BNT162b2 mRNA Covid-19 vaccine has proved to be effective and safe. There have been described some adverse effects, although most of them are mild. Axillary lymphadenopathies ipsilateral to the vaccine injection is one of the adverse effects, recorded in 0.3% of patients.1 There were not identified lymphadenopathies in other locations.
Axillary lymphadenopathy in women older than 30 years must be evaluated by ultrasonography.2 Lymphadenopathy malignancy probability is estimated according to the Amonkar sonographic criteria from normal appearance (UN1) to very high suspicion malignant appearance (UN5).3 The findings taken into account in order to point to the malignancy probability are: lymph morphology, cortical thickness, position of the fatty hilum and distribution of the lymph vascularization.2,3
Core needle biopsy (CNB) or fine needle aspiration (FNA) puncture are the indicated procedures when suspicious malignant lymphadenopathy is identified by ultrasonography.3
BNT162b2 mRNA Covid-19 vaccination commenced in our hospital (Hospital Clínico Universitario Virgen de la Arrixaca, Murcia, Spain) on January 11, 2021. Patients consulting because of axillary lymphadenopathy have risen on January 18, 2021 in our hospital, the reason why an ultrasonography was requested for these patients. A total number of 1117 workers were vaccinated between those days.
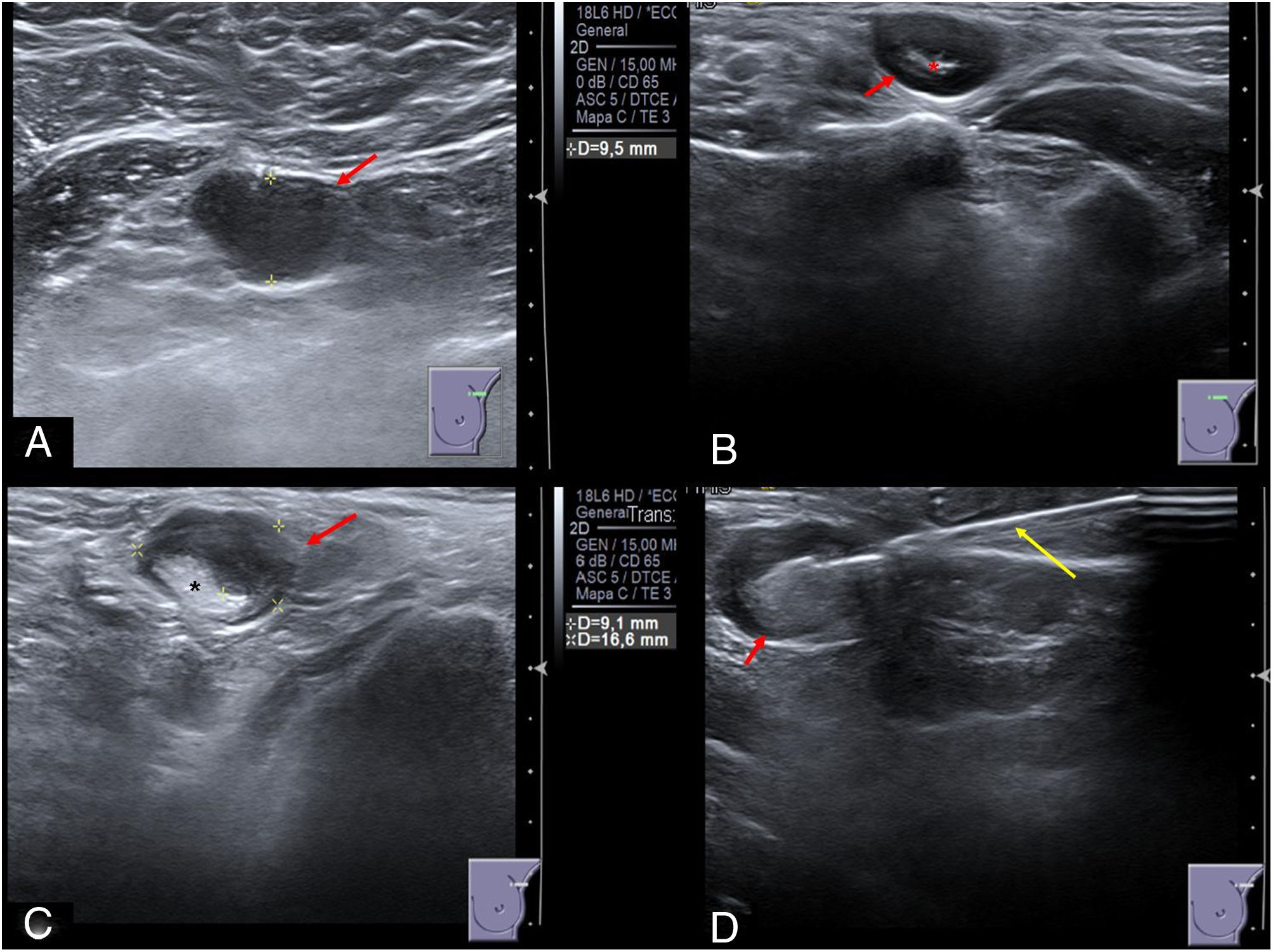
From January 12, 2021 to January 21, 2021, 11 of the 1117 vaccinated workers (0.985%) were studied by ultrasound due to painful axillary or supraclavicular lymphadenopathy ipsilateral to the shoulder where BNT162b2 mRNA Covid-19 vaccination was injected. The ultrasound findings (Fig. 1) were suspicious malignant lymphadenopathies (UN4 or UN5) according to Amonkar sonographic criteria thus an ultrasound-guided CNB was performed in each patient. The samples were analyzed by the Department of Pathology and the pathologist reported reactive paracortical/interfollicular hyperplasia, which was attributable to benign reactive lymphadenopathies.
Sonographic appearance of post vaccine lymphadenopathies (red arrows). (A) Axillary lymphadenopathy UN5 with rounded morphology, marked cortical thickness and absence of fatty hilum. (B) Supraclavicular lymphadenopathy UN4 with rounded morphology, diffuse cortical thickness and scarce fatty hilum (red asterisk). (C) Axillary lymphadenopathy UN4 with rounded morphology, nodular cortical thickness and displaced fatty hilum (black asterisk). (D) Ultrasound-guided core needle biopsy (yellow arrow) of a malignant appearance axillary lymphadenopathy.
Axillary lymphadenopathy might be expected as reactive lymphadenopathy in the setting of Covid-19 vaccine.1 However, there is no description in the available evidence about the sonographic appearance of the reactive lymph nodes after a vaccination and our sonographic findings were of high suspicion of malignancy lymphadenopathies (UN4 or UN5).3
Furthermore, we found lymphadenopathy more frequently (0.9875%) than reported in the BNT162b2 mRNA Covid-19 vaccine safety study (0.3%).1 Despite our smaller population under study, we think this difference might be even bigger secondary to the fact that, facing this suspicious finding in symptomatic patients, we also performed ultrasonography in asymptomatic workers who received the vaccination on the same days than the symptomatic patients. We identified similar lymphadenopathies even in asymptomatic patients, who might not be included in the 0.3% patients with symptomatic lymphadenopathies reported in the BNT162b2 mRNA Covid-19 vaccine safety study.1
Supraclavicular lymphadenopathies were identified, which was not described in previous articles about BNT162b2 mRNA Covid-19 vaccine.1
Nevertheless, the pathologist report disclosed benignity, so an alternative diagnosis must be investigated. The common event shared by all these patients was the recent BNT162b2 mRNA Covid-19 vaccination ipsilateral to the identified lymphadenopathies. The relationship between the axillary lymphadenopathy and the BNT162b2 mRNA Covid-19 vaccine is plausible owing to the early immune response in the first nodes which receive the pathogen after its inoculation.1 Furthermore, this relationship is highly probable according to the reports in the efficacy and safety studies about SARS-CoV-2 and other pathogens vaccines1,4 in which the reported histological appearance was also similar to benign reactive lymphadenopathies.5
The lymphadenopathies are expected to disappear within ten days after BNT162b2 mRNA Covid-19 vaccination.1 Similar benign evolution of reactive lymphadenopathies is shown posterior to other vaccines.4
The current vaccination process against Covid-19 demands the clinicians to expect the potential lymphadenopathy occurrence, even in asymptomatic patients or unusual locations as the supraclavicular region, and the radiologists to know its delicate sonographic appearance. We consider this information to be urgent according to prevent false positives, unnecessary lymph node biopsies and patient concern.
To conclude, we would like to suggest:
- •
Clinicians might recommend clinical follow-up to check lymphadenopathy remission and must dissuade their patients from an unjustified disavowal to the vaccine second dose. Ultrasonography may be requested just in case of persistence longer than 10 days after the vaccination. However, longer evidence is needed to accurately set the suitable management.
- •
Radiologists should report reactive lymphadenopathies in case of coming across with suspicious malignant lymphadenopathy during an ultrasonography in recently vaccinated patients.