Until now, the coronavirus disease 2019 (COVID-19) pandemic has affected more than 2.5 million individuals worldwide, with approximately 170,000 deaths. Currently, no treatments with robust evidence of clinical benefit exist, and utilization of experimental agents have been recommended by national and international guidelines as a part of clinical studies.
MethodIn this retrospective study, a total of 323 patients severe acute respiratory syndrome due to PCR-documented COVID-19 infection admitted in our unit were included. Patients were categorized into two groups as those who did or did not receive high dose intravenous vitamin C. we examined the effect of high dose intravenous vitamin C administered in addition to other commonly used agents on prognosis in patients with COVID-19 pneumonia.
ResultsAs compared to patients who did not receive vitamin C, those in the VC group were not significantly different in terms of the length of hospital stay (p=0.05), re-admission rate (p=0.943), admission to intensive care, need for advanced oxygen support (p=0.488), need for advanced medical treatment (p<0.001), and mortality (p=0.52).
ConclusionThe limited evidence based on small samples precludes definitive conclusions regarding the potential efficacy of high dose vitamin C in these patients, indicating the need for further assessment within the context of clinical research.
Hasta ahora, la pandemia de la enfermedad por coronavirus 2019 (COVID-19) ha afectado a más de 2,5 millones de individuos en todo el mundo, con aproximadamente 170.000 muertes. En la actualidad, no existen tratamientos con evidencias sólidas de beneficio clínico, y la utilización de agentes experimentales ha sido recomendada por las guías nacionales e internacionales como parte de los estudios clínicos.
MétodoEn este estudio retrospectivo se incluyeron un total de 323 pacientes con síndrome respiratorio agudo severo por infección por COVID-19 documentada por PCR, ingresados en nuestra unidad. Los pacientes se clasificaron en 2 grupos, según recibieran o no dosis elevadas de vitamina C intravenosa. Se examinó el efecto de la administración de dosis elevadas de vitamina C intravenosa, además de otros agentes utilizados habitualmente, sobre el pronóstico de los pacientes con neumonía por COVID-19.
ResultadosEn comparación con los pacientes que no recibieron vitamina C, los del grupo que recibieron vitamina C no fueron significativamente diferentes en cuanto a la duración de la estancia hospitalaria (p=0,05), la tasa de reingreso (p=0,943), el ingreso en cuidados intensivos, la necesidad de soporte avanzado de oxígeno (p=0,488), la necesidad de tratamiento médico avanzado (p<0,001) y la mortalidad (p=0,52).
ConclusionesLas limitadas evidencias basadas en muestras pequeñas impiden sacar conclusiones definitivas sobre la potencial eficacia de la vitamina C en dosis altas en estos pacientes, lo que indica la necesidad de una mayor evaluación en el contexto de la investigación clínica.
Until now, the coronavirus disease 2019 (COVID-19) pandemic has affected more than 2.5 million individuals worldwide, with approximately 170,000 deaths.1 Currently, no treatments with robust evidence of clinical benefit exist, and utilization of experimental agents have been recommended by national and international guidelines as a part of clinical studies.2,3 On the other hand, the evidence indicating a potential benefit of vitamin support for prevention and treatment of viral infections in populations with vitamin deficiencies is mounting.4 Vitamin C (VC) is a ubiquitous and essential nutrient with antioxidant properties that has potential benefits in viral infections, including COVID-19. Cytokine storm is e phenomenon that can be observed in both viral and bacterial infections,2 resulting in increased oxidative stress via a common and non-specific pathway. Since oxidative stress can be prevented and managed in most instances as suggested by three different clinical studies involving a total of 146 patients with sepsis, it is plausible to assume that VC may also offer certain benefits in patients with COVID.5
In fact, high dose VC has been utilized clinically for several decades, and in a recent document prepared by a NIH (National Institutes of Health) Expert Panel, clearly states that VC regimens up to 1.5g/kg represent safe approach without significant side effects.6 This is particularly important, when one considers the fact that development of effective vaccines and anti-viral agents is a time-consuming process and the fact that VC and other antioxidants are among possible therapeutic options for alleviating COVID-19 related ARDS.
Therefore, we examined the effect of high dose intravenous vitamin C administered in addition to other commonly used agents on prognosis in patients with COVID-19 pneumonia.
Materials and methodsIn this retrospective study, a total of 323 patients severe acute respiratory syndrome due to PCR-documented COVID-19 infection admitted between 1 and 30 September 2020 in our unit were included. Patients were categorized into two groups as those who did or did not receive high dose intravenous vitamin C. Vitamin C was administered to those who were hospitalized in the first 15 days of September. Vitamin C was not administered to those who went to bed in the second half of the month. One-hundred and fifty-three patients received 2g/day intravenous vitamin C, while 170 patients received no vitamin C. Demographic characteristics, comorbid conditions and chest X-ray and computed tomography findings were recorded. Also, basic inflammatory markers such as d-dimer, ferritin, and C-reactive protein (CRP) were compared before and after vitamin C administration. Length of hospital stay, history of re-admission, need for advanced oxygen support and medical treatment, admission to intensive care, and mortality rates were examined.
Statistical analysisStatistical analysis was performed with SPSS 21.0 (Statistical Package for Social Sciences for Windows, Inc., Chicago, Illinois, USA). The compatibility of the data to normal distribution was investigated by Kolmogorov–Smirnov test. Data showing continuous variables are expressed as mean±standard deviation or median (minimum–maximum), and categorical data are expressed as number and percentage (%). Independent groups were compared using Student t test or Mann–Whitney U test. Intra-group comparisons were made using the Wilcoxon test. Categorical variables were compared using the Chi-square test. A value of p<0.05 was considered statistically significant.
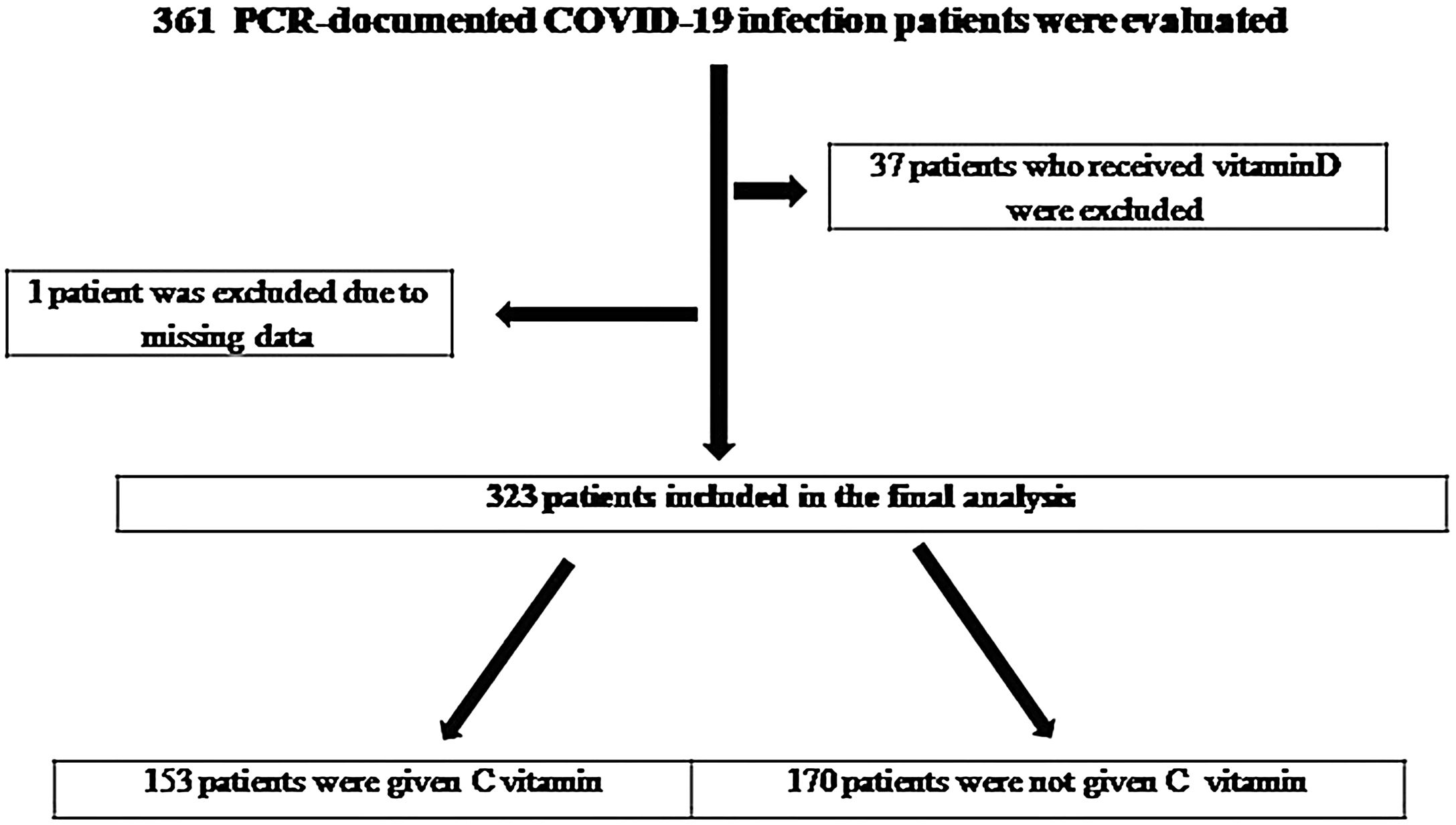
Results361 PCR-documented COVID-19 infection patients were evaluated. 37 patients were excluded due to received Vit D and one patient was due to missing data (Fig. 1). A total of 323 patients (204 male, 63.15%; 119 female, 36.38%) were included. Vitamin C was given to 153 of these patients (66.7% male, 33.3% female).
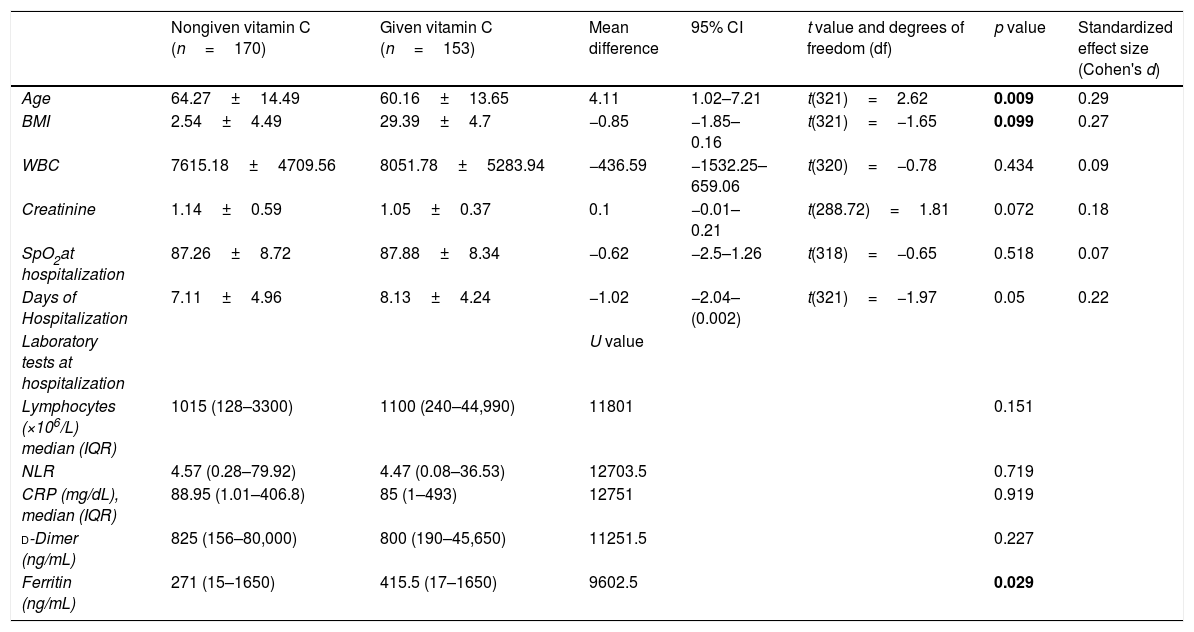
The mean age in VC group was (60.16±13.65) years, and the body mass index was (29.39±4.7). Hypertension, diabetes mellitus, coronary artery disease, and COPD were present in 39.9%, 32%, 16.3%, and 12.4%, respectively (Table 1). Vitamin C treatment at a dose of 2g/day was initiated within a median duration of 3 days following admission.
Baseline characteristics of patients according to given vitamin C.
| Nongiven vitamin C (n=170) | Given vitamin C (n=153) | Mean difference | 95% CI | t value and degrees of freedom (df) | p value | Standardized effect size (Cohen's d) | |
|---|---|---|---|---|---|---|---|
| Age | 64.27±14.49 | 60.16±13.65 | 4.11 | 1.02–7.21 | t(321)=2.62 | 0.009 | 0.29 |
| BMI | 2.54±4.49 | 29.39±4.7 | −0.85 | −1.85–0.16 | t(321)=−1.65 | 0.099 | 0.27 |
| WBC | 7615.18±4709.56 | 8051.78±5283.94 | −436.59 | −1532.25–659.06 | t(320)=−0.78 | 0.434 | 0.09 |
| Creatinine | 1.14±0.59 | 1.05±0.37 | 0.1 | −0.01–0.21 | t(288.72)=1.81 | 0.072 | 0.18 |
| SpO2at hospitalization | 87.26±8.72 | 87.88±8.34 | −0.62 | −2.5–1.26 | t(318)=−0.65 | 0.518 | 0.07 |
| Days of Hospitalization | 7.11±4.96 | 8.13±4.24 | −1.02 | −2.04–(0.002) | t(321)=−1.97 | 0.05 | 0.22 |
| Laboratory tests at hospitalization | U value | ||||||
| Lymphocytes (×106/L) median (IQR) | 1015 (128–3300) | 1100 (240–44,990) | 11801 | 0.151 | |||
| NLR | 4.57 (0.28–79.92) | 4.47 (0.08–36.53) | 12703.5 | 0.719 | |||
| CRP (mg/dL), median (IQR) | 88.95 (1.01–406.8) | 85 (1–493) | 12751 | 0.919 | |||
| d-Dimer (ng/mL) | 825 (156–80,000) | 800 (190–45,650) | 11251.5 | 0.227 | |||
| Ferritin (ng/mL) | 271 (15–1650) | 415.5 (17–1650) | 9602.5 | 0.029 | |||
| Gender | |||
| Male | 102 (60%) | 102 (66.7%) | 0.215 |
| Female | 68 (40%) | 51 (33.3%) | |
| Comorbidity | |||
| Hypertension | 75 (44.1%) | 61 (39.9%) | 0.44 |
| Diabetes mellitus | 47 (27.6%) | 49 (32%) | 0.39 |
| Coronary artery disease | 29 (17.1%) | 25 (16.3%) | 0.863 |
| Heart failure | 12 (7.1%) | 5 (3.3%) | 0.208 |
| COPD | 27 (15.9%) | 19 (12.4%) | 0.465 |
| Asthma | 12 (7.1%) | 10 (6.5%) | ns |
| Malignancy | 16 (9.5%) | 13 (8.5%) | 0.913 |
| Renal failure | 7 (4.1%) | 4 (2.6%) | 0.662 |
| İnterstitial lung disease | 1 (0.6%) | 1 (0.7%) | ns |
| Rheumatological disease | 4 (2.4%) | 6 (3.9%) | 0.526 |
| Need for intensive care | 12 (7.1%) | 11 (7.2%) | ns |
| Need for advanced oxygen therapy | 18 (10.6%) | 21 (13.7%) | 0.488 |
| Need for advanced medical treatment | 15 (8.8%) | 37 (24.2%) | <0.001 |
| Result | |||
| Exitus | 24 (14.1%) | 17 (11.1%) | 0.52 |
| Discharge | 146 (85.9%) | 136 (88.9%) | |
The mean lymphocyte count at presentation was 1100×103/μl (240–44,990), CRP was 85mg/dL (1–493), ferritin was 415.5ng/mL (17–1650), and mean d-dimer was 800ng/mL (190–45,650). Also, the mean oxygen saturation (SPO2) at presentation was (87.88±8.34, 95% CI: −2.5–1.26). Fifty-nine point five percent of the patients had bilateral lung involvement, and 93.5% had ground glass opacities.
As an initial treatment for COVID-19 infection, patients had received 8mg of dexamethasone once daily. Favipiravir was given with a loading dose of 1600mg twice daily for the first day, followed by 600mg twice daily for maintenance, for a total duration of 5, 7, or 10 days.
The mean duration of hospital stay in VC group was (8.13±4.24, 95% CI: −2.04–0.002) days, the admission rate to intensive care was 7.2%, the need for advanced oxygen support was 13.7%, and the need for advanced medical treatment was 24.2%. While 136 of these patients were discharged, 17 died.
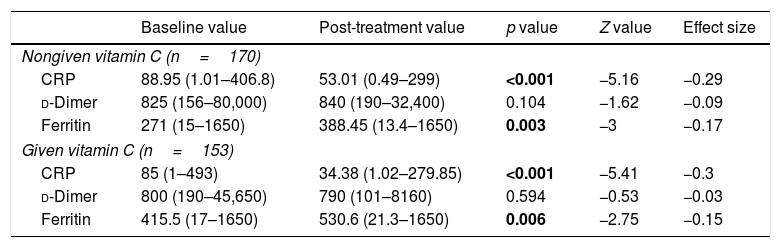
A comparison of CRP, d-dimer, and ferritin between baseline and post-treatment did not show any significant differences in the VC group (Table 2).
Baseline and post-treatment values.
| Baseline value | Post-treatment value | p value | Z value | Effect size | |
|---|---|---|---|---|---|
| Nongiven vitamin C (n=170) | |||||
| CRP | 88.95 (1.01–406.8) | 53.01 (0.49–299) | <0.001 | −5.16 | −0.29 |
| d-Dimer | 825 (156–80,000) | 840 (190–32,400) | 0.104 | −1.62 | −0.09 |
| Ferritin | 271 (15–1650) | 388.45 (13.4–1650) | 0.003 | −3 | −0.17 |
| Given vitamin C (n=153) | |||||
| CRP | 85 (1–493) | 34.38 (1.02–279.85) | <0.001 | −5.41 | −0.3 |
| d-Dimer | 800 (190–45,650) | 790 (101–8160) | 0.594 | −0.53 | −0.03 |
| Ferritin | 415.5 (17–1650) | 530.6 (21.3–1650) | 0.006 | −2.75 | −0.15 |
Similarly, as compared to patients who did not receive vitamin C, those in the VC group were not significantly different in terms of the length of hospital stay (p=0.05), re-admission rate (p=0.943), admission to intensive care, need for advanced oxygen support (p=0.488), need for advanced medical treatment (p<0.001), and mortality (p=0.52).
No adverse effects associated with the use of high dose vitamin C treatment were recorded.
DiscussionHere, we report a group of vulnerable and elderly COVID-19 patients with multiple comorbidities who received IV vitamin C in addition to the treatments recommended in national guidelines. In this retrospective study, we basically examined the effect of high dose intravenous vitamin C treatment in COVID-19 patients in three categories: (i) inflammatory response; (ii) prognosis (need for advanced medical treatment, need for intensive care unit), and (iii) mortality.
Previous studies in COVID-19 patients have established a relationship between disease severity and the intensity of the inflammatory response as measured by interleukin-6, ferritin, C-reactive protein (CRP), and d-dimer.7
In our study, elevated CRP levels at baseline were consistent with previous reports describing the characteristics of COVID-19 patients.8 In a study by Zhao et al. significant decline in CRP was observed in severe and critically ill patients following high dose intravenous vitamin C (median dose 162.7mg/kg/day and 178.6mg/kg/day in severely ill and critically ill patients, respectively).9 Hiedra et al., administered intravenous vitamin c at a dose of 1g every 8h for 3 days in 17 patients infected with COVID-19, and found reduction in inflammatory markers such as ferritin, and d-dimer.10 In contrast with these publications, no significant reduction in CRP ferritin, and d-dimer was seen following vitamin C administration.
Previous studies showed that intravenous VC (IVC) could achieve higher plasma concentrations as compared to oral VC due to elimination of intestinal absorption and tissue transportation phases as well as renal reabsorption.11 Several clinical studies have established the efficacy and safety of intravenously administered high dose VC in critically ill patients.9,10
Vitamin C together with corticosteroids and thiamine was found to reduce the risk of progressive organ dysfunction, including acute kidney injury, in addition to reducing mortality in patients with severe sepsis or septic shock.12,13 Current literature contains clear descriptions of how severe sepsis, ARDS, and cytokine storm can become in COVID-19 patients,14 who have tissue injury due to oxidative stress or free radicals generated by septic shock and cytokine storm. Since the prevention and management of oxidative stress requires potentially high doses of antioxidants, vitamin C may represent a plausible option in this settings.15 In the most recent CITRIS-ALI study, high dose IVC (50mg/kg bodyweight every 6h, for 96h) was found to reduce all-cause mortality in the septic patient cohort (29.8% vs. 46.3%).13 In China, successful use of high dose intravenous VC has been reported in a total of 50 COVID-19 patients with moderate or severe disease. In that study, the dose range was between 10 and 20g administered every 8–10h. An improvement in oxygenation index was reported, and all patients were discharged home without mortality.16 However, since no significant clinical benefits were found in another recent randomized controlled study, the evidence seems controversial.17 Although high dose intravenous vitamin C was associated with favorable effects, particularly in more severe patients, we also failed to observe statistically significant differences in terms of inflammatory markers, need for advanced medical treatment and oxygen support, and mortality.
The COVID-19 Treatment Guidelines Panel states that there is no clear-cut evidence for or against the use of vitamin C in critical or non-critical COVID-19 patients.18 Currently, there are no completed controlled vitamin C trials in COVID-19 patients, and the existing observational data is scarce and inconclusive. Since COVID-19 patients with mild disease are less likely to have cytokine storm due to oxidative stress or severe inflammation, the role of vitamin C in these settings is also unknown.18
A significant concern regarding the use of high dose VC involves potential side effects. However, most of the reported side effects of high dose VC are mild.19 High dose VC has been reported to lead to hemolysis, acute renal injury, and acute oxalate nephropathy in patients with glucose 6 phosphate dehydrogenase (G6PD) deficiency.20,21 However, these side effects have been reported in a few cases only, and have been associated with very high doses, or use of non-standard administration practices in high risk patients with underlying disorders. In this study, no adverse reactions have been observed in association with IVC administration.
In conclusion, the limited evidence based on small samples precludes definitive conclusions regarding the potential efficacy of high dose vitamin C in these patients, indicating the need for further assessment within the context of clinical research. However, considering the safety of high dose VC, healthcare professionals should more closely inspect this potential therapeutic opportunity. Obviously, well-designed clinical studies are warranted to develop standard protocols for rout.
Conflict of interestThe authors declare that they have no conflict of interest.