Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the latest therapeutic option available for the treatment of type 2 diabetes mellitus (DM2).1 The most common side effects of this therapeutic class are genitourinary infections in relation to glycosuria induced through its mechanism of action. Data from clinical trials, case reports, and observational studies have indicated that its use may be associated with serious adverse events, including amputations, diabetic ketoacidosis (DKA), and acute kidney failure.2 The U.S. Food and Drug Administration was the first agency to issue a warning in 2015 about the increased risk of DKA with the use of these drugs.3 DKA is a severe acute complication of DM, rare in patients with DM2, which can occasionally be fatal. DKA has been reported with the 3SGLT2 inhibitors available and the incidence was less than 1/1000 in randomized controlled trials and 1.6/1000 person/year in cohort studies.4 In the CANVAS program, patients randomized to canagliflozin experienced significantly higher rates of lower limb amputation compared to placebo.3
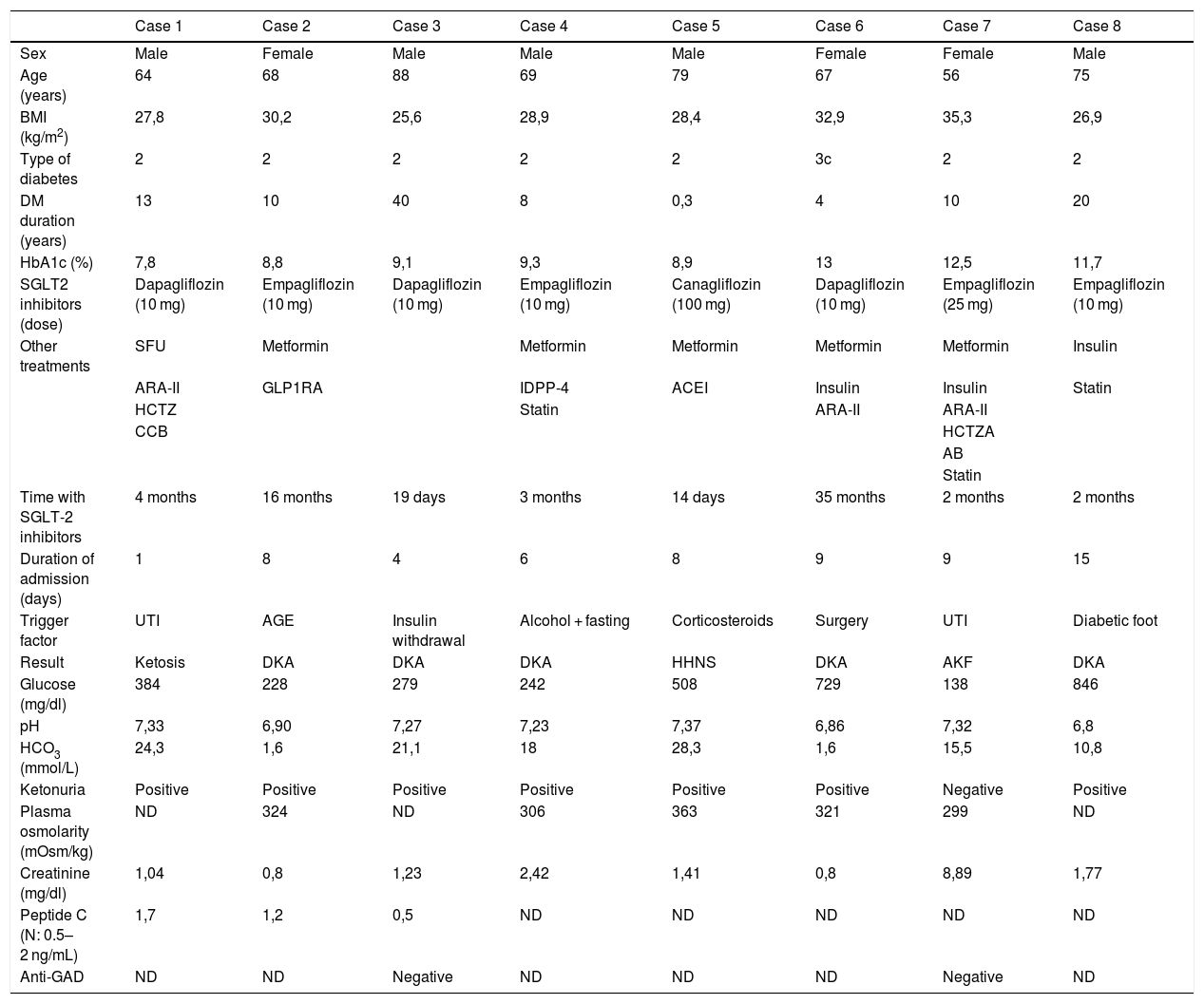
A retrospective study was conducted from January 2014 to December 2018. Eight subjects with acute decompensations were found in treatment with SGLT2 inhibitors: Five cases of diabetic ketoacidosis, one hyperosmolar hyperglycaemic nonketotic syndrome, one ketotic hyperglycaemia and one acute renal failure. Patient characteristics, concomitant treatment, triggers and laboratory data are collected in Table 1. Regarding risk factors for the development of ketoacidosis, 2 subjects had a history of diabetic ketoacidosis and another of alcohol use. The triggering factors were: insulin withdrawal, acute infections (acute gastroenteritis, respiratory infection, urinary tract infection, perforating plantar ulcer), glucocorticoids, previous surgery and alcohol consumption with starvation. Two subjects required ICU admission for severe ketoacidosis, one required haemodialysis and the other, transmetatarsal amputation of the left foot.
Characteristics of the patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male | Female | Female | Male |
| Age (years) | 64 | 68 | 88 | 69 | 79 | 67 | 56 | 75 |
| BMI (kg/m2) | 27,8 | 30,2 | 25,6 | 28,9 | 28,4 | 32,9 | 35,3 | 26,9 |
| Type of diabetes | 2 | 2 | 2 | 2 | 2 | 3c | 2 | 2 |
| DM duration (years) | 13 | 10 | 40 | 8 | 0,3 | 4 | 10 | 20 |
| HbA1c (%) | 7,8 | 8,8 | 9,1 | 9,3 | 8,9 | 13 | 12,5 | 11,7 |
| SGLT2 inhibitors (dose) | Dapagliflozin (10 mg) | Empagliflozin (10 mg) | Dapagliflozin (10 mg) | Empagliflozin (10 mg) | Canagliflozin (100 mg) | Dapagliflozin (10 mg) | Empagliflozin (25 mg) | Empagliflozin (10 mg) |
| Other treatments | SFU | Metformin | Metformin | Metformin | Metformin | Metformin | Insulin | |
| ARA-II | GLP1RA | IDPP-4 | ACEI | Insulin | Insulin | Statin | ||
| HCTZ | Statin | ARA-II | ARA-II | |||||
| CCB | HCTZA | |||||||
| AB | ||||||||
| Statin | ||||||||
| Time with SGLT-2 inhibitors | 4 months | 16 months | 19 days | 3 months | 14 days | 35 months | 2 months | 2 months |
| Duration of admission (days) | 1 | 8 | 4 | 6 | 8 | 9 | 9 | 15 |
| Trigger factor | UTI | AGE | Insulin withdrawal | Alcohol + fasting | Corticosteroids | Surgery | UTI | Diabetic foot |
| Result | Ketosis | DKA | DKA | DKA | HHNS | DKA | AKF | DKA |
| Glucose (mg/dl) | 384 | 228 | 279 | 242 | 508 | 729 | 138 | 846 |
| pH | 7,33 | 6,90 | 7,27 | 7,23 | 7,37 | 6,86 | 7,32 | 6,8 |
| HCO3 (mmol/L) | 24,3 | 1,6 | 21,1 | 18 | 28,3 | 1,6 | 15,5 | 10,8 |
| Ketonuria | Positive | Positive | Positive | Positive | Positive | Positive | Negative | Positive |
| Plasma osmolarity (mOsm/kg) | ND | 324 | ND | 306 | 363 | 321 | 299 | ND |
| Creatinine (mg/dl) | 1,04 | 0,8 | 1,23 | 2,42 | 1,41 | 0,8 | 8,89 | 1,77 |
| Peptide C (N: 0.5–2 ng/mL) | 1,7 | 1,2 | 0,5 | ND | ND | ND | ND | ND |
| Anti-GAD | ND | ND | Negative | ND | ND | ND | Negative | ND |
AB: alpha blocking; CCB: calcium-channel blockers; Anti-GAD: antibodies to glutamic acid decarboxylase; ARA-II: angiotensin II receptor antagonist; DKA: diabetic ketoacidosis; HHNS: Hyperosmolar Hyperglycaemic Nonketotic Syndrome; AGE: acute gastroenteritis; GLP1RA: GLP-1 receptor agonists; HCTZ: hydrochlorothiazide; DPP4i: dipeptidyl peptidase-4 inhibitor; ACEI: angiotensin-converting enzyme inhibitor; AKF: acute kidney failure; UTI: urinary tract infection; ND: not described; SFU: sulfonylurea.
Most patients with DKA have a precipitating cause, which includes reduced oral intake, fasting, omission or reduction in insulin dose, intercurrent events, alcohol abuse, and the use of corticosteroids, among others; however, in many cases, triggers cannot be identified.3
It seems that people with a poorer reserve of cells®, longer duration of diabetes, poorer diabetes control and lower BMI are more susceptible to euglycemic DKA.4 In the different published case series, most patients had latent autoimmune diabetes of adults (LADA) or DM1 in which SGLT2 inhibitors was prescribed out of guideline, or they were erroneously diagnosed as T2DM, and in other cases they are long-standing DM2 patients with a significant pancreatic reserve deficiency.5 In our series, all patients had non-autoimmune diabetes.
Furthermore, a great variability is observed in the duration of treatment with SGLT2 inhibitors before the onset of DKA, which ranges from very few days to months or years (1–182 days).3
Treatment with iSLGT2 is associated with some volume depletion and reduced blood pressure levels. In this context, it is reasonable to temporarily discontinue these drugs during surgery or breakthrough disease, especially if vomiting or diarrhoea occurs, and to adjust diuretic and hypotensive treatment.4
Ketoacidosis, kidney failure, and amputations are rare but serious complications of SGLT2 inhibitors. Proper indication of iSLGT2, as in any drug, is the key to minimize serious complications; it is of great importance to rule out that the patient does not have insulin deficiency (DM1, latent autoimmune diabetes in adults or advanced DM2). More clinical trials and observational studies are needed to determine the risk of developing DKA in patients treated with SGLT2 inhibitors and to identify the most susceptible patients more accurately.
The patients in our series belong to the same hospital center with a reference area of 300,000 inhabitants, all of them with non-autoimmune DM; it is the largest series published nationally.
Please cite this article as: Díaz Trastoy O, Sánchez Sobrino P, Rego Iraeta AL. Complicaciones agudas graves por inhibidores del cotransportador sodio-glucosa tipo 2 (sglt2): un problema real y no tan infrecuente. Med Clin (Barc). 2020;155:138–139.