The development of vaccines against SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), the causative agent of COVID-19 (coronavirus disease-19), is an important epidemiological tool for pandemic control. People with systemic autoimmune diseases (SAD) are at increased risk of severe forms and mortality from COVID-19.1,2 The risk factors associated with greater severity are, on the one hand, the same as in the general population, such as age over 65, male sex, high blood pressure with cardiac comorbidity and chronic lung or kidney disease.2 On the other hand, there are factors inherent to SAD. In the international COVID-19 Global Rheumatology Alliance physician-reported registry study, the highest risk of mortality was associated with moderate-high activity, use of a dose greater than 10 mg/day of prednisone, sulfasalazine or rituximab.2
The increased morbidity and mortality places people with SAD among the priority groups of patients who would benefit from vaccination against COVID-19.3 To date, the data regarding their efficacy and adverse reactions are unknown since these groups of patients were excluded from the clinical studies that led to the approval of these vaccines. However, the position of different scientific societies is in favour of offering these patients vaccination against COVID-19.3–6 From a clinical perspective, the following questions are raised to guide the decisions to be made:
- 1)
What types of COVID-19 vaccines are currently available and how effective are they in patients with SAD?
- 2)
Can COVID-19 vaccines trigger SAD or cause a disease flare-up?
- 3)
What potential adverse reactions can COVID-19 vaccines cause in patients with SAD?
- 4)
Are patients with SAD who receive adenovirus-vectored DNA vaccines at a higher risk of developing thrombosis?
- 5)
What is the most appropriate clinical setting to administer the COVID-19 vaccine in a patient with SAD?
- 6)
Is it necessary to stop immunosuppressive treatment before administering the COVID-19 vaccine?
- 7)
Type of vaccines against COVID-19 currently available and efficacy in patients with SAD.
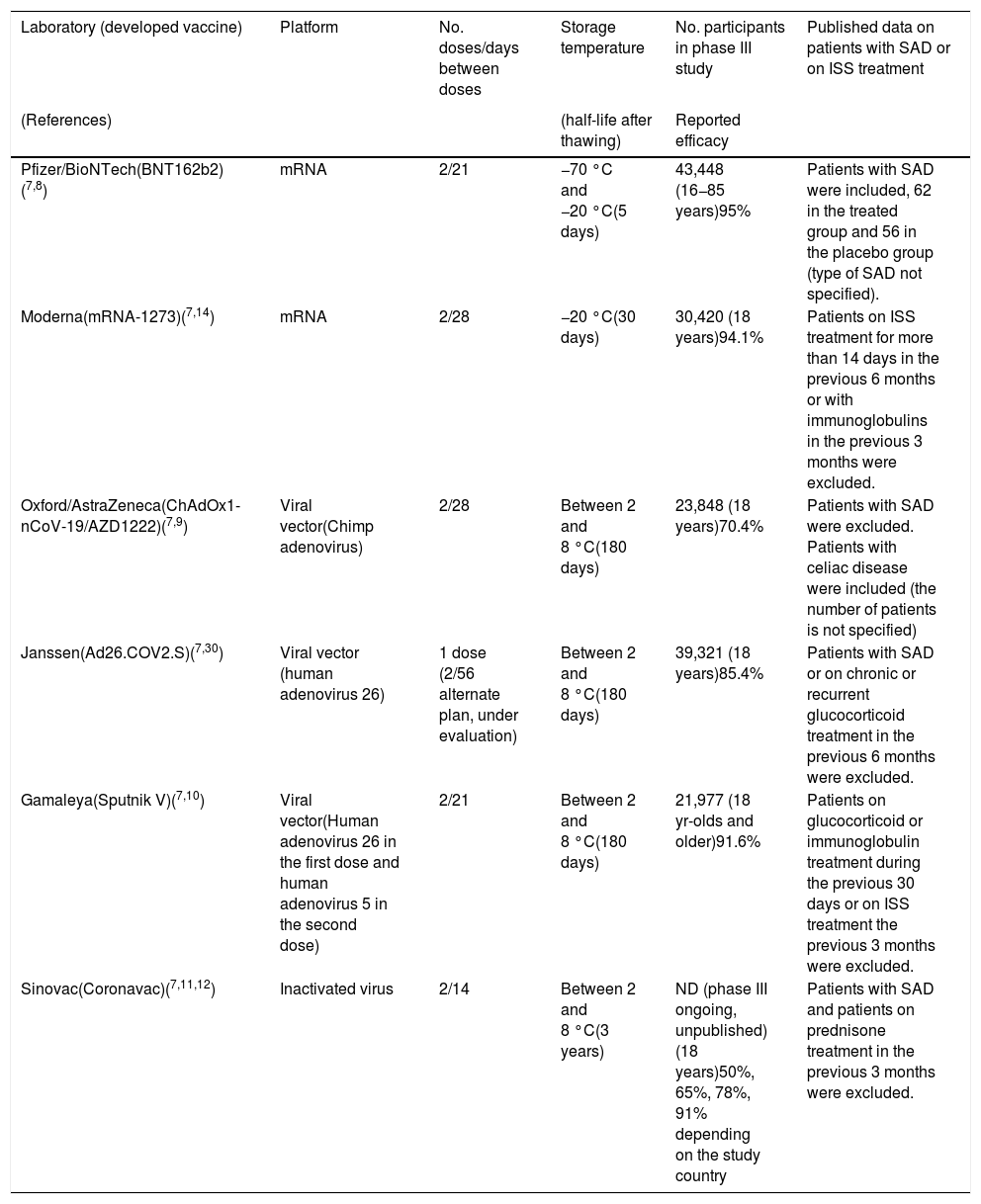
There are four different SARS-CoV-2 vaccine platforms evaluated in phase III clinical trials: (a) those based on messenger RNA (mRNA); (b) those using DNA via a viral vector; c) those using inactivated SARS-CoV-2 viruses; and (d) the fourth platform, consisting of vaccines using recombinant protein nanoparticles with adjuvants7 (Table 1).
Main characteristics of vaccines against COVID-19.
| Laboratory (developed vaccine) | Platform | No. doses/days between doses | Storage temperature | No. participants in phase III study | Published data on patients with SAD or on ISS treatment |
|---|---|---|---|---|---|
| (References) | (half-life after thawing) | Reported efficacy | |||
| Pfizer/BioNTech(BNT162b2)(7,8) | mRNA | 2/21 | −70 °C and −20 °C(5 days) | 43,448 (16−85 years)95% | Patients with SAD were included, 62 in the treated group and 56 in the placebo group (type of SAD not specified). |
| Moderna(mRNA-1273)(7,14) | mRNA | 2/28 | −20 °C(30 days) | 30,420 (18 years)94.1% | Patients on ISS treatment for more than 14 days in the previous 6 months or with immunoglobulins in the previous 3 months were excluded. |
| Oxford/AstraZeneca(ChAdOx1-nCoV-19/AZD1222)(7,9) | Viral vector(Chimp adenovirus) | 2/28 | Between 2 and 8 °C(180 days) | 23,848 (18 years)70.4% | Patients with SAD were excluded. Patients with celiac disease were included (the number of patients is not specified) |
| Janssen(Ad26.COV2.S)(7,30) | Viral vector (human adenovirus 26) | 1 dose (2/56 alternate plan, under evaluation) | Between 2 and 8 °C(180 days) | 39,321 (18 years)85.4% | Patients with SAD or on chronic or recurrent glucocorticoid treatment in the previous 6 months were excluded. |
| Gamaleya(Sputnik V)(7,10) | Viral vector(Human adenovirus 26 in the first dose and human adenovirus 5 in the second dose) | 2/21 | Between 2 and 8 °C(180 days) | 21,977 (18 yr-olds and older)91.6% | Patients on glucocorticoid or immunoglobulin treatment during the previous 30 days or on ISS treatment the previous 3 months were excluded. |
| Sinovac(Coronavac)(7,11,12) | Inactivated virus | 2/14 | Between 2 and 8 °C(3 years) | ND (phase III ongoing, unpublished) (18 years)50%, 65%, 78%, 91% depending on the study country | Patients with SAD and patients on prednisone treatment in the previous 3 months were excluded. |
MRNA: Messenger RNA; SAD: systemic autoimmune disease; ISS: immunosuppressant; ND: no data.
mRNA-based vaccines include in their sequence the S protein (spike protein) of SARS-CoV-2 and a lipid layer of nanoparticles as a protective coating for the mRNA. This facilitates its entry into the cell cytoplasm and the initiation of translation of the mRNA into the viral protein, which then leads to the immune response generating neutralising antibodies against the S protein. This strategy is used by the Pfizer-BioNTech and Moderna/National Institute of Allergy and Infectious Diseases7 laboratories. The Pfizer-BioNTech vaccine studies included individuals aged 16 years and older and very few patients with rheumatic diseases (62 in the treated group and 56 in the placebo group), representing only 0.3% of the sample. To date, no specific data have been published for this group of patients.8
DNA-based vaccines use adenoviruses whose replicative capacity has been eliminated and, additionally, have had the S protein's DNA of the SARS-CoV-2 inserted. It is therefore a vaccine that introduces a replication-incompetent adenovirus but allows entry into the cell nucleus of the DNA necessary for the synthesis of the S protein, which generates the immune response. This is the strategy used (with different adenoviruses) by the laboratories University of Oxford/AstraZeneca (chimpanzee adenovirus), Gamaleya National Research Centre for Epidemiology and Microbiology (human adenoviruses 5 and 26), CanSino Biological Inc/Beijing Institute of Biotechnology (human adenovirus 5) and Janssen Pharmaceutical Companies (human adenovirus 26).7 The University of Oxford/AstraZeneca vaccine studies included patients aged 18 years or older and excluded patients with SAD (except those with celiac disease).9 Patients on glucocorticoid or immunoglobulin therapy or immunosuppressive therapy in the 3 months prior to vaccine administration were excluded from the Gamaleya vaccine study.10
The third group is made up of vaccines that use inactivated viruses as their basis. Chemically inactivated SARS-CoV-2 viral particles are used, administered together with an excipient (adjuvant) that enhances their immunogenicity. This results in an immune response with production of neutralising antibodies against the S protein and other less represented antigens, which are considered non-neutralising antibodies. This is the strategy used by the SinoVac (Coronavac), Biotech laboratory, whose phase I/II study results and phase III protocol have been published. This included people aged 18 years or older and excluded patients with SAD, as well as those who received prednisone in the 3 months prior to vaccine administration.7,11,12
The fourth vaccine platform is built on the basis of recombinant protein nanoparticles (subunits) associated with an adjuvant that enhances their immune response. This is the strategy used by the Novavax laboratory, with published data from phase I/II studies (phase III ongoing).7,13
In terms of efficacy, i.e., patients without SARS-CoV-2 infection after completing the vaccination course, the percentages are 95% for the Pfizer/BioNTech vaccine, 94.1% for Moderna, 91.6% for Gamaleya, 70.4% for Oxford/AstraZeneca, and Sinovac has reported variable efficacy of 50%, 65%, 78% and 91% depending on the country where the study was conducted, respectively.7,14
In terms of effectiveness in the production of antibodies in people without AAS, mRNA and DNA viral vector vaccines provide mechanisms to enhance the immune response (innate and acquired) by activation of intracellular pathways (such as interferon and toll-like receptor activation), effects not observed with inactivated virus vaccines. While all COVID-19 vaccine strategies generate memory cells, the levels of antibody production achieved by mRNA or viral vector DNA vaccines are (at least 4-fold) higher than those obtained by inactivated virus vaccines.
It should be noted that the exact production of antibodies against SARS-CoV-2 in patients with SAD after vaccination is not known. The first data on the response to mRNA vaccines in patients with SAD have recently been published.15,16 The first study involving 26 patients with different autoimmune diseases, showed that the response defined by the production of IgG antibodies directed against SARS-CoV-2 protein S and neutralising antibodies was found in all patients. However, the titers were significantly lower in patients than in healthy controls. Furthermore, the authors found no differences in response to vaccines between patients treated with anti-TNF agents and those receiving disease-modifying drugs.15 The second study involving 123 patients detected the generation of anti-SARS-CoV-2 antibodies in 74%.16 Treatment with mycophenolate or rituximab was associated with an increased risk of not generating a response to the vaccine.17
- 1)
Potential for COVID-19 vaccines to trigger a SAD or cause a disease flare-up.
Autoimmune diseases (Guillain-Barré syndrome, immune thrombocytopenia, and antiphospholipid syndrome, among others)18 as well as the presence of antinuclear antibodies, antibodies against extractable nuclear antigens, antimitochondrial antibodies, antiphospholipid antibodies and rheumatoid factor have been described during or immediately after COVID-19.18 In turn, cross-reactivity has been demonstrated between antibodies directed against the SARS-CoV-2 S protein and different tissue antigens.19 This raises the hypothesis that the SARS-CoV-2 S protein could, by molecular mimicry, trigger the development of a SAD.
In the same way that mRNA vaccines achieve a heightened immune response by activating additional cytoplasmic pathways, it is feasible that they may trigger a cascade of immune mechanisms leading to aberrant activation of the immune response (innate or acquired) and hypothetically induce or activate a SAD. In this sense, one of the serious adverse reactions reported in the Moderna vaccine has been the development of rheumatoid arthritis in one of the participants of the treated group, the only case described in the study with 30,000 volunteers included.20
The non-inclusion of patients with SAD in the clinical studies of the different vaccines makes it impossible to offer scientific evidence in this regard. However, the molecular mimicry mechanisms associated with the presence of the SARS-CoV-2 S protein, as well as the activation of aberrant pathways of the immune response, would leave the door open to mechanisms responsible for the activation of a SAD as a result of COVID-19 vaccination. Therefore, there is a theoretical risk of the autoimmune disease developing a flare-up or worsening after vaccination. However, the benefit of COVID-19 vaccine protection against severe disease outweighs the potential risk of disease relapse.3
- 3)
Potential adverse reactions of COVID-19 vaccines in patients with SAD.
The most common adverse reactions (variable in frequency depending on the type of vaccine) are pain at the puncture site, erythema, headache, transient oral paraesthesia, adynamia, and adenomegaly. With the exception of the case of rheumatoid arthritis reported in the Moderna studies, no adverse reactions related to the development of SAD or autoinflammatory diseases have been reported.8,13 The first results of the study of vaccines in patients with SAD sponsored by the "COVID-19 Global Rheumatology Alliance" should be made public shortly. The study has now collected data from more than 2600 patients with SAD who have received at least one dose of the vaccine.21 Another similar study is the "VACOLUP study", sponsored by RESO (Centre de Référence des maladies auto-immunes systémiques rares Est Sud-Ouest) in France. In this case, there are updated data from 339 patients with systemic lupus erythematosus. 49% had some side effect after the first dose (81% mild-moderate) and 49% after the second dose (79% mild-moderate). Only 3% have had a disease flare-up and there have been no cases of SARS-CoV-2 infection in vaccinated patients.22
In addition to the adverse reactions described in the general population, the potential lung damage related to the use of vaccines against COVID-19 generates particular interest in patients with SAD, given the high prevalence of diffuse interstitial lung disease (DILD) in these patients. The damage would occur through a mechanism known as antibody-dependent enhancement (ADE), in which there would be an increase in cellular entry and replication of the virus favoured by the presence of vaccine-induced antibodies. Once the vaccinated individual is confronted with the infection for which he/she was immunised, the presence of vaccine-induced antibodies would generate an antigen-antibody reaction with immunocomplex formation and consequent tissue damage. This type of damage leads to so-called vaccine antibodies enhanced respiratory disease (VAERD), described during the development of respiratory syncytial virus vaccines and in models closer to COVID-19, such as vaccines against SARS-CoV-1 and MERS-CoV (Middle East Respiratory Syndrome coronavirus).7,23
It should be noted that the VAERD phenomenon is related to both mRNA, DNA vectors, and inactivated virus vaccines. However, the risk could potentially be higher with inactivated virus vaccines, as the patient is exposed to multiple antigens leading to the production of non-neutralising antibodies and/or the synthesis of antibodies directed against the S protein in non-neutralising conformations. This increases the diversity of targets to be recognized and would grant a greater capacity to develop ADE phenomena.7,23
- 4)
Risk of thrombosis in patients with SAD who receive adenovirus-vectored DNA vaccines.
Since February 2021, several cases of cerebral venous thrombosis or splanchnic vein thrombosis together with thrombocytopenia have been reported in people who have received the Oxford/AstraZeneca vaccine.24–26 This atypical location of thrombosis and thrombocytopenia after the administration of the vaccine has been called vaccine-induced immune thrombotic thrombocytopenia (VITT). Its pathogenetic mechanism is similar to that of heparin induced thrombocytopenia (HIT) characterised by the presence of antibodies targeting platelet factor 4 that trigger platelet activation (among other mechanisms), resulting in thrombosis together with a decrease in platelet numbers.24–26 The European Medicines Agency (EMA) has reported on the plausibility of the relationship between the administration of this vaccine and this adverse reaction without having been able to identify the risk factors that predispose its occurrence. Overall, the EMA establishes that the estimated risk of this adverse reaction is 1 case per 100,000 people who have received the first dose of the Oxford/AstraZeneca vaccine.27
The data published as of April 2021 includes 39 patients who received the Oxford/AstraZeneca vaccine and none of them had a previous history of SAD, thrombocytopenia or thrombosis.24–26 The study to rule out other causes of thrombosis and thrombocytopenia revealed 2 cases with antiphospholipid antibodies, one of them with von Willebrand's disease and a heterozygous factor V Leiden mutation. The other case had anticardiolipin antibodies. In the series including 23 patients (the largest series), antinuclear antibodies were negative in all patients and lupus anticoagulant was present in 5 of the 10 cases in which it was measured. The authors relate this finding to the state of disseminated intravascular coagulation that existed in these patients.24–26 More recently, 6 cases of VITT have been described in association with the Janssen adenovirus vector vaccine, and the same pathogenic mechanism has been proposed by the EMA.28
The development of these thromboses after the administration of both vaccines has been interpreted by regulatory agencies as an adverse reaction probably related to adenovirus vector vaccines. On the other hand, since none of the cases had a previous history of SAD, thrombocytopenia, or thrombosis, it can be inferred that there is no evidence to suggest that this type of vaccine may represent an increased thrombotic risk in patients with SAD. However, more pharmacovigilance studies are necessary to reaffirm or refute the existence of this risk.
- 5)
Most appropriate clinical setting for COVID-19 vaccine administration.
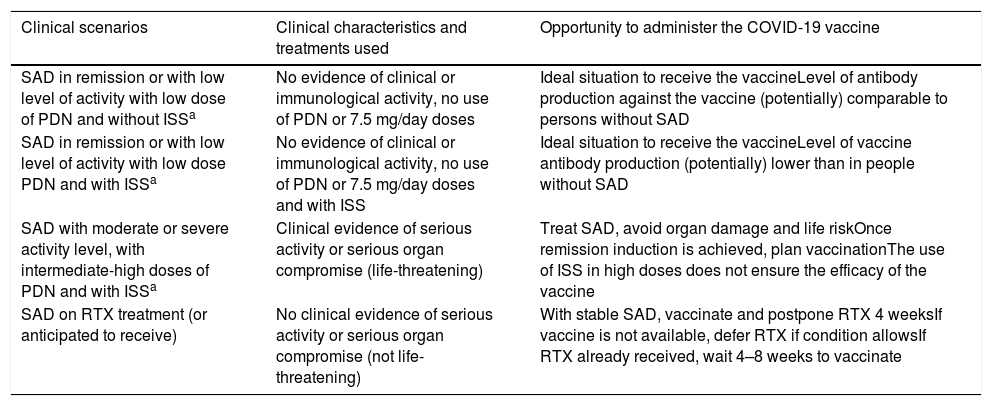
Ideally, patients with SAD should receive any vaccine during remission or a period of low activity, or before planned immunosuppressive therapy, in particular B-cell depletion therapy.29 Regarding the timing of vaccination and the type of ongoing immunosuppressive therapy, the general recommendation is not to change the treatment of SAD, since there is no evidence in this regard.5,6 Patients with SAD could be divided into four scenarios according to their clinical situation at the time of offering the vaccine (Table 2):
- •
Patient with disease in remission or low disease activity, on low-dose glucocorticoids and no immunosuppressants. This group is in the ideal situation, where the disease has no activity and glucocorticoid levels are low (≤7.5 mg/day). It is the best scenario to administer the vaccine and achieve antibody levels comparable to people without SAD.
- •
Patient with disease in remission or low disease activity, on low-dose glucocorticoid therapy, conventional immunosuppressive and/or biologic therapy or Janus kinase inhibitors. In this group of patients, immunosuppression levels could determine the lower production of antibodies or lower development of memory cells. However, this condition should not preclude vaccination, considering the pandemic scenario, where the potential benefits outweigh the possible adverse reactions.
- •
Patient with moderate or severe activity, in treatment with intermediate or high doses of glucocorticoids, with conventional immunosuppressants and/or biological treatment or Janus kinase inhibitors or considering any of these treatments. In this situation it is reasonable to treat SAD first and postpone vaccination as high doses of immunosuppressants are commonly used, which can significantly decrease the production of antibodies induced by COVID-19 vaccines. In addition, the potential adverse reactions to vaccination may overlap with the patterns of disease activity, which may make it difficult to differentiate which symptoms are due to which cause.3
- •
Patient on (or scheduled to receive) rituximab. The use of rituximab is associated with an overall decrease in immunoglobulins (especially IgG isotype), as well as lower levels of specific antibodies after vaccination.17,29 Regarding the use of rituximab as part of the treatment, the following should be considered: (1) if clinically feasible and the vaccine is available, vaccinate the patient and defer rituximab for 4 weeks; (2) if the patient has already received rituximab, a reasonable option would be to vaccinate against COVID-19 4–8 weeks after receiving it; (3) if the vaccine is not yet available and it is clinically safe to defer rituximab, rituximab administration should be delayed or an alternative treatment plan should be used; (4) if the vaccine is not yet available and it is not safe to defer rituximab for 4 weeks, rituximab should be administered and the vaccine should be offered when available.6
- 6)
Need for discontinuation of immunosuppressive treatment prior to administration of COVID-19 vaccine.
Clinical Scenarios of Patients with Systemic Autoimmune Disease at the Time of COVID-19 Vaccine Administration.
| Clinical scenarios | Clinical characteristics and treatments used | Opportunity to administer the COVID-19 vaccine |
|---|---|---|
| SAD in remission or with low level of activity with low dose of PDN and without ISSa | No evidence of clinical or immunological activity, no use of PDN or 7.5 mg/day doses | Ideal situation to receive the vaccineLevel of antibody production against the vaccine (potentially) comparable to persons without SAD |
| SAD in remission or with low level of activity with low dose PDN and with ISSa | No evidence of clinical or immunological activity, no use of PDN or 7.5 mg/day doses and with ISS | Ideal situation to receive the vaccineLevel of vaccine antibody production (potentially) lower than in people without SAD |
| SAD with moderate or severe activity level, with intermediate-high doses of PDN and with ISSa | Clinical evidence of serious activity or serious organ compromise (life-threatening) | Treat SAD, avoid organ damage and life riskOnce remission induction is achieved, plan vaccinationThe use of ISS in high doses does not ensure the efficacy of the vaccine |
| SAD on RTX treatment (or anticipated to receive) | No clinical evidence of serious activity or serious organ compromise (not life-threatening) | With stable SAD, vaccinate and postpone RTX 4 weeksIf vaccine is not available, defer RTX if condition allowsIf RTX already received, wait 4–8 weeks to vaccinate |
SAD: systemic autoimmune disease; PDN: prednisone; ISS: immunosuppressants; RTX rituximab.
There is no consensus on this point. Recommendations from the American College of Rheumatology suggest discontinuing treatment with methotrexate, Janus kinase inhibitors, abatacept, and intravenous cyclophosphamide pulses for up to one week after each dose of the vaccine is administered (as long as the disease is stable and if clinically feasible).3 This recommendation is based on the results of the immune response to other vaccines such as influenza or pneumococcal vaccines in patients with SAD on immunosuppressive therapy. However, other scientific societies recommend that treatment should not be discontinued when administering the COVID-19 vaccine.5,6
Variables to consider when making decisions regarding vaccination against COVID-19As a general rule, none of the available and approved COVID-19 vaccines are based on live attenuated SARS-CoV-2 viruses and are therefore not formally contraindicated in patients with SAD. It can be inferred that, in some cases, the response to the vaccine will be less intense and therefore protection will be weaker. This underlines the need to maintain physical protective measures, such as face mask use, hand hygiene and continued physical distancing, as vaccination does not prevent infection, but prevents severe forms of infection and therefore does not exempt the patient from complying with physical preventive measures. In the same way, vaccination against COVID-19 of SAD patient cohabitants is a way to reduce the risks of infection.3
The act of recommending vaccination against COVID-19 should be a shared decision between the physician and the patient, who should assess whether the benefits outweigh the potential risks of infection. In cases of patients with SAD who have already had COVID-19 symptoms, it seems reasonable to suggest the same regimen as for the general population and to wait 6 months before administering the vaccine.
The scientific societies that have taken a position on this issue agree to offer vaccination against COVID-19 to all patients with SAD, with any of the vaccination platforms approved by the drug regulatory agencies and emphasise that the potential benefits of avoiding a severe form of COVID-19 outweigh the risks of potential adverse reactions.3–6 With all this information, when prescribing vaccination against COVID-19 in patients with SAD, it is important to consider the following factors that can potentially influence both the levels of protection and the development of adverse reactions:
- 1)
The degree of SAD activity and the type and intensity of immunosuppressive treatment used at the time of offering vaccination.
- 2)
Patients with risk factors associated with severe COVID-19 could benefit from the use of mRNA or viral vector DNA vaccines, since they offer additional intracellular mechanisms to enhance the immune response. This would allow for higher antibody titres, considering that due to age or immunosuppressive treatment they might develop a less intense immune response against SARS-CoV-2.
- 3)
Inactivated virus vaccines potentially have a higher risk of developing lung damage due to VAERD, a fact to be considered in patients with SAD and respiratory comorbidity such as the presence of ILD. This could be an important factor in the choice of mRNA or viral vector DNA vaccines, which induce the production of neutralising antibodies in a specific way and have a lower risk of inducing non-neutralising antibodies (VAERD-associated).
- 4)
mRNA, viral vector DNA, inactivated virus or recombinant protein nanoparticles vaccines could be suitable options in patients with SAD in remission or with low activity, absence, or low dose of immunosuppressants and absence of ILD.
- 5)
To date, there is not enough scientific evidence to support modifying the treatment of SAD before or after the administration of the COVID-19 vaccine.
- 6)
Once the vaccination schedule of the chosen option has been completed, since it is not possible to predict the titre of neutralising antibodies against COVID-19 and to determine with certainty the level of protection that the patient will have, it is suggested to assess the magnitude of the response acquired after vaccination. Future studies will probably show whether this is possible by detecting in serum (e.g., by ELISA techniques) IgG isotype antibodies against the S protein of SARS-CoV-2 3–6 months after administration of the vaccine.
- 7)
Last but not least, vaccination against COVID-19 in patients with SAD does not exempt them from maintaining the recommended physical and social protective measures, as the levels of protection that the new COVID-19 vaccines will offer in patients with SAD are unknown.
Promoting vaccination against COVID-19 in patients with SAD is putting the high risk of developing a severe form of the infection ahead of the potential risk of side effects. It should be noted that these serious side effects are very rare. However, due to the scarcity of specific evidence, the patient must be involved in the decision to receive the vaccine. In this process, the patient should be advised by the treating physician, who will inform him/her of the benefit-risk ratio derived from his/her disease, the degree of activity and the type of underlying treatment. In any case, it is very important that the patient continues to maintain the general measures that have proven to be effective in preventing the spread of the virus, as well as maintaining the treatment of his/her SAD.
FundingThis article has not received any type of funding.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Cairoli E, Espinosa G. Enfermedades autoinmunes y vacunas contra la COVID-19. Toma de decisiones en escenarios de incertidumbre. Med Clin (Barc). 2021;157:247–252.