During the COVID-19 pandemic, several strategies were suggested for the management of the disease, including pharmacological and non-pharmacological treatments such as convalescent plasma (CP). The use of CP was suggested due to the beneficial results shown in treating other viral diseases.
ObjectiveTo determine the efficacy and safety of CP obtained from whole blood in patients with COVID-19.
MethodsPilot clinical trial in patients with COVID-19 from a general hospital. The subjects were separated into three groups that received the transfusion of 400 ml of CP (n = 23) or 400 ml of standard plasma (SP) (n = 19) and a non-transfused group (NT) (n = 37). Patients also received the standard available medical treatment for COVID 19. Subjects were followed up daily from admission to day 21.
ResultsCP did not improve the survival curve in moderate and severe variants of COVID-19, nor did it reduce the degree of severity of the disease evaluated with the COVID-19 WHO and SOFA clinical progression scale. No patient had a severe post-transfusion reaction to CP.
ConclusionsTreatment with CP does not reduce the mortality of patients even when its administration has a high degree of safety.
Durante la pandemia de COVID-19 surgieron diversas estrategias para el manejo de la enfermedad, incluidos los tratamientos farmacológicos y no farmacológicos como el plasma convaleciente (PC). El uso de PC se sugirió debido a los resultados benéficos mostrados al tratar otras enfermedades virales.
ObjetivoDeterminar la eficacia y seguridad de la administración de PC obtenido de sangre total en pacientes con COVID-19.
MétodosEnsayo clínico piloto en pacientes con COVID-19 de un hospital general. Los sujetos se separaron en tres grupos que recibieron la transfusión de 400 ml de PC (n = 23) o 400 ml de plasma estándar (PE) (n = 19) y un grupo no transfundido (NT) (n = 37). Los pacientes recibieron además el tratamiento médico estándar disponible para COVID 19. El seguimiento de los sujetos se llevó a cabo diariamente desde el ingreso hasta el día 21.
ResultadosEl PC no mejoró la curva de supervivencia en las variantes moderadas y graves de COVID-19, ni disminuyó el grado de severidad de la enfermedad evaluado con la escala de progresión clínica COVID-19 OMS y SOFA. Ningún paciente presentó una reacción postransfusional severa al PC.
ConclusionesEl tratamiento con PC no disminuye la mortalidad de los pacientes, aun cuando su administración tiene un alto grado de seguridad.
SARS-CoV-2 infection disease was declared a pandemic in March 2020, and given the urgency of the situation, global health institutions allowed the use of products that could have potential benefits for patients.1 For this reason, the use of convalescent plasma (CP) in COVID-19 was initiated in clinical trials, based on the concept of passive immunity, which relates to the protection that an individual acquires after receiving antibodies from previously infected persons.2
Some of the first studies on the use of CP in COVID-19 found beneficial effects, such as a decrease in temperature, viral load, and SOFA scores, favourable changes in PaO2/FiO2 and improved tomographic imaging from the third day of transfusion.3 Another study found that CP reduced mortality in critically ill patients, reduced clinical symptoms, increased neutralising antibody titres and reduced the presence of SARS-CoV-2 in most patients.4 In contrast, another trial reported that 28-day mortality did not differ significantly between those transfused with CP and those receiving standard care.5
Regarding the safety of transfusion therapy, it is well known that plasma administration is always linked to the risk of adverse post-transfusion reactions which can be immediate or delayed.6 In a previous study with severely ill participants, 2 mild and non-severe transfusion reactions were reported.7 Although the exact risk of CP administration in the treatment of COVID-19 is not known, the main serious adverse events (SAEs) observed have been allergic, respiratory, thrombotic, thromboembolic and cardiac.8,9 A low risk for transfusion-related adverse reactions is reported as the incidence of all SAEs was <1%.10
Multiple international publications indicate that the use of plasma in convalescent patients may be a potentially useful treatment modality for COVID-19, however, to date, there is no consensus on the benefits of this therapy that fully confirms its efficacy and safety. The objective of this pilot study was to determine the efficacy and safety of administering CP obtained from whole blood in patients with COVID-19.
MethodsParticipantsPatients with acute respiratory distress syndrome (ARDS) due to COVID-19, admitted to the Hospital General de Zona (HGZ) No. 46 of the Instituto Mexicano del Seguro Social (IMSS) in Villahermosa, Tabasco, from 1 February to 30 October, 2021, aged 18 years or older, with a diagnosis of SARS-CoV-2 infection confirmed by RT-PCR positive nasopharyngeal swab, with partial oxygen saturation (SpO2) ≤ 94% and/or supplemental oxygen requirement and with radiological evidence of pulmonary involvement with CORADS classification 5 or 6 by chest computed tomography (CT) scan were included. Patients with negative test for COVID-19, with CORADS < 5 by chest CT, pregnant or lactating women; diagnosed with bacterial, fungal or tuberculosis infection; haemodialysis or on peritoneal dialysis; liver failure; heart failure; oncological diagnosis with prognosis of life <3 months; history of allergic reaction to transfusion; chronic obstructive pulmonary disease on home oxygen therapy; presence of positive serology for HIV, hepatitis B and C; and diagnosis of autoimmune diseases were excluded.
Study design and treatmentsThe present study is a pilot clinical trial conducted in hospitalised patients with COVID-19 who met the selection criteria. Out of 211 hospitalised patients, 46 patients were randomised to receive treatment with standard plasma (SP, n = 23) (control) or CP, n = 23. Another comparison group consisted of subjects who met the inclusion and exclusion criteria and agreed to participate in the study, but did not receive the transfusion (NT, n = 37). The method used to generate the randomisation sequence for transfusion with plasma was performed using a computer-based online random number generator (www.random.org). Participants and investigators were blinded to the type of plasma that was transfused. The treatment allocation sequence was implemented by blood bank administrative staff, who were not involved in the trial. One investigator registered the participants and received the different plasmas without knowing whether they were SP or CP. The plasma units were placed in custom-made black plastic bags, which prevented identification of the convalescent or non-convalescent donor, with labels for identification that included the blood bank's internal coding number and the following wording: "COVID-19 patient use only".
Patients enrolled in the SP and CP groups were transfused with 200 ml of the respective compatible plasma (AB0 and Rh system) intravenously over 1 h, on 2 occasions separated by 24 h. In addition, all subjects received standard treatment, according to the best available evidence at the time and the procedural guidelines for patients with COVID-19.11 Within this treatment, patients received oxygen at different volumes and pressures adjusted to their ventilatory needs, as well as 8 mg dexamethasone/2 times daily from hospital admission. Baseline data were recorded (day 0), and from the first transfusion onwards, follow-up continued on days 3, 5, 7, 14 and 21. Follow-up was completed until the day of clinical improvement and discharge or voluntary discharge or loss to follow-up until day 21 or death.
COVID-19 convalescent donor selectionDonors were subjects recovered from COVID-19 with no history of pregnancy or transfusion, who were diagnosed by positive RT-PCR of nasopharyngeal swab. CP donors ranged from 21 days to 60 days after onset with mild symptoms and out-of-hospital management, asymptomatic at the time of donation, with subsequent negative RT-PCR test and meeting the selection criteria of NOM-253-SSA-01-2012. Plasma was obtained by fractionation of whole blood using the standard procedure according to the official blood bank guidelines established by the Mexican Ministry of Health.
Efficacy evaluation of convalescent plasma in patients with COVID-19The efficacy of CP was determined by 21-day survival analysis and clinical improvement. Clinical improvement was assessed using the WHO COVID-19 clinical progression scale12 and the Sequential Organ Failure Assessment Scale (SOFA).13 The former defines improvement as patient discharge or a 2-point reduction according to the following classification: 6 points, death; 5 points, hospitalisation plus use of extracorporeal membrane oxygenation (ECMO) or invasive mechanical ventilation; 4 points, hospitalisation plus non-invasive ventilation or high-flow supplemental oxygen; 3 points, hospitalisation plus supplemental oxygen (other than high-flow or non-invasive ventilation); 2 points, hospitalisation without supplemental oxygen; 1 point, hospital discharge; the second assesses severity by organ damage indicators such as respiration, coagulation, liver, cardiovascular, nervous system and renal function.
Safety assessment of convalescent plasma in patients with COVID-19CP safety was assessed by considering the presence of adverse effects such as: fever, chills, pruritus, hives, hypotension, up to severe situations such as anaphylactic shock, transfusion-related acute lung injury or cardiovascular system volume overload; or allergic reaction to citrate. An adverse transfusion reaction was considered when it occurred within 48 h after the last transfusion. All biochemical determinations were performed using the Architect i2000SR® (Abbott Diagnostics, Abbott Park, Illinois, USA). AB0/Rh D group typing was performed on the Echo Lumena kit® (Immucor®, Cerdanyola del Vallès, Barcelona, Spain) and blood cytometry on the Medonic M32B series kit® (Boule Diagnostics AB®, Spånga, Sweden) with the reagents for the respective kits.
Statistical analysisTo determine whether the variables followed a parametric distribution, the D'Agostino-Pearson test was used. Measures of central tendency were expressed as mean and standard deviation (SD) or median (25th percentile and 75th percentile). The one-way ANOVA and Tukey test was used to compare variables with parametric distribution. The Chi-square test was used for the comparison of non-parametric variables. Survival of subjects during the 21-day period was analysed using Kaplan–Meier curves. Multiple comparisons were performed using Mantel-Cox regression (log rank regression) and Gehan–Wilcoxon regression. Time-courses of both WHO and SOFA clinical progression scale-related values were analysed by mixed-effects modelling using log-transformed values to normalise distributions and Tukey's test for multiple comparisons. In this way, the effects of time, treatment and interactions on these variables were analysed. Multivariate logistic regression testing was used to investigate factors associated with mortality, with the "enter" method for variable selection. In all cases a p < 0.05 value was accepted as statistically significant. Data were processed in GraphPad Prism v.7.0 statistical software for Windows and IBM SPSS® Statistics v.26.
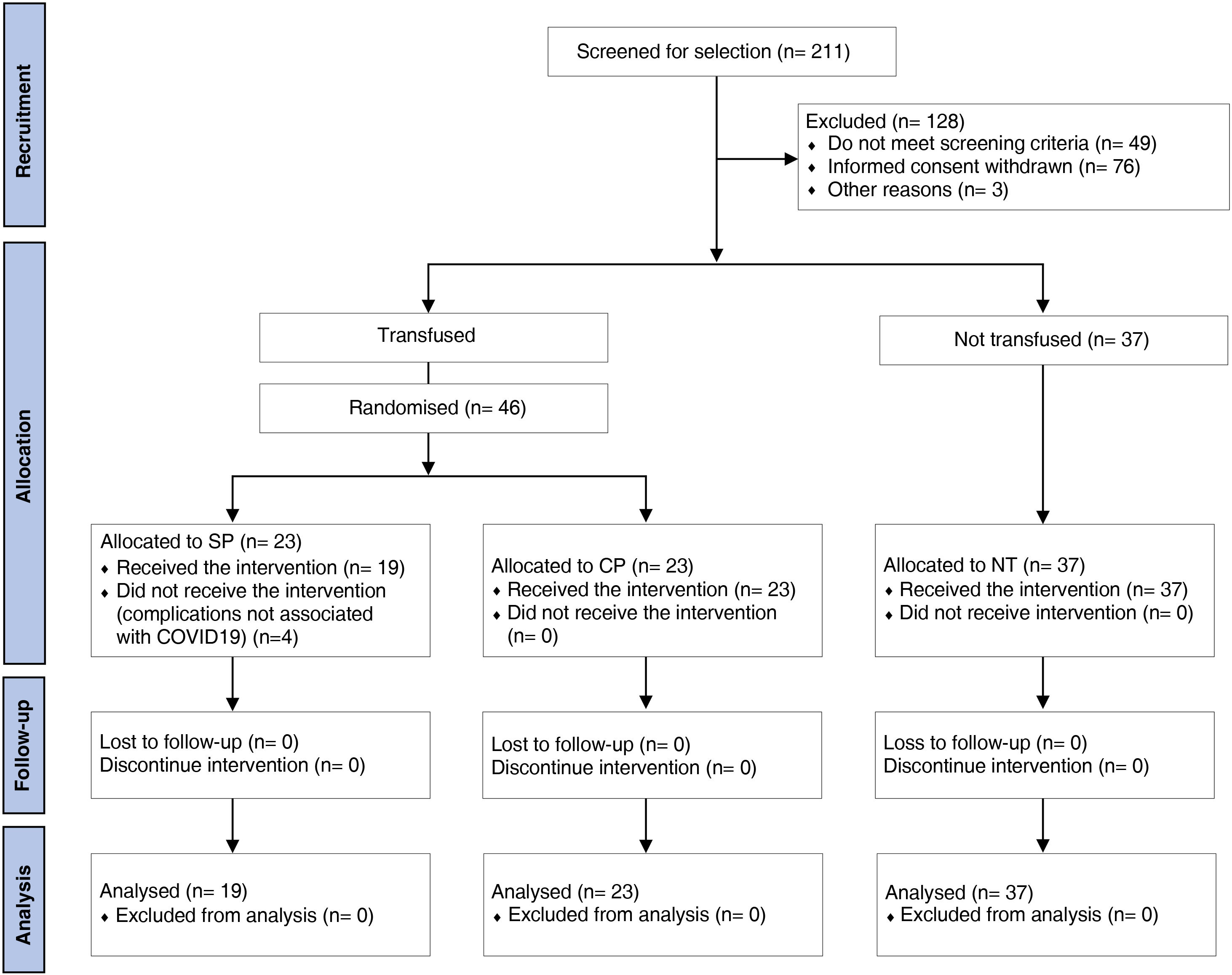
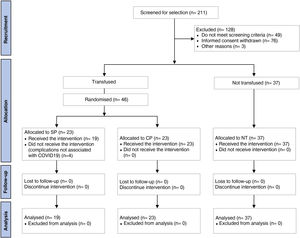
ResultsCharacteristics of the participantsOf all patients admitted to hospital during the study period, 211 were assessed as eligible to participate. Those who did not meet the inclusion criteria (n = 53), withdrew informed consent (n = 76) or other reasons (n = 3) were excluded. Thus, 83 patients were included, of whom 46 were randomised to receive transfusions with SP (n = 23) or CP (n = 23). In the SP group, 4 patients were withdrawn before transfusion because of complications not associated with COVID-19. Thirty-seven patients who did not receive any plasma transfusion were included in the NT group (Fig. 1).
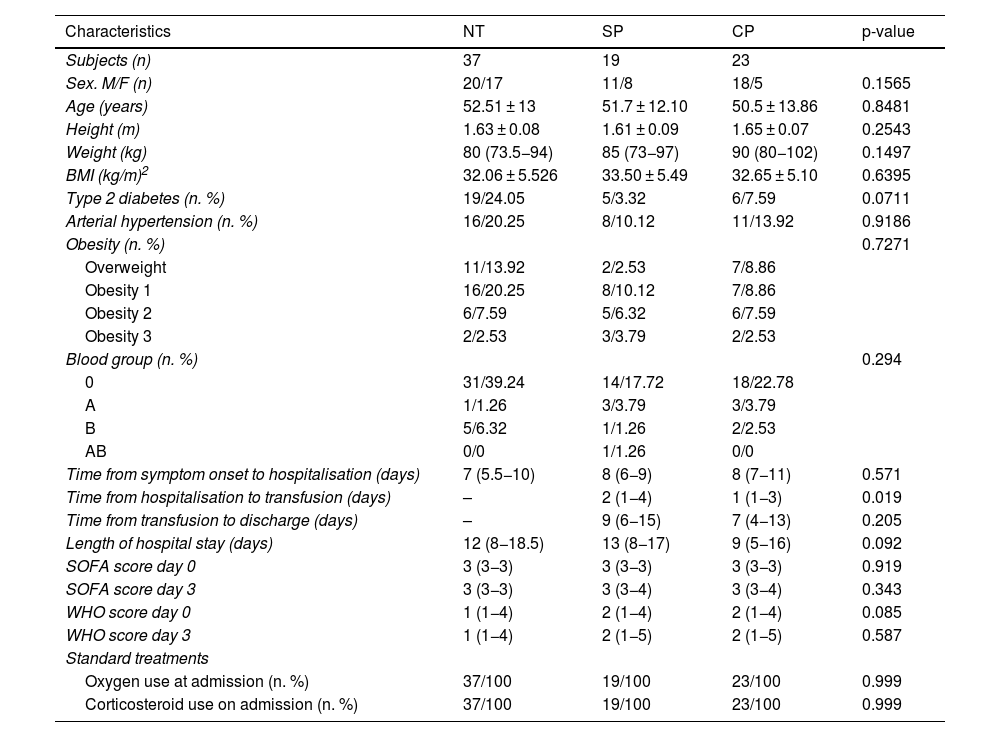
The clinical characteristics of the subjects studied are shown in Table 1. Overall, homogeneity was observed between the groups for the different variables studied. Ages ranged from 24 to 82 years, with a mean of 51.07 ± 12.95 years and 62% of them were male. The median weight of the participants was 85 kg (76−97), of which 25.31% were overweight and 69.62% were obese. Of all subjects, 45.2% were hypertensive and 34.96% had a diagnosis of diabetes. Participants who were transfused spent an average of 2.17 days between hospitalisation and plasma transfusion, with this time period being longer for the SP group (2.89 days; 95% CI: 1.87–3.92) compared to CP (1.57 days; 95% CI: 1.03–2.10) (p = 0.019).
Clinical characteristics of participants.
| Characteristics | NT | SP | CP | p-value |
|---|---|---|---|---|
| Subjects (n) | 37 | 19 | 23 | |
| Sex. M/F (n) | 20/17 | 11/8 | 18/5 | 0.1565 |
| Age (years) | 52.51 ± 13 | 51.7 ± 12.10 | 50.5 ± 13.86 | 0.8481 |
| Height (m) | 1.63 ± 0.08 | 1.61 ± 0.09 | 1.65 ± 0.07 | 0.2543 |
| Weight (kg) | 80 (73.5−94) | 85 (73−97) | 90 (80−102) | 0.1497 |
| BMI (kg/m)2 | 32.06 ± 5.526 | 33.50 ± 5.49 | 32.65 ± 5.10 | 0.6395 |
| Type 2 diabetes (n. %) | 19/24.05 | 5/3.32 | 6/7.59 | 0.0711 |
| Arterial hypertension (n. %) | 16/20.25 | 8/10.12 | 11/13.92 | 0.9186 |
| Obesity (n. %) | 0.7271 | |||
| Overweight | 11/13.92 | 2/2.53 | 7/8.86 | |
| Obesity 1 | 16/20.25 | 8/10.12 | 7/8.86 | |
| Obesity 2 | 6/7.59 | 5/6.32 | 6/7.59 | |
| Obesity 3 | 2/2.53 | 3/3.79 | 2/2.53 | |
| Blood group (n. %) | 0.294 | |||
| 0 | 31/39.24 | 14/17.72 | 18/22.78 | |
| A | 1/1.26 | 3/3.79 | 3/3.79 | |
| B | 5/6.32 | 1/1.26 | 2/2.53 | |
| AB | 0/0 | 1/1.26 | 0/0 | |
| Time from symptom onset to hospitalisation (days) | 7 (5.5−10) | 8 (6−9) | 8 (7−11) | 0.571 |
| Time from hospitalisation to transfusion (days) | – | 2 (1−4) | 1 (1−3) | 0.019 |
| Time from transfusion to discharge (days) | – | 9 (6−15) | 7 (4−13) | 0.205 |
| Length of hospital stay (days) | 12 (8−18.5) | 13 (8−17) | 9 (5−16) | 0.092 |
| SOFA score day 0 | 3 (3−3) | 3 (3−3) | 3 (3−3) | 0.919 |
| SOFA score day 3 | 3 (3−3) | 3 (3−4) | 3 (3−4) | 0.343 |
| WHO score day 0 | 1 (1−4) | 2 (1−4) | 2 (1−4) | 0.085 |
| WHO score day 3 | 1 (1−4) | 2 (1−5) | 2 (1−5) | 0.587 |
| Standard treatments | ||||
| Oxygen use at admission (n. %) | 37/100 | 19/100 | 23/100 | 0.999 |
| Corticosteroid use on admission (n. %) | 37/100 | 19/100 | 23/100 | 0.999 |
Data expressed as mean ± standard deviation or median (25th and 75th percentiles), or as frequency (n or %).
BMI: body mass index; NT: not transfused; WHO: World Health Organisation; CP: convalescent plasma; SP: standard plasma; SOFA: Sequential Organ Failure Assessment Scale.
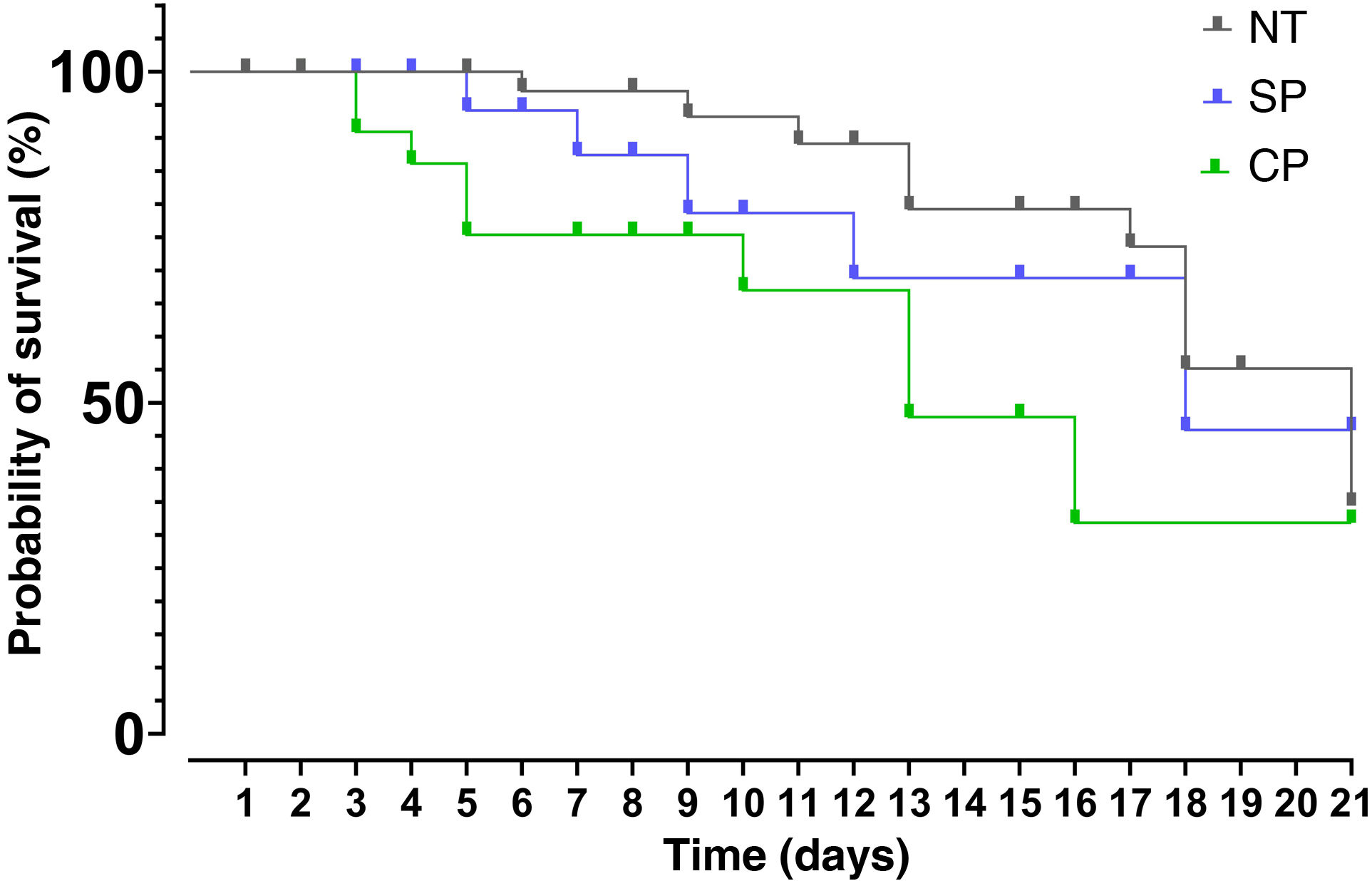
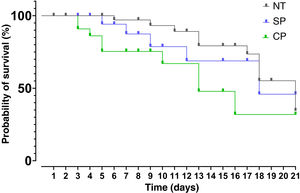
After data analysis using Kaplan–Meier curves it was found that the median survival for the group receiving SP was 18 days, 13 days for the CP group and 21 days for the NT group. The results of the Mantel-Cox test showed that there are no significant differences between treatments (95% CI: 15.54–20.46; p > 0.05). The Chi-square value was 5.608 with one degree of freedom and a value of p = 0.061 (Fig. 2).
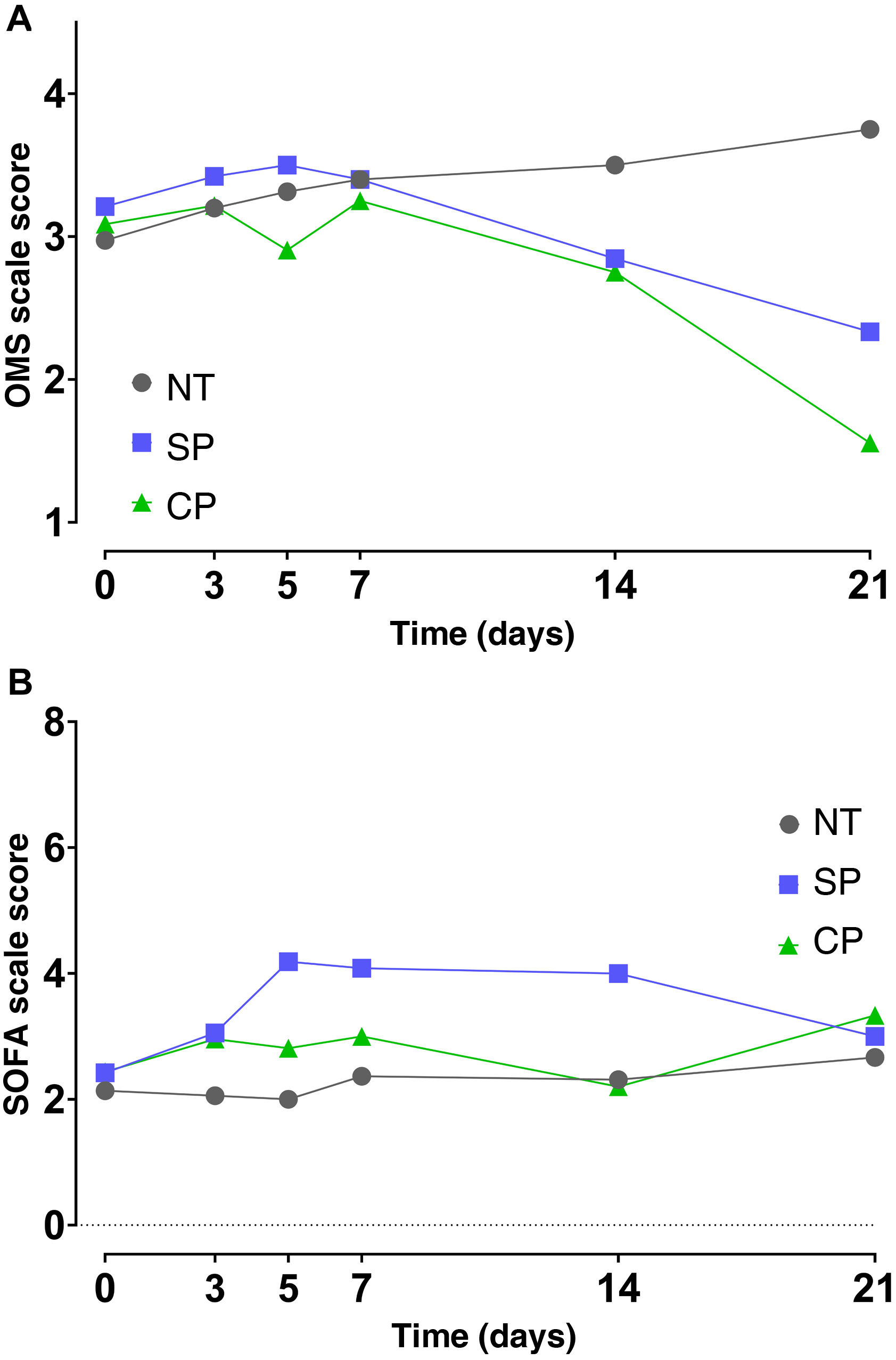
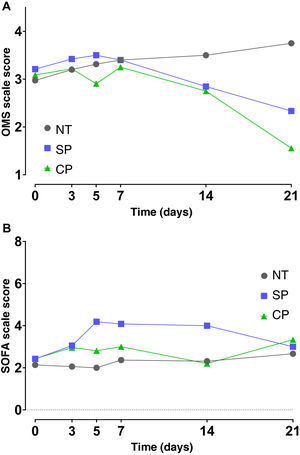
Effect of convalescent plasma administration on disease severity in COVID-19 patientsThe results of the mixed effects model showed that there were no differences in the time course (days 0, 3, 5, 7, 14 and 21) of the SOFA and OMS scale values between the different interventions. Even though differences in the time factor were found for the SOFA scale (p = 0.011) and for the OMS scale (p < 0.001), multiple comparisons using Tukey's test and confidence interval analysis showed a lack of evidence to reject the lack of effects of CP effects (p > 0.05) (Fig. 3A and B).
Disease severity in patients with COVID 19 from transfusion baseline to 21 days using the WHO COVID-19 clinical progression scale (A) and the SOFA scale (B). Median and standard error values are shown. NT (n = 37), SP (n = 19) and CP (n = 23). NT: not transfused; CP, convalescent plasma; SP, standard plasma.
After a post hoc ANOVA and Tukey test no CP effects were observed on some inflammatory parameters such as: WBCs (F = 0,440; p = 0,646); lymphocytes (F = 0,152; p = 0,859); LDH (F = 0,233; p = 0,801); CRP (F = 0,754; p = 0,475); procalcitonin (F = 0,335; p = 0,717); D-dimer (F = 0,400; p = 0,672) and ferritin (F = 1,646; p = 0,206) determined after 3 days of transfusion.
Logistic regressionThe multivariate logistic regression analysis for the study of factors affecting mortality showed that plasma type, length of hospital stay, degree of obesity, presence of type 2 diabetes and hypertension were not significant. However, the variable age with a B = 0.086 and an OR of 1.090 (95% CI: 1.009–1.177) indicates that the older the age, the higher the risk of death (p = 0.028). Also, the shorter the time from symptoms to hospital admission, the higher the probability of survival, B = 0.412 with an OR = 0.662 (95% CI: 0.496−0.884; p = 0.005).
Safety of convalescent plasma administration in COVID-19 patientsNo transfusion reactions were observed in patients transfused with CP. However, in the SP group, one patient was reported with a mild adverse reaction characterised by hives, generalised reddish wheals and pruritus, which subsided immediately after discontinuing the transfusion and following the administration of a single dose of 25 mg diphenhydramine intravenously. The comorbidity associated with this subject was arterial hypertension of 5 years' progression, under control at the time of COVID-19. In addition, the patient had never been transfused and had no allergies.
DiscussionThe results of the present study show that CP is not effective in reducing mortality and severity of patients in severe and very severe stages of COVID-19.
Contrary to expectations, mortality of patients receiving CP tended to be higher than in the SP and NT groups. These results are partially similar to a previous study, where mortality was reported to be higher in the CP group than in the standard care group. They suggest that a possible explanation for the lack of effect could be the variation in antibody levels of each donor.14 Similarly, other researchers observed no significant difference in mortality risk or overall hospital discharge rate between the CP group and the standard care group.15 In contrast, a recent meta-analysis reported that patients transfused with CP exhibited a 51% reduction in mortality rate over standard treatment, while others reported that the group receiving CP with high antibody concentrations was associated with a lower risk of death in non-intubated patients compared to the use of plasma with low antibody levels.16,17 Quantification of neutralising antibodies could not be performed in our laboratory in the present study, so it is not known whether low levels of neutralising antibodies could have affected our findings. On the other hand, CP units were collected between days 21 and 60 after the onset of mild COVID-19 symptoms, so we assume that the antibody titre was also variable and dependent on the characteristic immune status of each individual.18,19
Other possible reasons for the lack of effects of CP on mortality could be attributed to the use of a small sample size in this study. It should be mentioned that this study was originally designed to be a randomised controlled clinical trial with an estimated sample size of 100 participants based on the decreased mortality variable. However, due to the lack of altruistic CP donation and with the administration of the first COVID-19 vaccines in potential donors, it was not possible to complete this sample size, so it was decided to introduce a secondary comparison group consisting of subjects with the same characteristics, but who had not received any transfusion.
Analysis of the effects of CP on the degree of severity assessed by the WHO scale and the SOFA scale showed that there is no difference between treatment groups over time. Although there are other scales with higher sensitivity and specificity, previous studies have used the SOFA scale to predict morbidity and mortality in COVID-19 patients in intensive care.20
Some studies have shown that symptoms of hypoxaemia and respiratory failure in patients with severe and very severe presentation start from the second week of disease onset, and that CP treatment in this period may not be able to reverse the fatal outcome.20 Furthermore, in another trial in patients with moderate COVID, CP transfusion did not induce a decrease in mortality or progression to severe disease, even when undergoing follow-up to day 28 after admission. They propose that the use of CPs in early disease may not be useful, as there is a high rate of autonomous recovery in patients with mild disease.21
The comorbidities most commonly observed in the participants of this study were hypertension and diabetes, with the highest percentage of diabetes observed in the NT group. Similarly, a retrospective study reported that 48% of their patients had comorbidities, with hypertension being the most common with 30%, followed by diabetes with 19% and coronary heart disease with 8%. That study reports that increased risk of in-hospital death is associated with older age and a higher SOFA score.22
Another important observation is that standard treatments in all groups were not prescribed in the protocol, as they consist of supportive care, treatments for respiratory symptoms and currently available treatments for COVID-19 suggested by WHO, which have been reported in several clinical trials.5,11
In relation to safety, our results indicate that the proportion of subjects with adverse post-transfusion reaction was very low. The only patient who had a transfusion reaction was in the SP group, which was mild and subsided with the administration of a single dose of diphenhydramine. It is important to mention that premedication with 8 mg dexamethasone/2 times a day in all participants may have contributed to reduce some of the potential post-transfusion reactions. This treatment protocol was implemented from March 2021 for patients in the early stages of the inflammatory phase of the disease because it demonstrated a beneficial effect on survival rate in patients receiving invasive or non-invasive mechanical ventilation.23
It is well known that transfusion reactions to any blood product can range from mild to severe, and even lead to death.6 Mild reactions reported in studies specifically using CP for COVID-19 have been hives, pruritus and fever. Other studies with different designs have reported severe reactions such as transfusion-related acute lung injury (TRALI) and anaphylactic reaction.24 However, these events may be related to the presence of concomitant diseases and/or other treatments, and not to the transfusion itself.8 In another study evaluating safety, severe transfusion-related adverse events were reported to be less than 1%.25
The strength of the present study is that it was conducted at a time of extreme epidemiological urgency and under conditions of high transmission, when different therapeutic measures were being investigated to try to combat this epidemic. In addition, the study was double-blind as neither the participants nor the researchers knew which type of intervention was assigned.
In conclusion, the present study did not show any beneficial effect of CP treatment on COVID-19 mortality or disease severity. CP administration had a high level of safety as evidenced by the absence of post-transfusion reactions after 72 h. Further studies are recommended to clarify other variables involved in these results.
Ethical considerationsThe research protocol was approved by the National Committee for Scientific Research (CNIC) and the Health Research Ethics Committee (CEIS) of the IMSS with registration number R-2020-785-139. All participants signed an informed consent form for this study. Blood collection was performed under the guidelines of the Official Mexican Standard NOM-253-SSA1-2012, for the management of human blood and its components for therapeutic purposes, as well as the Official Mexican Standard NOM-012-SSA3-2012, which sets out the criteria for the conduct of research projects for health in human subjects.
FundingThis research did not receive specific support from public, private or non-profit organisations.
Conflict of interestThe authors declare that they have no conflicts of interest.
Special thanks are extended to all the volunteers who participated in this study. To Dr. Rita Rivera, Q.F.B. Mateo Castillo and Q.F.B. Pablo Ramos for technical support at the Blood Bank of the Hospital General de Zona No. 46 (IMSS) and to the staff of the Centro Estatal de Hemoterapia for providing units of fresh frozen plasma.