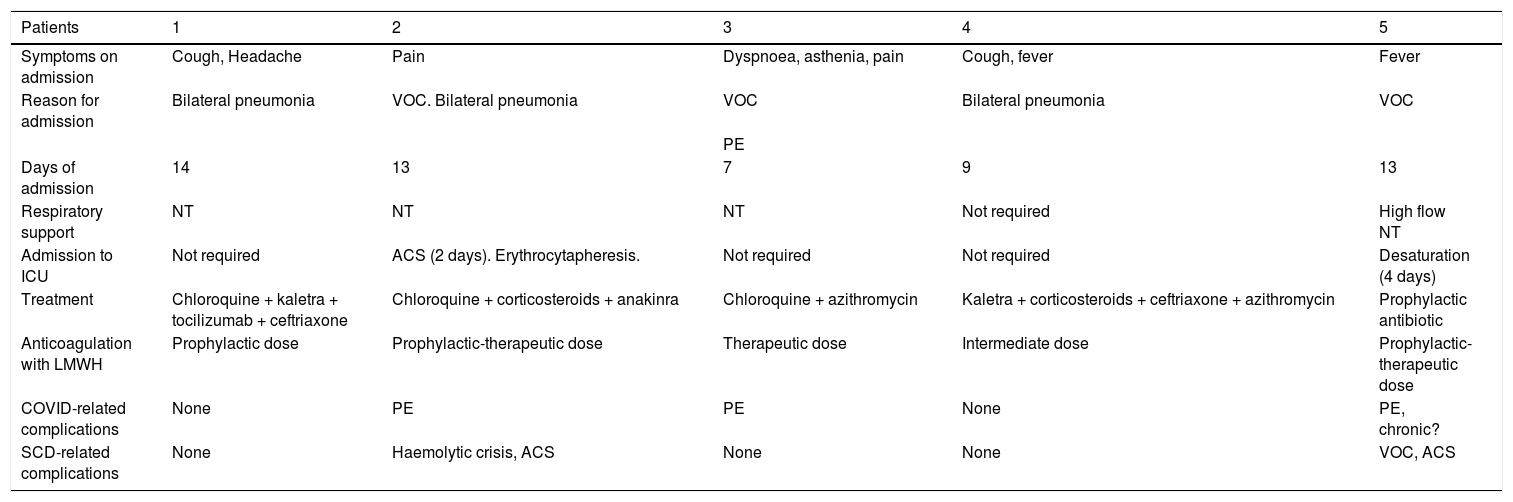
Patients with sickle cell disease (SCD) suffer from recurrent chronic haemolytic anaemia and vaso-occlusive crises (VOC) leading to multi-organ involvement and asplenia. VOC cases are secondary to hypoxia and infections and are therefore considered patients at risk for the development of complications during COVID-19 infection. In addition, the high incidence of thromboembolic complications in COVID-19 and the inherent thrombophilia in SCD are well known.
Thus, we designed a descriptive, observational, multicentre, retrospective study to analyse the incidence and course of COVID-19 infection in patients with SCD, as well as its relationship with the development of haemoglobinopathy-related complications. Thirteen Spanish centres with 289 patients (174 infants and 115 adults) with SS, SC, or S® thalassaemia phenotype were involved. Among them, seven cases (2.4%) were diagnosed with COVID-19. Two had mild symptomatology, without requiring hospital admission: those who were older and without active treatment. The course of the five patients who required admission, the therapy received, and their complications are summarised in Table 1. The low need for invasive respiratory support and the high incidence of thromboembolic complications stand out, despite prophylaxis with low molecular weight heparin (LMWH). Patient 3 had pulmonary embolism (PE) on admission. Treatment included chloroquine, lopinavir-ritonavir (Kaletra®), corticosteroids, and anti-interleukin 6 (IL-6). One patient did not require treatment for COVID-19; another did not receive chloroquine due to glucose-6-phosphate dehydrogenase (G-6-PDH) deficiency. Two required admission to the Intensive Care Unit (ICU): one for erythrocytapheresis due to acute chest syndrome, another for respiratory failure requiring non-invasive mechanical ventilation (NIMV). Admissions were short and there were no deaths.
Course and treatment of patients.
| Patients | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Symptoms on admission | Cough, Headache | Pain | Dyspnoea, asthenia, pain | Cough, fever | Fever |
| Reason for admission | Bilateral pneumonia | VOC. Bilateral pneumonia | VOC | Bilateral pneumonia | VOC |
| PE | |||||
| Days of admission | 14 | 13 | 7 | 9 | 13 |
| Respiratory support | NT | NT | NT | Not required | High flow NT |
| Admission to ICU | Not required | ACS (2 days). Erythrocytapheresis. | Not required | Not required | Desaturation (4 days) |
| Treatment | Chloroquine + kaletra + tocilizumab + ceftriaxone | Chloroquine + corticosteroids + anakinra | Chloroquine + azithromycin | Kaletra + corticosteroids + ceftriaxone + azithromycin | Prophylactic antibiotic |
| Anticoagulation with LMWH | Prophylactic dose | Prophylactic-therapeutic dose | Therapeutic dose | Intermediate dose | Prophylactic-therapeutic dose |
| COVID-related complications | None | PE | PE | None | PE, chronic? |
| SCD-related complications | None | Haemolytic crisis, ACS | None | None | VOC, ACS |
VOC: Vasoocclusive crisis; NT: Nasal tubes; ACS: Acute chest syndrome; PE: Pulmonary embolism.
In this small series, the incidence of COVID-19 infection was not higher than the rest of the Spanish population and no increase in mortality was observed. Respiratory involvement by COVID-19 showed little severity, although the incidence of thromboembolic disease was very high despite LMWH prophylaxis. Complications inherent to SCD were not more serious than expected.
Similar results are found in the literature. McCloskey et al.1 conducted a study of 10 patients with SCD and COVID-19 in the UK. Only one individual with previous comorbidities died, and none required NIMV or admission to the ICU. They did not report any thrombosis, performing prophylaxis with LMWH. Arlet et al.2 analysed a cohort of 83 patients with SCD and COVID-19 in a French multicenter study. Seventeen people (20%) were admitted to ICU, nine of whom required NIMV. The ICU admission rate was higher in patients with the SC genotype (63%), two of whom died from COVID-19-related lung disease. Only one case of thrombosis was reported, but antithrombotic prophylaxis was not specified. Abdulrahman et al.,3 in population screening by polymerase chain reaction (PCR) test in Bahrain, reported a COVID-19 infection rate of 1.6% in SCD patients (six in total). Of these, only three had symptoms and only one patient required oxygen therapy. No deaths were reported.
Ramachandran et al.4 studied nine hospitalized patients with COVID-19 and SCD, comparing them with a cohort without SCD. Only one with SCD was admitted to the ICU (10 vs. 36% in the control group) and none required intubation (8% in the control group). There were no deaths among sickle cell patients, compared to a mortality of 5.6% in the control group. However, the study presented by Mucalo et al.5 at the ASH 2020 congress, with 218 patients with SCD and COVID-19, describes a higher mortality in all age groups, with an age-adjusted mortality ratio 7.7 times higher in people with SCD compared to the African-American population without SCD. The incidence of thrombosis is not described.
Studies do not seem to find a higher incidence of COVID-19 infection in patients with SCD. In terms of outcomes, it is unclear whether this is a risk group for the development of COVID-19 and SCD-associated complications. The discrepancy in results could be due to different biases: low number of cases in the series, underestimation of less severe cases in areas without seroprevalence studies, multicenter studies with different management protocols, and inadequate control cohorts. It is therefore necessary to wait the results of series with larger and more homogeneous patient samples before definitive conclusions can be drawn.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Argüello-Marina M, López-Rubio M, Morado M. Infección por SARS-CoV-2 en pacientes con enfermedad falciforme. Med Clin (Barc). 2021;157:345–346.