To determine the prevalence of potential interactions in COVID19 patients receiving lopinavir/ritonavir (LPV/r). The secondary objective was to develop recommendations and identify the risk factors associated with presenting potential interactions with LPV/r.
Subjects and methodsCross-sectional and multicenter study with the participation of 2 hospitals. COVID 19 patients over 18 years of age, admitted to hospital and under treatment with LPV/r were included. A screening of potential interactions related to LPV/r and home and hospital medication was carried out. Lexicomp® (Uptodate), HIV-drug interactions and COVID-drug interactions were used as the query database.
Results361 patients with a mean age of 62.77 ± 14.64 years were included, where 59.6% (n = 215) were men. 62.3% (n = 225) had 1 or more potential interactions and 26, 87% (n = 97) 2 or more. The independent variables associated with presenting ≥1 potential interactions were age (>65) (OR 1.95; 95% CI 1.06–3.59, P = .033), ICU admission (OR 9.22; CI 95% 1.98–42.93; P = .005), previous respiratory pathology (OR 2.90; 95% CI 1.15–7.36; P = .024), psychiatric (OR 4.14; 95 CI% 1.36–12.61; P = .013), dyslipidemia (OR 3.21; 95% CI 1.63–6.35; P = .001) and the number of drugs prescribed (OR 4.33; 95% CI 2.40–7.81; P = .000).
ConclusionThe prevalence of potential interactions in COVD 19 patient undergoing treatment with LPV/r is high, with age (>65), ICU admission, previous respiratory and psychiatric pathology, dyslipidemia and the number of prescribed drugs acting as risk factors.
Determinar la prevalencia de interacciones potenciales en pacientes COVID19 en tratamiento con lopinavir/ritonavir (LPV/r). El objetivo secundario fue elaborar recomendaciones e identificar los factores de riesgo asociados a presentar interacciones potenciales con LPV/r.
Sujetos y métodosEstudio transversal y multicéntrico con la participación 2 hospitales. Se incluyeron pacientes COVID 19 mayores de 18 años, con ingreso hospitalario y en tratamiento con LPV/r. Se realizó un cribado de las interacciones potenciales relacionadas con LPV/r y la medicación domiciliaria y hospitalaria. Se utilizó como base de datos de consulta Lexicomp® (Uptodate), HIV-drug interacctions y COVID-drug interacctions.
ResultadosSe incluyeron 361 pacientes con una media de edad de 62,77 ± 14,64 años, donde el 59,6% (n = 215) fueron hombres. El 62,3% (n = 225) tuvieron 1 o más interacciones potenciales y el 26, 87% (n = 97) 2 o más. Las variables independientes asociadas a presentar ≥ 1 interacciones potenciales fueron la edad (> 65) (OR 1,95; IC 95% 1,06–3,59; P = ,033), el ingreso en UCI (OR 9,22; IC 95% 1,98–42,93; P = ,005), la patología previa respiratoria (OR 2,90; IC 95% 1,15–7,36; P = ,024), psiquiátrica (OR 4,14; IC 95% 1,36–12,61; P = ,013), la dislipemia (OR 3,21; IC 95% 1.63–6,35; P = ,001) y el número de fármacos prescrito (OR 4,33; IC 95% 2,40–7,81; P = ,000).
ConclusiónLa prevalencia de interacciones potenciales en paciente COVD 19 en tratamiento con LPV/r es elevada, comportándose como factores de riesgo asociados la edad (>65), el ingreso en UCI, la patología previa respiratoria, psiquiátrica y la dislipemia y el número de fármacos prescritos.
COVID-19 is the name given to the disease caused by the "Severe Acute Respiratory Syndrome Coronavirus 2" (SARS-CoV-2), officially declared a global pandemic by the World Health Organization.1
Currently, there is no effective drug against COVID-19. One of the treatment alternatives used is lopinavir/ritonavir (LPV/r), which showed in vitro activity and positive clinical outcomes against coronaviruses in previous epidemics (SARS and MERS).2 A recent study in a severe COVID-19 patient showed no clinical benefit from delayed initiation of LPV/r, so further adequately conducted studies are needed to establish the usefulness of LPV/r.3
It is a combined antiretroviral therapy, which acts as a protease inhibitor (PI), used in the treatment of human immunodeficiency virus (HIV) infection.4 Lopinavir has antiviral activity, while ritonavir acts as a lopinavir enhancer, increasing its plasma concentrations by inhibiting CYP3A4. A relevant drawback of LPV/r is its high profile of interactions, due to its ability to modify the hepatic metabolism of other drugs, through the inhibition of CYP3A4 or the induction of CYP2C9 and 2C19 and glucuronidation reactions. On the other hand, it inhibits the activity of membrane transporter proteins such as BCR, OATP1B1 and glycoprotein-P, involved in intestinal and hepatic drug clearance.5
The population most susceptible to developing potential interactions with a clinical impact are elderly patients, with comorbidity and exposed to polypharmacy.6 On the other hand, the population with the highest risk of suffering from severe disease due to COVID-19 are patients over 60 years of age and with a medical history, such as cardiovascular disease.1
The main objective of the study was to determine the prevalence and report the potential interactions in COVID-19 patients receiving LPV/r therapy. The secondary objective was to develop recommendations for each interaction according to the sources consulted and to identify risk factors associated with potential interactions with LPV/r.
Material and methodsCross-sectional and multicenter study with the participation of a secondary-level and a primary-level hospital. The data collection period was 15 days, from 20th March to 4th April 2020. The prevalence of potential interactions in COVID-19 patients treated with LPV/r was recorded and analysed. All patients older than 18 with hospital admission diagnosed with pneumonia due to COVID-19 or with clinical suspicion, who received treatment with LPV/r, were included in the study. All cases with acute respiratory symptoms including, among other symptoms, fever, persistent cough, odynophagia or respiratory distress and that met hospital admission criteria, were considered as suspected COVID-19 patients. The diagnosis of COVID-19 pneumonia was confirmed by positive PCR in a nasopharyngeal swab sample. An interaction related to LPV/r and any prescribed or home medication was defined as the existence of any qualitative or quantitative modification in the effect of the prescribed or home medication or LPV/r, with the concomitant administration of both. A review and screening of the interactions between LPV/r, prescribed and home medication, was carried out using the following identification and consultation databases: Lexicomp® (Uptodate),7 HIV-drug interactions8 and COVID-drug interactions.9 Potential drug interactions were those classified according to risk level: X (avoid combination), D (consider therapy modification) and C (monitor therapy). The latter were partially recorded, that is, only risk C interactions, with a recommendation to modify the therapy, in addition to monitoring it. Subsequently, the frequency was analysed and each interaction was reported according to the mechanism, the level of risk, if it had an impact on LPV/r, on the other drug involved in the interaction or both, if it could compromise the efficacy or cause toxicity and the recommendation, in each case.
Patient demographics, age, sex, and medical history were recorded. The number of medications prescribed during their hospitalization, as well as the number of home medications, were also recorded. ≥5 home drugs were considered as polypharmacy. The clinical history software and the electronic assisted prescription software of each centre were used to extract the data. Verbal consent was requested from all patients for the administration of LPV/r and for participation in the study.
For the statistical analysis, the IBM SPSS Statistics software, version 26.0 was used. Categorical variables were expressed with absolute frequencies and percentages, while continuous variables were expressed as mean, standard deviation, and range. To assess whether there were differences in the number of interactions between patients of different sex, the Mann-Whitney test was used. To determine the risk factors associated with having interactions, a univariate logistic regression analysis was performed with all demographic and clinical variables. The dependent variable was having ≥1 potential interactions, while the independent variables were age, admission to the ICU, polypharmacy, number of drugs prescribed and previous illness. A multivariate analysis was performed for the variables previously associated in a statistically significant way. A P-value of 5% (P < .05) was considered of significance.
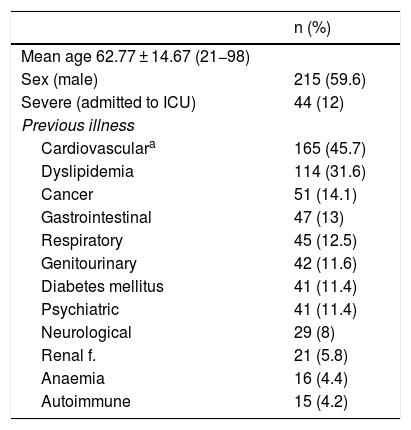
ResultsOf an initial population of 383 patients, 22 were excluded due to lack of data in the electronic clinical history. Finally, 361 patients with hospital admission secondary to COVID-19 pneumonia under treatment with LPV/r were included in the analysis. 59.6% (n = 215) were men with a mean global age of 62.77 ± 14.64 years. The demographic characteristics of the population are summarized in Table 1. 18.3% (n = 66) were patients taking 5 or more home drugs (polypharmacy) and 179 (49.6%) of the patients had ≥11 drugs prescribed during admission. There were no clinically significant differences in the number of potential interactions between patients of different sex.
Demographic characteristics of patients with COVID-19 treated with LPV/r.
| n (%) | |
|---|---|
| Mean age 62.77 ± 14.67 (21−98) | |
| Sex (male) | 215 (59.6) |
| Severe (admitted to ICU) | 44 (12) |
| Previous illness | |
| Cardiovasculara | 165 (45.7) |
| Dyslipidemia | 114 (31.6) |
| Cancer | 51 (14.1) |
| Gastrointestinal | 47 (13) |
| Respiratory | 45 (12.5) |
| Genitourinary | 42 (11.6) |
| Diabetes mellitus | 41 (11.4) |
| Psychiatric | 41 (11.4) |
| Neurological | 29 (8) |
| Renal f. | 21 (5.8) |
| Anaemia | 16 (4.4) |
| Autoimmune | 15 (4.2) |
Renal f.: renal failure; LPV/r: lopinavir/ritonavir; ICU: intensive care unit.
A total of 3931 prescription lines were analysed during admission, with a mean of 10.89 ± 2.68 (6–19) prescribed drugs/patient and 895 home drugs, with a mean of 2.47 ± 2.85 (0–17) medications/patient.
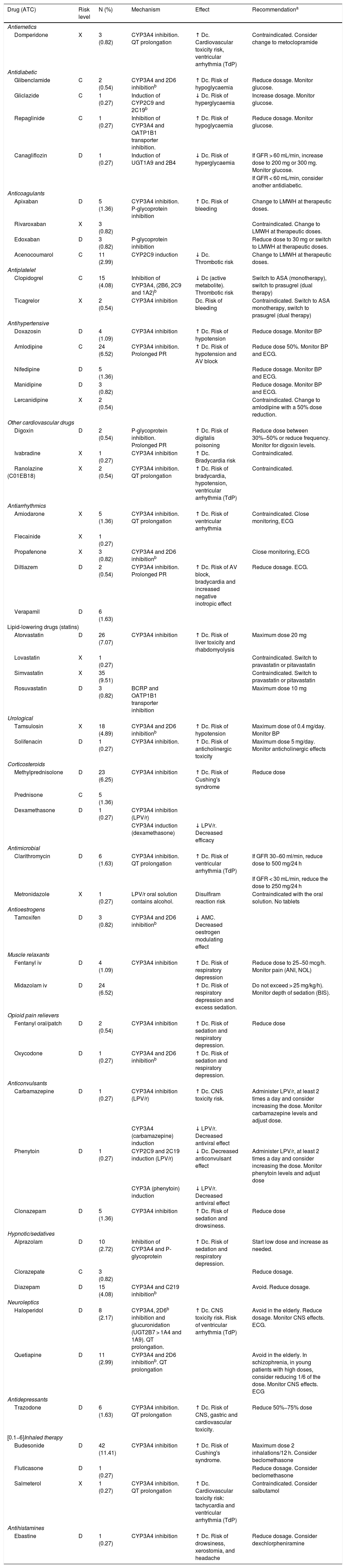
A total of 369 potential interactions were recorded with 52 different drugs. 20.92% (n = 78) were X-risk interactions, 63.32% (n = 233), D-risk and the remaining 15.76% (n = 58), C-risk. The drug with the highest rate of interaction was inhaled budesonide (11.41%, n = 42), followed by simvastatin (9.51%, n = 35) and atorvastatin (7.07%, n = 26). The rates of interactions by drug, together with the description of each interaction according to: mechanism, risk level, impact, and recommendation of each interaction, are summarized in Table 2. 37.7% (n = 136) of the patients had no interaction, 62.3% (n = 225) had one or more potential interactions, and 26.87% (n = 97) had 2 or more interactions.
List of registered potential interactions associated with LPV/r by drug, therapeutic group, severity, frequency, interaction mechanism, effect, and recommendation.
| Drug (ATC) | Risk level | N (%) | Mechanism | Effect | Recommendationa |
|---|---|---|---|---|---|
| Antiemetics | |||||
| Domperidone | X | 3 (0.82) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Cardiovascular toxicity risk, ventricular arrhythmia (TdP) | Contraindicated. Consider change to metoclopramide |
| Antidiabetic | |||||
| Glibenclamide | C | 2 (0.54) | CYP3A4 and 2D6 inhibitionb | ↑ Dc. Risk of hypoglycaemia | Reduce dosage. Monitor glucose. |
| Gliclazide | C | 1 (0.27) | Induction of CYP2C9 and 2C19b | ↓ Dc. Risk of hyperglycaemia | Increase dosage. Monitor glucose. |
| Repaglinide | C | 1 (0.27) | Inhibition of CYP3A4 and OATP1B1 transporter inhibition. | ↑ Dc. Risk of hypoglycaemia | Reduce dosage. Monitor glucose. |
| Canagliflozin | D | 1 (0.27) | Induction of UGT1A9 and 2B4 | ↓ Dc. Risk of hyperglycaemia | If GFR > 60 mL/min, increase dose to 200 mg or 300 mg. Monitor glucose. |
| If GFR < 60 mL/min, consider another antidiabetic. | |||||
| Anticoagulants | |||||
| Apixaban | D | 5 (1.36) | CYP3A4 inhibition. P-glycoprotein inhibition | ↑ Dc. Risk of bleeding | Change to LMWH at therapeutic doses. |
| Rivaroxaban | X | 3 (0.82) | Contraindicated. Change to LMWH at therapeutic doses. | ||
| Edoxaban | D | 3 (0.82) | P-glycoprotein inhibition | Reduce dose to 30 mg or switch to LMWH at therapeutic doses. | |
| Acenocoumarol | C | 11 (2.99) | CYP2C9 induction | ↓ Dc. Thrombotic risk | Change to LMWH at therapeutic doses. |
| Antiplatelet | |||||
| Clopidogrel | C | 15 (4.08) | Inhibition of CYP3A4, (2B6, 2C9 and 1A2)b | ↓ Dc (active metabolite). Thrombotic risk | Switch to ASA (monotherapy), switch to prasugrel (dual therapy) |
| Ticagrelor | X | 2 (0.54) | CYP3A4 inhibition | Dc. Risk of bleeding | Contraindicated. Switch to ASA monotherapy, switch to prasugrel (dual therapy) |
| Antihypertensive | |||||
| Doxazosin | D | 4 (1.09) | CYP3A4 inhibition | ↑ Dc. Risk of hypotension | Reduce dosage. Monitor BP |
| Amlodipine | C | 24 (6.52) | CYP3A4 inhibition. Prolonged PR | ↑ Dc. Risk of hypotension and AV block | Reduce dose 50%. Monitor BP and ECG. |
| Nifedipine | D | 5 (1.36) | Reduce dosage. Monitor BP and ECG. | ||
| Manidipine | D | 3 (0.82) | Reduce dosage. Monitor BP and ECG. | ||
| Lercanidipine | X | 2 (0.54) | Contraindicated. Change to amlodipine with a 50% dose reduction. | ||
| Other cardiovascular drugs | |||||
| Digoxin | D | 2 (0.54) | P-glycoprotein inhibition. Prolonged PR | ↑ Dc. Risk of digitalis poisoning | Reduce dose between 30%−50% or reduce frequency. Monitor for digoxin levels. |
| Ivabradine | X | 1 (0.27) | CYP3A4 inhibition | ↑ Dc. Bradycardia risk | Contraindicated. |
| Ranolazine (C01EB18) | X | 2 (0.54) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Risk of bradycardia, hypotension, ventricular arrhythmia (TdP) | Contraindicated. |
| Antiarrhythmics | |||||
| Amiodarone | X | 5 (1.36) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Risk of ventricular arrhythmia | Contraindicated. Close monitoring, ECG |
| Flecainide | X | 1 (0.27) | |||
| Propafenone | X | 3 (0.82) | CYP3A4 and 2D6 inhibitionb | Close monitoring, ECG | |
| Diltiazem | D | 2 (0.54) | CYP3A4 inhibition. Prolonged PR | ↑ Dc. Risk of AV block, bradycardia and increased negative inotropic effect | Reduce dosage. ECG. |
| Verapamil | D | 6 (1.63) | |||
| Lipid-lowering drugs (statins) | |||||
| Atorvastatin | D | 26 (7.07) | CYP3A4 inhibition | ↑ Dc. Risk of liver toxicity and rhabdomyolysis | Maximum dose 20 mg |
| Lovastatin | X | 1 (0.27) | Contraindicated. Switch to pravastatin or pitavastatin | ||
| Simvastatin | X | 35 (9.51) | Contraindicated. Switch to pravastatin or pitavastatin | ||
| Rosuvastatin | D | 3 (0.82) | BCRP and OATP1B1 transporter inhibition | Maximum dose 10 mg | |
| Urological | |||||
| Tamsulosin | X | 18 (4.89) | CYP3A4 and 2D6 inhibitionb | ↑ Dc. Risk of hypotension | Maximum dose of 0.4 mg/day. Monitor BP |
| Solifenacin | D | 1 (0.27) | CYP3A4 inhibition. | ↑ Dc. Risk of anticholinergic toxicity | Maximum dose 5 mg/day. Monitor anticholinergic effects |
| Corticosteroids | |||||
| Methylprednisolone | D | 23 (6.25) | CYP3A4 inhibition | ↑ Dc. Risk of Cushing's syndrome | Reduce dose |
| Prednisone | C | 5 (1.36) | |||
| Dexamethasone | D | 1 (0.27) | CYP3A4 inhibition (LPV/r) | ||
| CYP3A4 induction (dexamethasone) | ↓ LPV/r. Decreased efficacy | ||||
| Antimicrobial | |||||
| Clarithromycin | D | 6 (1.63) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Risk of ventricular arrhythmia (TdP) | If GFR 30−60 ml/min, reduce dose to 500 mg/24 h |
| If GFR < 30 mL/min, reduce the dose to 250 mg/24 h | |||||
| Metronidazole | X | 1 (0.27) | LPV/r oral solution contains alcohol. | Disulfiram reaction risk | Contraindicated with the oral solution. No tablets |
| Antioestrogens | |||||
| Tamoxifen | D | 3 (0.82) | CYP3A4 and 2D6 inhibitionb | ↓ AMC. Decreased oestrogen modulating effect | |
| Muscle relaxants | |||||
| Fentanyl iv | D | 4 (1.09) | CYP3A4 inhibition | ↑ Dc. Risk of respiratory depression | Reduce dose to 25−50 mcg/h. Monitor pain (ANI, NOL) |
| Midazolam iv | D | 24 (6.52) | ↑ Dc. Risk of respiratory depression and excess sedation. | Do not exceed > 25 mg/kg/h). Monitor depth of sedation (BIS). | |
| Opioid pain relievers | |||||
| Fentanyl oral/patch | D | 2 (0.54) | CYP3A4 inhibition | ↑ Dc. Risk of sedation and respiratory depression. | Reduce dose |
| Oxycodone | D | 1 (0.27) | CYP3A4 and 2D6 inhibitionb | ↑ Dc. Risk of sedation and respiratory depression. | |
| Anticonvulsants | |||||
| Carbamazepine | D | 1 (0.27) | CYP3A4 inhibition (LPV/r) | ↑ Dc. CNS toxicity risk. | Administer LPV/r, at least 2 times a day and consider increasing the dose. Monitor carbamazepine levels and adjust dose. |
| CYP3A4 (carbamazepine) induction | ↓ LPV/r. Decreased antiviral effect | ||||
| Phenytoin | D | 1 (0.27) | CYP2C9 and 2C19 induction (LPV/r) | ↓ Dc. Decreased anticonvulsant effect | Administer LPV/r, at least 2 times a day and consider increasing the dose. Monitor phenytoin levels and adjust dose |
| CYP3A (phenytoin) induction | ↓ LPV/r. Decreased antiviral effect | ||||
| Clonazepam | D | 5 (1.36) | CYP3A4 inhibition | ↑ Dc. Risk of sedation and drowsiness. | Reduce dose |
| Hypnotic/sedatives | |||||
| Alprazolam | D | 10 (2.72) | Inhibition of CYP3A4 and P-glycoprotein | ↑ Dc. Risk of sedation and respiratory depression. | Start low dose and increase as needed. |
| Clorazepate | C | 3 (0.82) | Reduce dosage. | ||
| Diazepam | D | 15 (4.08) | CYP3A4 and C219 inhibitionb | Avoid. Reduce dosage. | |
| Neuroleptics | |||||
| Haloperidol | D | 8 (2.17) | CYP3A4, 2D6b inhibition and glucuronidation (UGT2B7 > 1A4 and 1A9). QT prolongation. | ↑ Dc. CNS toxicity risk. Risk of ventricular arrhythmia (TdP) | Avoid in the elderly. Reduce dosage. Monitor CNS effects. ECG. |
| Quetiapine | D | 11 (2.99) | CYP3A4 and 2D6 inhibitionb. QT prolongation | Avoid in the elderly. In schizophrenia, in young patients with high doses, consider reducing 1/6 of the dose. Monitor CNS effects. ECG | |
| Antidepressants | |||||
| Trazodone | D | 6 (1.63) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Risk of CNS, gastric and cardiovascular toxicity. | Reduce 50%−75% dose |
| [0.1−6]Inhaled therapy | |||||
| Budesonide | D | 42 (11.41) | CYP3A4 inhibition | ↑ Dc. Risk of Cushing's syndrome. | Maximum dose 2 inhalations/12 h. Consider beclomethasone |
| Fluticasone | D | 1 (0.27) | Reduce dosage. Consider beclomethasone | ||
| Salmeterol | X | 1 (0.27) | CYP3A4 inhibition. QT prolongation | ↑ Dc. Cardiovascular toxicity risk: tachycardia and ventricular arrhythmia (TdP) | Contraindicated. Consider salbutamol |
| Antihistamines | |||||
| Ebastine | D | 1 (0.27) | CYP3A4 inhibition | ↑ Dc. Risk of drowsiness, xerostomia, and headache | Reduce dosage. Consider dexchlorpheniramine |
ASA: acetylsalicylic acid; ANI: analgesia nociception index; AV: atrioventricular; Dc: drug concentration; AMC: active metabolite concentration; CYP: cytochrome P450; ECG: electrocardiogram; GFR: glomerular filtration rate; LMWH: low molecular weight heparin; LPV/r: lopinavir/ritonavir; NOL: nociception index level; PR: PR interval; QT: QT interval; CNS: central nervous system; BP: blood pressure; TdP: torsade de pointes; UGT: UDP-glucuronyl transferases.
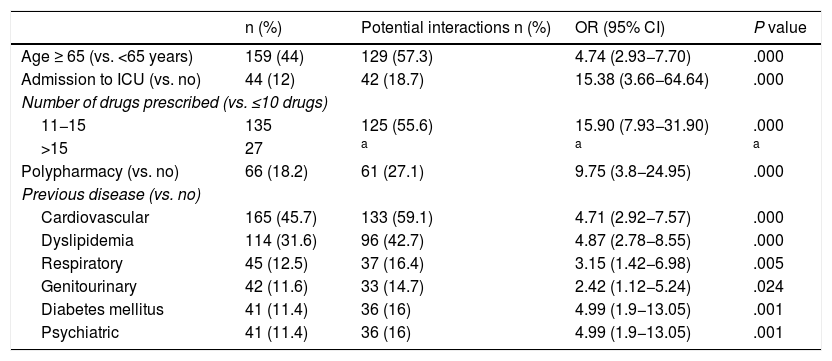
The risk factors associated with having ≥1 potential interactions obtained through a univariate logistic regression model are shown in Table 3. The independent variables that increase the probability of developing ≥1 potential interactions, using a multivariate regression model were age >65 years (OR 1.95; 95% CI 1.06–3.59; P = .033), admission to ICU (OR 9.22; 95% CI 1.98–42.93; P = .005), previous respiratory disease (OR 2.90; 95% CI 1.15–7.36; P = .024), psychiatric disease (OR 4.14; 95% CI 1.36–12.61; P = .013), dyslipidaemia (OR 3.21; 95% CI 1.63–6.35; P = .001) and the number of drugs prescribed during hospital admission (OR 4.33; 95% CI 2.40–7.81; P = .000).
Univariate binary logistic regression analysis. Variables associated with having ≥ 1 potential interactions with LPV/r in a patient with COVID-19.
| n (%) | Potential interactions n (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Age ≥ 65 (vs. <65 years) | 159 (44) | 129 (57.3) | 4.74 (2.93−7.70) | .000 |
| Admission to ICU (vs. no) | 44 (12) | 42 (18.7) | 15.38 (3.66−64.64) | .000 |
| Number of drugs prescribed (vs. ≤10 drugs) | ||||
| 11−15 | 135 | 125 (55.6) | 15.90 (7.93−31.90) | .000 |
| >15 | 27 | a | a | a |
| Polypharmacy (vs. no) | 66 (18.2) | 61 (27.1) | 9.75 (3.8−24.95) | .000 |
| Previous disease (vs. no) | ||||
| Cardiovascular | 165 (45.7) | 133 (59.1) | 4.71 (2.92−7.57) | .000 |
| Dyslipidemia | 114 (31.6) | 96 (42.7) | 4.87 (2.78−8.55) | .000 |
| Respiratory | 45 (12.5) | 37 (16.4) | 3.15 (1.42−6.98) | .005 |
| Genitourinary | 42 (11.6) | 33 (14.7) | 2.42 (1.12−5.24) | .024 |
| Diabetes mellitus | 41 (11.4) | 36 (16) | 4.99 (1.9−13.05) | .001 |
| Psychiatric | 41 (11.4) | 36 (16) | 4.99 (1.9−13.05) | .001 |
ICU: intensive care unit.
Our study represents a novel assessment in relation to COVID-19 infection. To our knowledge, no study has been published evaluating the prevalence of potential drug interactions with LPV/r therapy. We are facing a new scenario with a different disease profile, therefore, it is complex to make comparisons between COVID-19 and other infections such as HIV, where there is more experience with LPV/r. Our study shows a high rate of potential interactions, occurring in more than 60% of patients. One of the risk factors related to a higher rate of potential interactions in the HIV population was treatment with a boosted PI, with global prevalence between 27 and 40%.10–12 This difference can be explained by the fact that we analyse home medication and hospital medication together.
Age over 65 was one of the variables that increased the probability of having potential interactions in the multivariate analysis. In an aging Spanish cohort (>65 years) of HIV patients, it was observed that the number of potential interactions increases with the number of drugs prescribed and with the use of a PI in the antiretroviral regimen.13 Furthermore, we observed that admission to the ICU behaves as an independent variable associated with developing potential interactions with a probability of 9 times greater than in a non-serious patient. In a study of potential interactions carried out in the critical care unit, the presence of potential interactions was attributed to age (>60 years) and the number of drugs prescribed.14 Precisely, the number of drugs prescribed was another predictor variable for the occurrence of potential interactions, coinciding with what was previously reported in the literature.15,16
Inhaled budesonide was the drug with the highest rate of interaction with LPV/r in our cohort. Therefore, respiratory disease was a risk factor associated with developing interactions. Another comorbidity with a high probability of interaction was psychiatric disorder. A group of drugs commonly involved in potential interactions due to their hepatic metabolism (CYP 3A4) in the HIV population were those drugs used in psychiatric disorders.17 Almost half of the patients in our study had a cardiovascular history. This high prevalence is explained by the fact that we include arterial hypertension within this group. COVID-19 infection is associated with haemostasis abnormalities, which may imply an increased risk of thromboembolic cardiovascular disease.18 Therefore, it is recommended to maintain anticoagulation and antiplatelet therapy as long as they are not associated with bleeding episodes. The appropriate LPV/r-compatible, anticoagulant, or antiplatelet agent, should be selected and in case of contraindication, consider not starting or discontinuing LPV/r (Table 2).19
LPV/r has a moderate ability to slow cardiac repolarization and prolong the QT interval.5 In addition, hydroxychloroquine and azithromycin, drugs currently used in combination with LVP/r for the treatment of COVID 19, also prolong QT, increasing the risk of ventricular arrhythmia (torsade de pointes). Therefore, the QT interval should be monitored in those patients with risk factors, avoiding drugs that prolong QT as much as possible.20
Dyslipidemia was another independent variable associated with developing potential interactions with LPV/r in our study. Simvastatin and atorvastatin were 2 of the drugs with the highest rate of interaction, responsible for the previous association. Hypercholesterolemia and PI-statin interaction are also recurrent in HIV patients.21 On the other hand, there is currently not enough evidence to show that statins are beneficial or harmful in COVID-19. Therefore, because prior cardiovascular disease is associated with a worse prognosis of COVID-19,22 the immunomodulatory capacity of statins in the immune response23 and their prior benefit in viral pneumonia,24 it may be possible to consider keeping statins as indicated, by adjusting the dose or selecting those that are free of interaction with LPV/r (Table 3).
Our study has some limitations. In the screening of the interactions, we found slight discrepancies in some interactions between the different databases, which can make establishing recommendations difficult. On the other hand, databases report interactions between two drugs, while screening is carried out with multiple combinations that can have an unaccounted-for sum effect, such as pharmacodynamic interaction, prolongation of QT.
In conclusion, COVID-19 patients undergoing treatment with LPV/r are exposed to having a high prevalence of potential interactions, with age (>65), admission to the ICU, previous respiratory disease and psychiatric disorders, dyslipidaemia and the number of drugs prescribed behaving as associated risk factors. Knowledge of the potential interactions profile in a COVID-19 patient receiving LPV/r and his/her adequate treatment can improve the safety and effectiveness of treatments. Collaboration between hospital and clinical pharmacists is essential for the comprehensive approach and treatment of the COVID 19 pandemic.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Brandariz-Nuñez D, Correas-Sanahuja M, Guarc E, Picón R, García B, Gil R. Interacciones medicamentosas potenciales en pacientes COVID 19 en tratamiento con lopinavir/ritonavir. Med Clin (Barc). 2020;155:281–287.