Información de la revista
Vol. 158. Núm. 6.
Páginas 284-290 (marzo 2022)
Compartir
Descargar PDF
Más opciones de artículo
Vol. 158. Núm. 6.
Páginas 284-290 (marzo 2022)
Special article
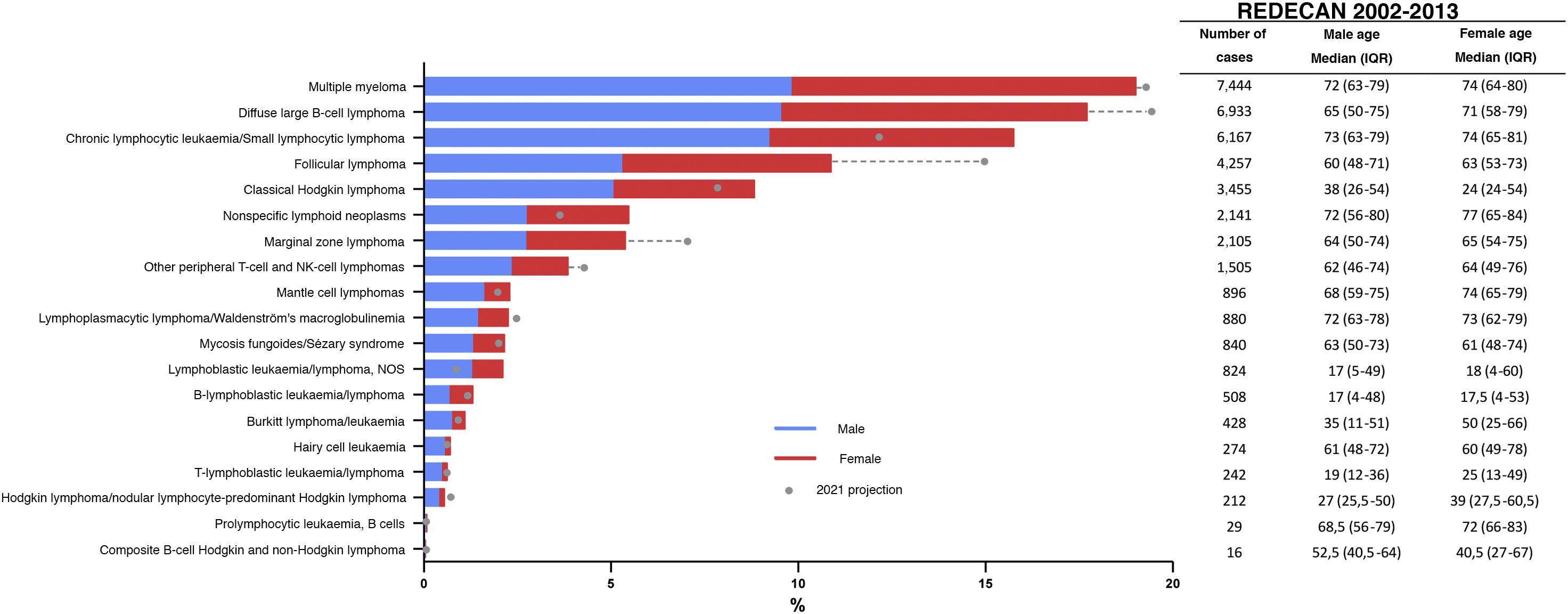
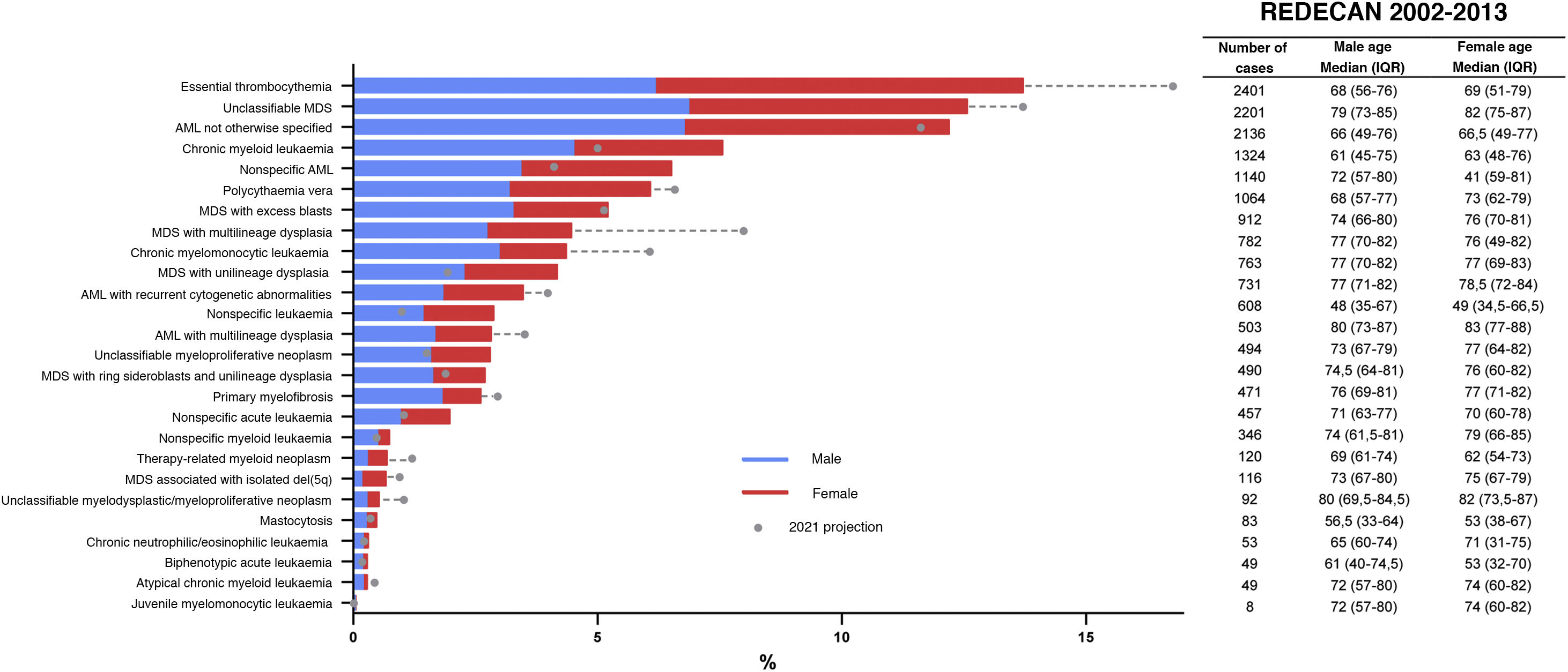
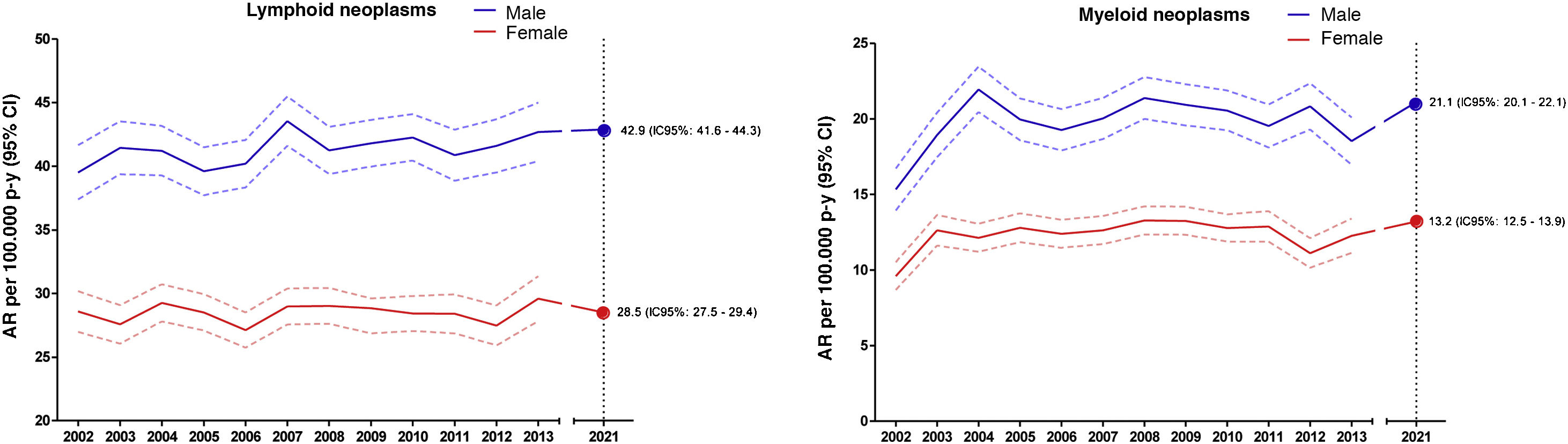
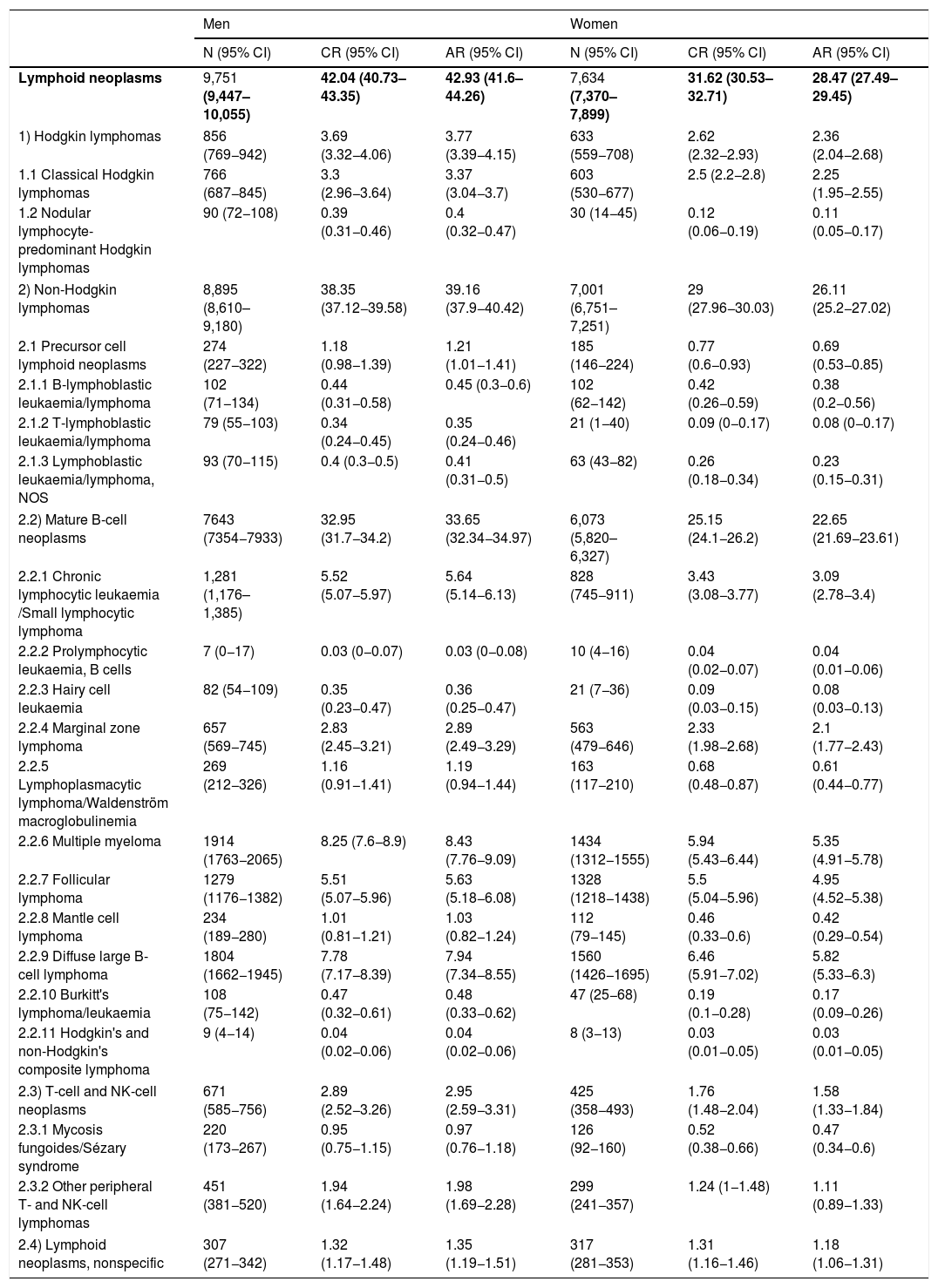
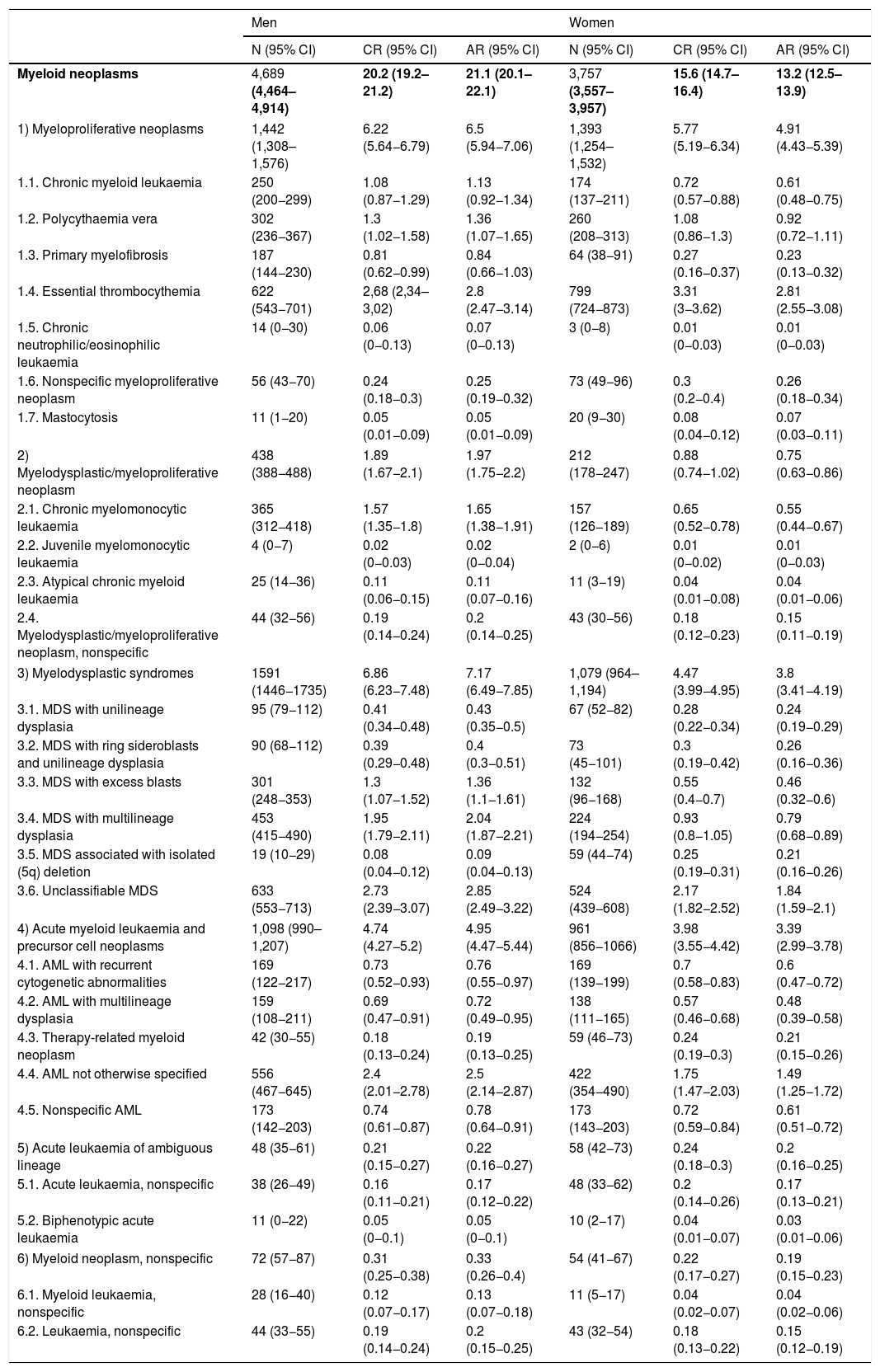
Estimates of the incidence of hematological neoplasms in Spain, 2021: Study of the Spanish Network of Cancer Registries (REDECAN)
Estimaciones de la incidencia de las neoplasias hematológicas en España, 2021: estudio de la Red Española de Registros de Cáncer (REDECAN)
Arantza Sanvisensa, Alberto Ameijideb, Marià Carullab, Rafael Marcos-Grageraa,c,d,
, Spanish Network of Cancer Registries (REDECAN) ◊
Autor para correspondencia
a Institut de Recerca contra la Leucèmia Josep Carreras, Unitat d’Epidemiologia i Registre del Càncer de Girona, Girona, Spain
b Registre del Càncer de Tarragona, Servei d’Epidemiologia i Prevenció del Càncer, Hospital Universitari Sant Joan de Reus, Tarragona, Spain
c Centro de Investigación Biomédica en Red: Epidemiología y Salud Pública (CIBERESP), Spain
d Unitat d’Epidemiologia i Registre del Càncer de Girona, Pla Director d’Oncologia, Institut Català d’Oncologia, Institut d’Investigació Biomèdica de Girona Dr. Josep Trueta (IDIBGI), Universitat de Girona, Girona, Spain
Información del artículo
Opciones para acceder a los textos completos de la publicación Medicina Clínica (English Edition)
Suscriptor
Suscribirse
Comprar
Contactar
Teléfono para suscripciones e incidencias
De lunes a viernes de 9h a 18h (GMT+1) excepto los meses de julio y agosto que será de 9 a 15h
Llamadas desde España
932 415 960
Llamadas desde fuera de España
+34 932 415 960
E-mail