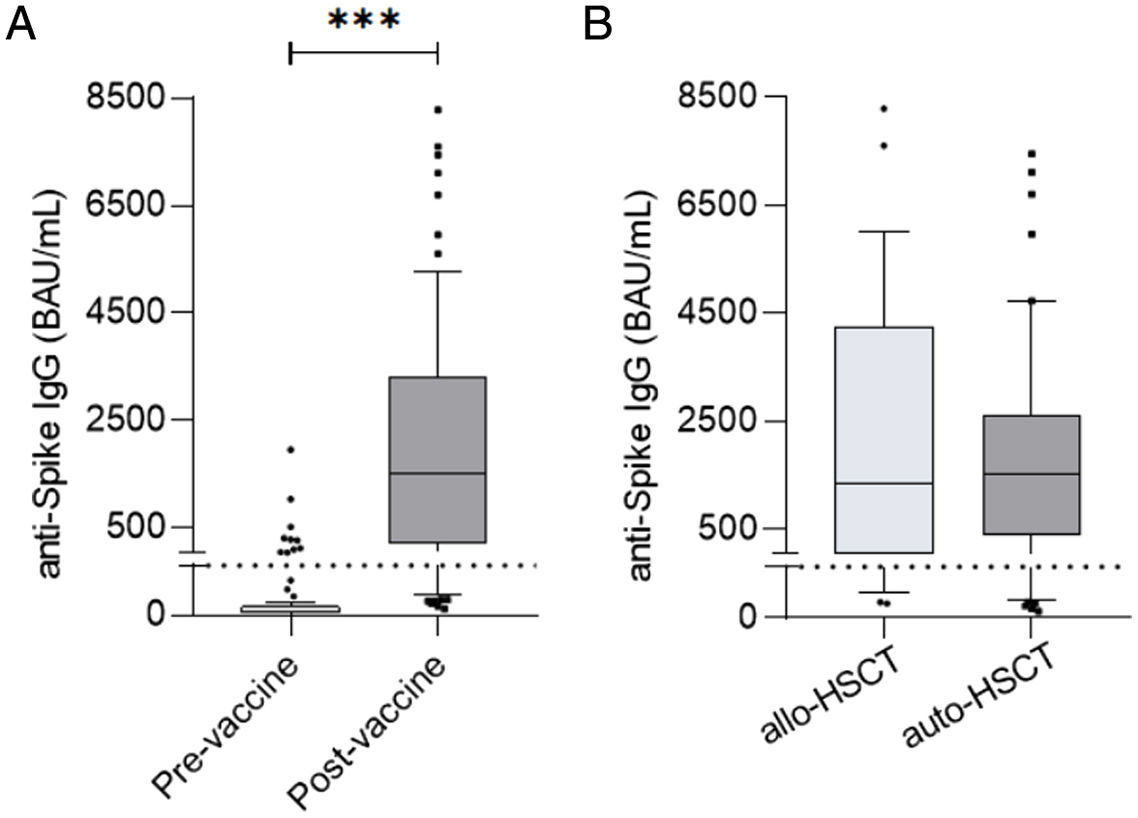
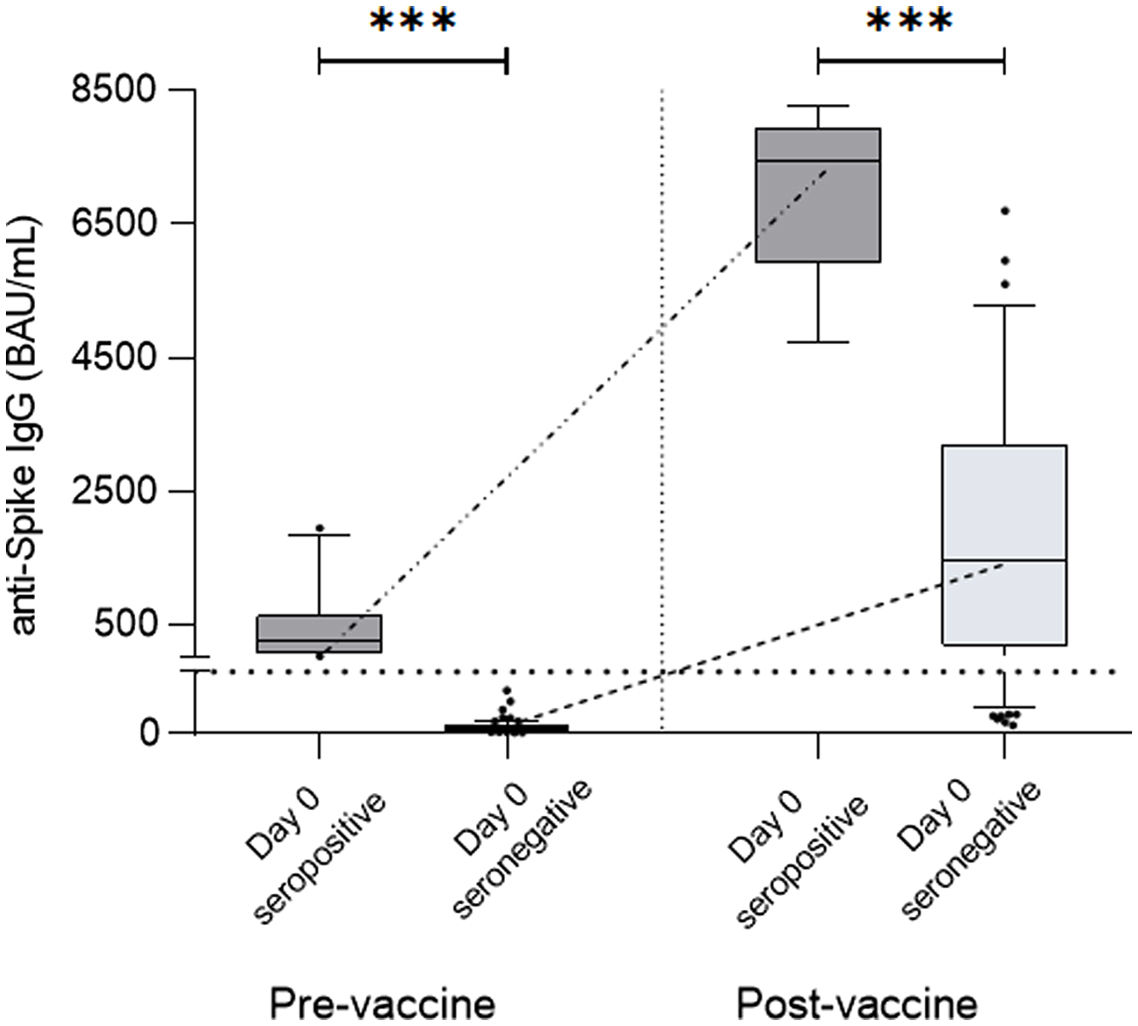
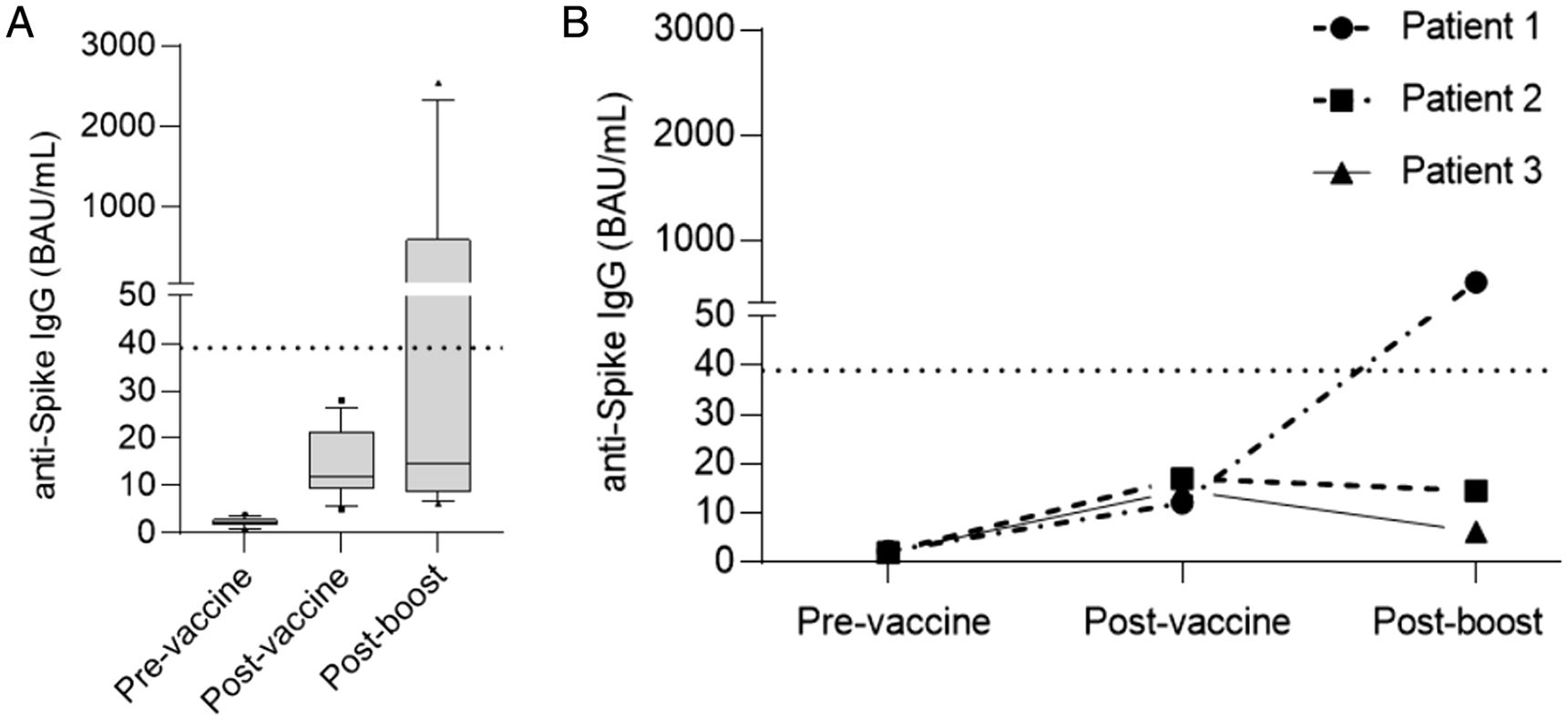
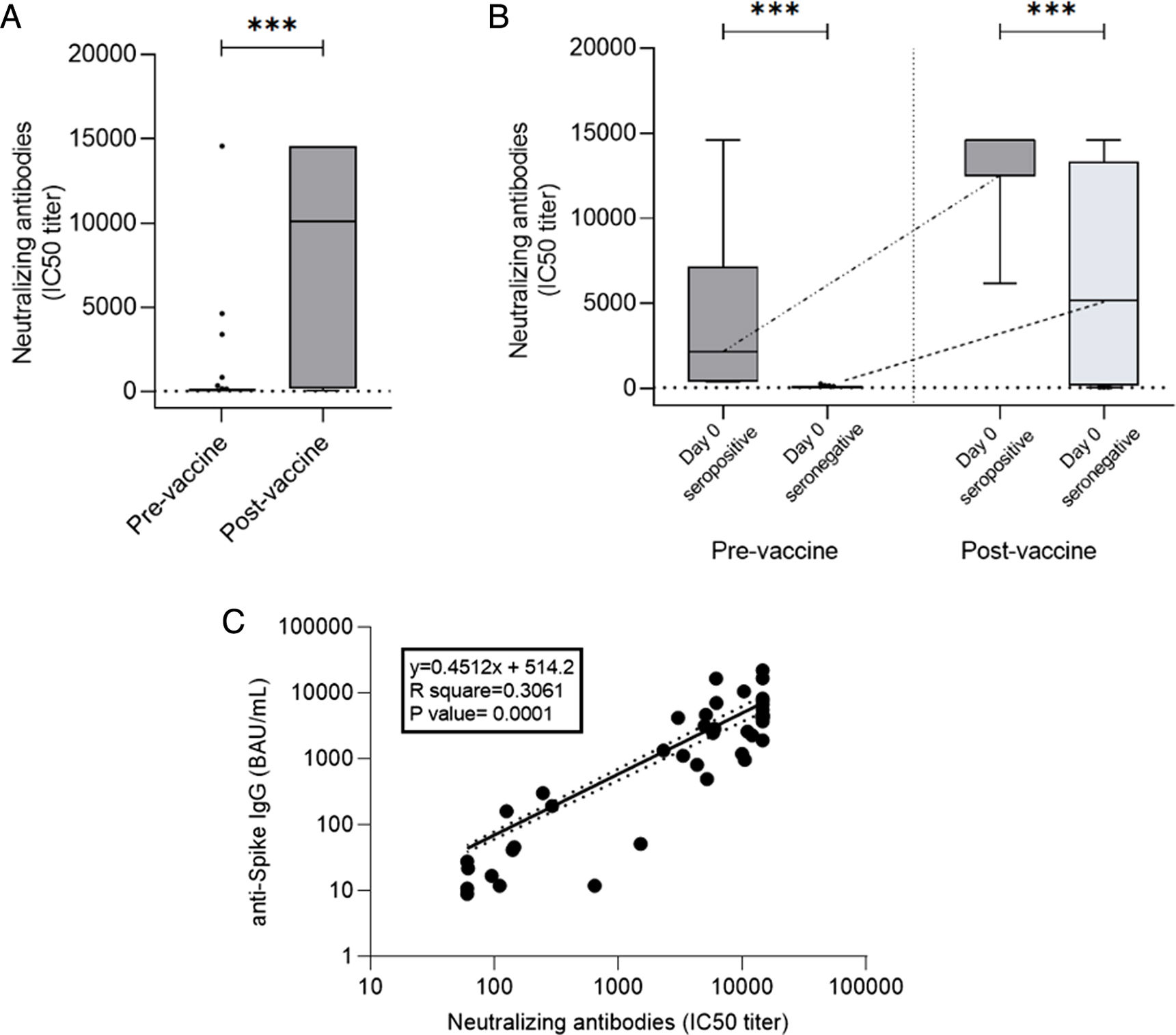
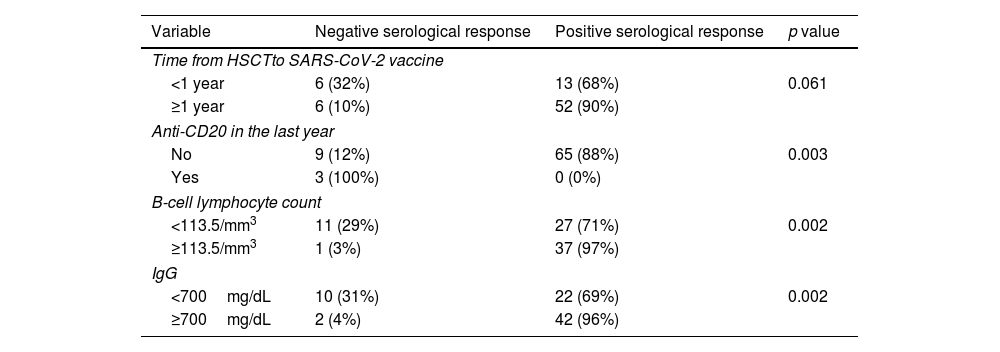
ResultsAt a median range of 75 days after the second vaccine dose, seroconversion rates were 84% and 85% for the autologous and allogeneic-HSCT groups, respectively. We confirmed some potential risk factors for a negative serological response, such as receiving anti-CD20 therapy in the previous year before vaccination, a low B-lymphocyte count and hypogammaglobulinemia. Neutralizing antibodies were quantified in 44 patients, with a good correlation with serological tests. Adverse events were minimal.
ConclusionmRNA1273 vaccination is safe and effective in HSCT recipients, especially in those presenting recovered immunity.
Entre los pacientes hematológicos, la COVID-19 se ha asociado a una mayor mortalidad. La vacunación frente a SARS-CoV-2 es la principal estrategia de prevención y ha demostrado eficacia en la reducción de la transmisión, de la hospitalización y de la tasa de mortalidad. Aun así, los pacientes oncohematológicos con un sistema inmunológico disfuncional podrían presentar una respuesta menor a la vacunación.
MétodosEstudio unicéntrico, prospectivo y observacional, con una cohorte de 31 receptores de un trasplante alogénico de progenitores hematopoyéticos y de 56 receptores de un trasplante autólogo que recibieron la vacunación frente a SARS-CoV-2 entre marzo de 2021 y mayo de 2021, con 2 dosis de la vacuna mRNA1273 (Moderna). Para poder determinar la tasa de seroconversión, se determinó el estado serológico previamente a la vacunación y posteriormente se monitorizó la respuesta serológica.
ResultadosCon un tiempo medio de seguimiento de 75 días después de la segunda vacuna, la tasa de seroconversión fue del 84%, y del 85% en el grupo receptor de trasplante autólogo y alogénico, respectivamente. Se confirmaron algunos potenciales factores de riesgo para la ausencia de respuesta serológica, como haber recibido terapias anti-CD20, un recuento bajo de linfocitos B y la hipogammaglobulinemia. En 44 pacientes se cuantificaron títulos de anticuerpos neutralizantes, con buena correlación con los test serológicos. Los efectos adversos de la vacuna fueron mínimos.
ConclusiónLa vacunación con mRNA1273 es segura y efectiva en los pacientes receptores de un trasplante de progenitores hematopoyéticos, especialmente en los que presentan reconstitución inmune previa.