The soluble urokinase-type plasminogen activator receptor (suPAR) potentially plays a role in immune-thrombosis, possibly by modulating plasmin activity or contributing to chemotaxis in a complex, poorly understood context. The role of suPAR levels in the short-term prognostic of patients with pulmonary embolism (PE) has not been evaluated.
Material and methodsThis observational, prospective, single-center study enrolled consecutive patients aged 18 and above with confirmed acute symptomatic PE and no prior anticoagulant therapy. The primary objective was to assess the prognostic capacity of suPAR levels measured at the time of diagnosis in terms of mortality.
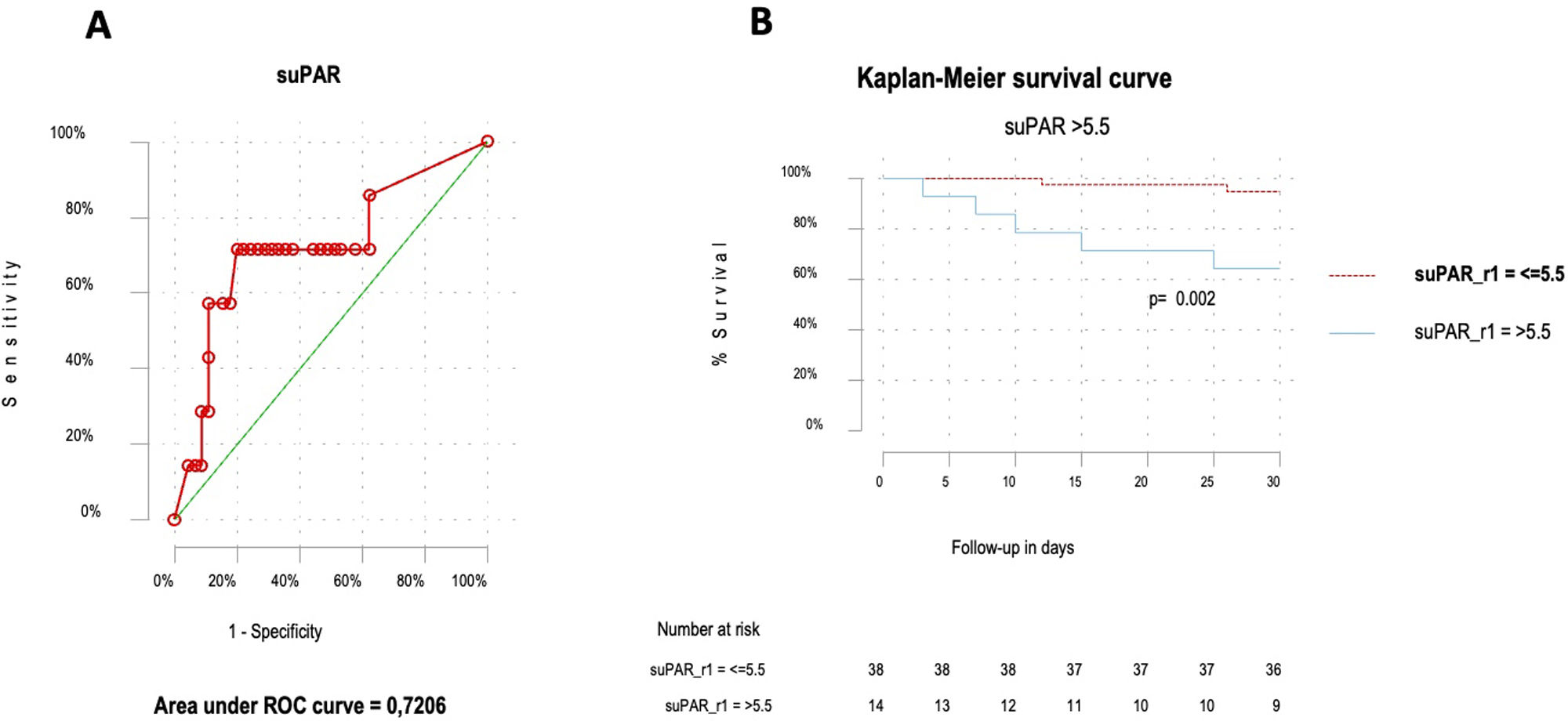
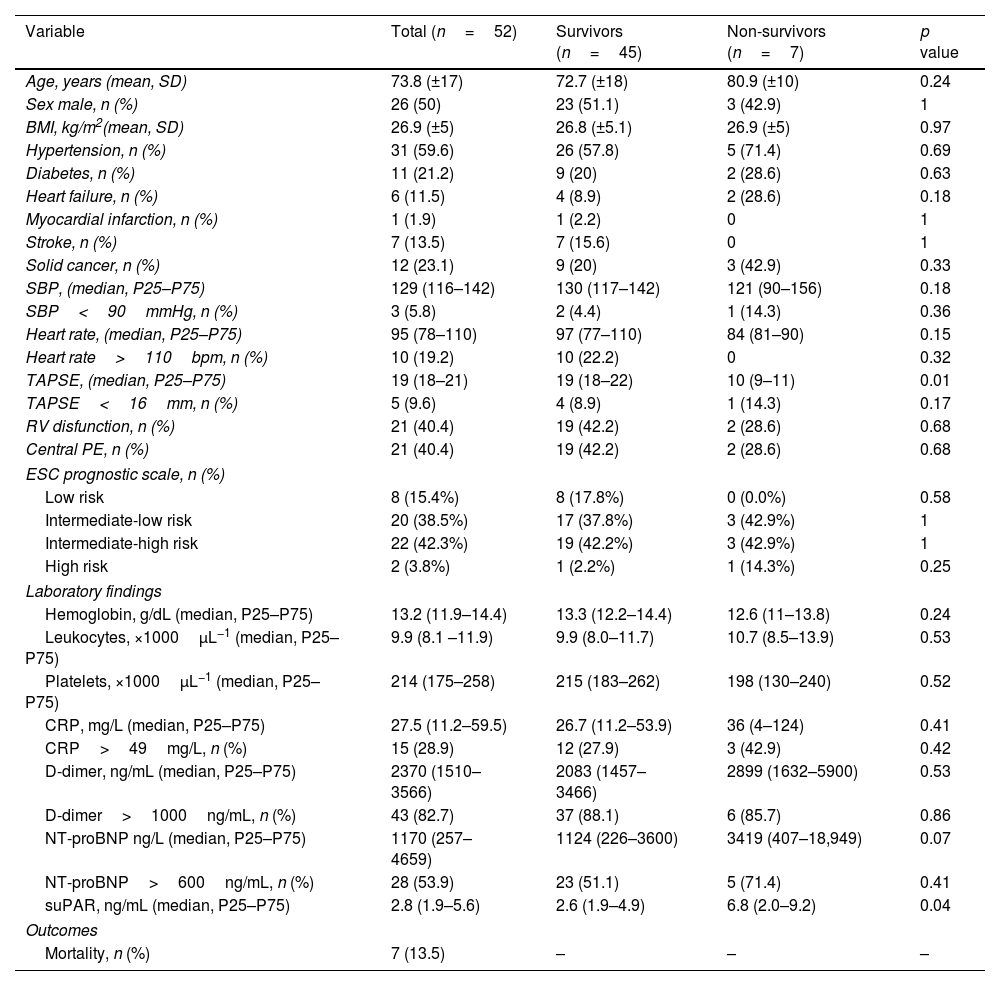
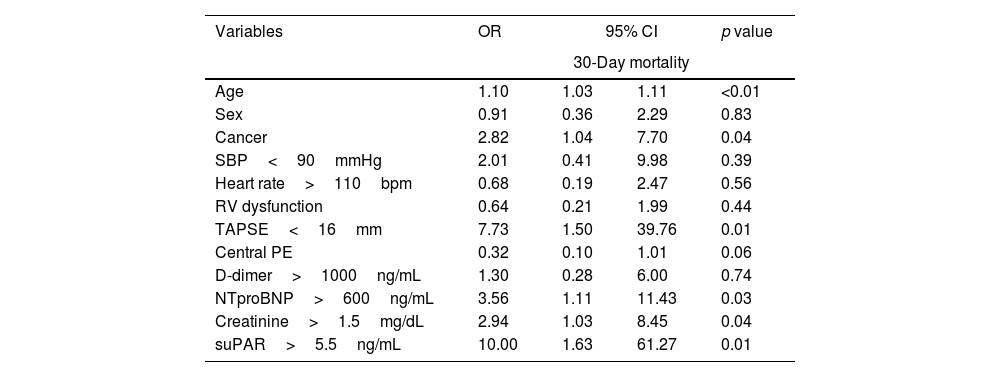
ResultsFifty-two patients, with a mean age of 73.8 years (±17), were included, with gender distribution evenly split at 50%. Seven (13.5%) patients died. The ROC curve for mortality yielded an AUC of 0.72 (95% CI 0.48–0.96), with an optimal suPAR cut-off of 5.5ng/mL. Bivariate analysis for suPAR>5.5ng/mL was associated with a crude odds ratio of 10 (95% CI 1.63–61.27; p=0.01) for 30-day mortality. Survival analysis showed a 30-day mortality hazard ratio of 8.33 (95% CI 1.69–40.99; p<0.01).
ConclusionsuPAR emerges as a potential biomarker for short-term mortality prediction and holds the potential for enhanced stratification in patients with acute symptomatic PE.
El receptor soluble activador del plasminógeno tipo uroquinasa (suPAR) podría participar en la denominada inmunotrombosis, posiblemente regulando la actividad de la plasmina o contribuyendo a la quimiotaxis por mecanismos complejos y aún no esclarecidos. El papel de los niveles de suPAR en el pronóstico a corto plazo en los pacientes con embolia pulmonar (EP) no ha sido evaluado.
Material y métodosEste estudio observacional, prospectivo y unicéntrico incluyó a pacientes mayores de 18 años, con EP sintomática confirmada y sin tratamiento anticoagulante previo. El objetivo primario fue evaluar la capacidad pronóstica de los niveles del suPAR medidos en el momento del diagnóstico, en términos de mortalidad.
ResultadosCincuenta y dos pacientes, con una edad media de 73,8 años (±17), fueron incluidos, con una distribución por sexos del 50%. Siete pacientes (13,5%) fallecieron. La curva ROC para mortalidad mostró un AUC de 0,72 (IC 95%: 0,48-0,96), con un punto de corte óptimo del suPAR de 5,5ng/ml. En el análisis bivariable, los niveles de suPAR>5,5ng/ml se asociaron con un odds ratio de 10 (IC 95%: 1,63-61,27; p=0,01) para mortalidad a 30 días. Los análisis de supervivencia mostraron una hazard ratio para mortalidad a los 30 días de 8,33 (IC 95%: 1,69-40,99; p<0,01).
ConclusiónEn los pacientes con embolia de pulmón aguda sintomática, el suPAR es un potencial biomarcador para la predicción de mortalidad precoz, y podría ayudar a mejorar la estratificación pronóstica en estos pacientes.