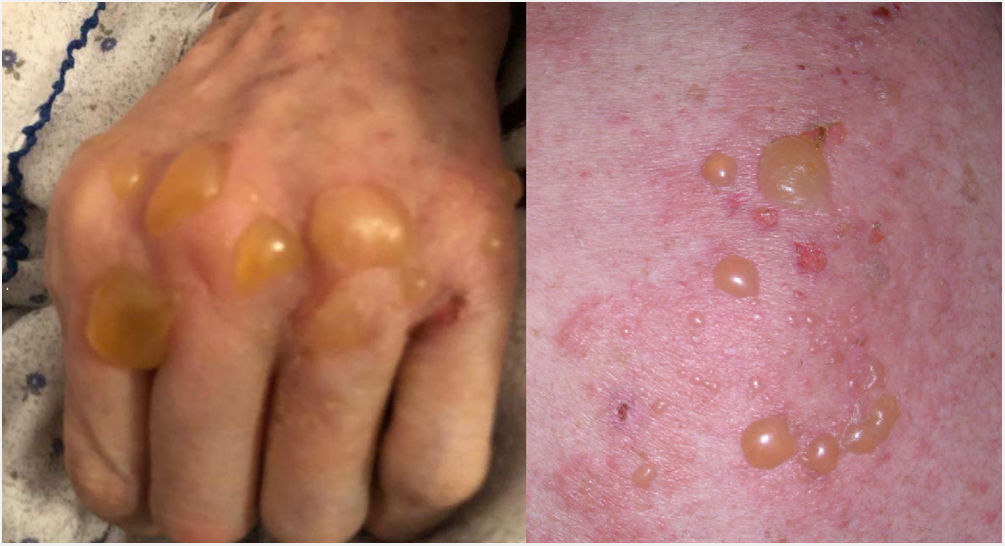
Bullous pemphigoid (BP) is an autoimmune skin disease characterized by the appearance of tense blisters over an erythematous base and the existence of circulating G immunoglobulins (IgGs) against hemidesmosome antigens BP180 and BP230.1 In this paper we present the case of a woman who developed BP after receiving the first dose of COMIRNATY (messenger ribonucleic acid [RNAm] Pfizer-BioNTech vaccine) and a subsequent reactivation after receiving the second one.
The case corresponds to a 78-year-old woman with diabetes mellitus, treated with insulin, and Alzheimer’s disease (global deterioration scale [GDS] score of 4), treated with memantine. She consulted the Dermatology Department due to a 1.5-month history of skin rash, reporting that the lesions had appeared three days after she received the COMIRNATY vaccine. Her symptoms stabilized within two weeks with the aid of high-potency topical corticosteroids. However, 21 days later, she experienced a significant reactivation after receiving the second dose of the vaccine (Fig. 1). A physical examination revealed tense blisters over an erythematous base on her face, trunk, and limbs. No lesions were detected on any of her mucous surfaces. A skin biopsy confirmed the diagnosis of BP, with both direct and indirect positive serum immunofluorescence. The remaining laboratory analyses yielded normal results, except for mild eosinophilia. We informed the Pharmacovigilance System and started treatment with prednisone 40 mg every 24 h, achieving a good clinical response.
The two BP antigens are found in the hemidesmosome, a cell adhesion complex that anchors the epithelium to the basement membrane. It has been proposed that IgG bound to these antigens activates the complement by inducing inflation and interrupting this bond.2 Genetic predisposition and certain triggering factors are involved in its etiopathogenesis. The association between BP and basement membrane alterations, such as traumas or burns; certain drugs, such as oral antidiabetics; or neurological diseases, such as Parkinson's disease or dementia, is well known.2,3 The latter association is partly explained by the autoimmune cross-reaction that occurs between the BP230 protein isoforms present in both the skin and the central nervous system.2 In relation to vaccines, cases of BP have been reported following the administration of vials against tetanus, diphtheria, pertussis, polio, rabies, hepatitis B, rotavirus, pneumococcus, or influenza.1–5 The latency period ranges between one day (or earlier in the case of the pediatric population)1 and one month following the vaccination.2,4,5 The mechanism by which the vaccine induces BP is not well understood, and it is unlikely that a vaccine itself would explain the association, as there are no similarities between the vaccine structure and the basement membrane antigens.2 One hypothesis that has previously been proposed is that the vaccination may trigger a greater autoimmune response in patients with a significant immunological predisposition,2,4 such as the case of our patient with Alzheimer’s disease, as the vaccine would activate B-cell immunity and, therefore, the production of antibodies,4 which is the main mechanism by which the lesions of this disease are generated.
In our patient, BP developed within three days of the administration of the first dose of the COMIRNATY vaccine against the 2019 coronavirus disease (COVID-19). The appearance of these lesions was followed by a period of clinical stabilization during the second week after the vaccination. This was followed by a significant reactivation of the lesions 21 days later, after she received the second dose of the vaccine. The timeline with respect to the administration of the first dose and the reactivation after the administration of the second one supports the hypothesis of a possible relationship between the COMIRNATY vaccine administered and the appearance of BP in our patient, with a score of 8 (possible causal reaction) in the Naranjo Adverse Drug Reaction Probability Scale.
Please cite this article as: Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Penfigoide ampolloso y vacuna COVID-19. Med Clin (Barc). 2021;157:e333–e334.