Chronic thrombo-embolic pulmonary hypertension (CTEPH) is a potentially curable form of pulmonary hypertension (PH) that develops in up to 3% of patients after pulmonary embolism (PE). In these patients, PE does not resolve, leading to organized fibrotic clots, with the development of precapillary PH as a result of the proximal obstruction of the pulmonary arteries. In addition, a distal microvasculopathy may also develop, contributing to the increase of pulmonary vascular resistance. Transthoracic echocardiography is the diagnostic tool that allows to establish the suspicion of PH. Ventilation–perfusion lung scintigraphy is the fundamental tool in the study of patients with suspected CTEPH; if it is normal, virtually rules out the diagnosis. Right heart catheterization is mandatory for the diagnosis of these patients. CTEPH is defined as the existence of symptoms, residual perfusion defects and precapillary PH after a minimum period of three months of anticoagulation. Pulmonary angiography helps determine the extent and surgical accessibility of thromboembolic lesions. CTEPH patients are candidates for long-term anticoagulation. Pulmonary endarterectomy is the treatment of choice, resulting in significant clinical and hemodynamic improvement. About 25% of patients have residual PH post-endarterectomy. Balloon pulmonary angioplasty is an endovascular technique that targets more distal lesions, being potentially useful for patients with inoperable CTEPH or persistent/recurrent PH post-endarterectomy. Both types of patients may also benefit from pharmacological treatment for PH. These three therapies are the cornerstone of CTEPH treatment, which has evolved towards a multimodal approach.
La hipertensión pulmonar tromboembólica crónica (HPTEC) es una forma potencialmente curable de hipertensión pulmonar (HP) que aparece hasta en 3% de los pacientes tras una embolia pulmonar (EP). En estos pacientes, la EP no se resuelve, dando paso a coágulos fibróticos organizados, con el desarrollo de HP precapilar debido a la obstrucción proximal de las arterias pulmonares. También puede desarrollarse una microvasculopatía distal que contribuye al aumento de la resistencia vascular pulmonar (RVP). La ecocardiografía transtorácica (ETT) es la exploración que permite establecer la sospecha de HP. La gammagrafía pulmonar de ventilación-perfusión (V/Q) es la herramienta fundamental en el estudio de los pacientes con sospecha de HPTEC; si es normal, prácticamente la descarta. El cateterismo cardiaco derecho es obligatorio para el diagnóstico. La HPTEC se define como la existencia de síntomas, defectos de perfusión residuales e HP precapilar tras un periodo mínimo de tres meses de anticoagulación. La angiografía pulmonar ayuda a determinar la extensión y la accesibilidad quirúrgica de las lesiones tromboembólicas. Las personas con HPTEC son candidatas a anticoagulación indefinida. La endarterectomía pulmonar es el tratamiento de elección, resultando en una mejoría clínica y hemodinámica significativa. Aproximadamente un 25% de los pacientes presentan HP residual postendarterectomía. La angioplastia pulmonar con balón (APB) es una técnica endovascular dirigida a lesiones más distales, de utilidad para sujetos con HPTEC inoperable o HP persistente/recidivante postendarterectomía. Ambos tipos de pacientes también se pueden beneficiar de tratamiento farmacológico para la HP. Las tres terapias constituyen los pilares de la terapia, que ha evolucionado hacia un enfoque multimodal.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary hypertension (PH) caused by obstruction and remodelling of the pulmonary arteries following an episode of pulmonary embolism (PE). These alterations result in a sustained increase in pulmonary vascular resistance (PVR), with subsequent development of PH and progression to right ventricular (RV) failure in the late stages of the disease. CTEPH falls into group 4 of the clinical classification of PH, developing after an episode of acute PE that does not adequately resolve.1 Although CTEPH is often considered a complication of symptomatic acute PE, only 50%–75% of patients have a history of acute PE.2,3
Although persistent perfusion defects after acute PE are common,4 their clinical relevance is highly variable, ranging from complete absence of symptoms to florid forms of CTEPH. The concept of chronic thromboembolic pulmonary disease (CTEPD) describes symptomatic patients with perfusion defects with preserved ventilation on ventilation/perfusion (V/Q) scintigraphy and organised fibrotic clots on contrast-enhanced computed tomography (CT angiography) or pulmonary angiography, after a minimum of three months of therapeutic anticoagulation.1 Patients with CTEPH may or may not have PH at rest. The term CTEPH remains the most appropriate term to define patients with CTEPD who have PH at rest.1
The diagnosis of CTEPH is established on the basis of three main criteria: the existence of clinical manifestations (exertional dyspnoea, fatigue or syncope), the detection of residual thrombotic lesions by imaging techniques and the demonstration of precapillary PH by right heart catheterisation.
The cumulative incidence of CTEPH is estimated to range between 0.1%–11.8% in the first two years after symptomatic PE.5 A recent meta-analysis including 10,249 patients with PE would suggest that the incidence of CTEPH in those who survive acute PE would be 2.7%.6 In subjects with a history of PE, incident cases of CTEPH usually occur within the first two years after the PE episode.7,8
PathophysiologyFollow-up studies of three to 12 months, primarily with V/Q scintigraphy, show that 23%–50% of patients still have perfusion defects.9–12 The hallmark of CTEPH is incomplete resolution and fibrotic transformation of the pulmonary arterial thrombus, with endothelialisation and organisation of the thrombus leading to mechanical obstruction of the larger pulmonary arteries. In addition to this proximal fibrotic obstruction, there is microvasculopathy in small pulmonary vessels (muscle-type pulmonary arteries of diameter between 50–500 μm).5 This microvasculopathy has histological lesions similar to other forms of PH (medial hypertrophy, intimal proliferation, thrombotic phenomena and plexiform lesions), affecting both vessel-dependent areas with thrombotic obstruction and healthy vessel-dependent areas.13 The combination of persistent macrovascular obstruction and microvasculopathy, together with vasoconstriction phenomena, results in the development of PH and RV pressure overload, which often exceeds that expected from the degree of macrovascular obstruction alone.13 The relative contribution of each mechanism to the increase in PVR is different in each patient and is of paramount importance in treatment planning.
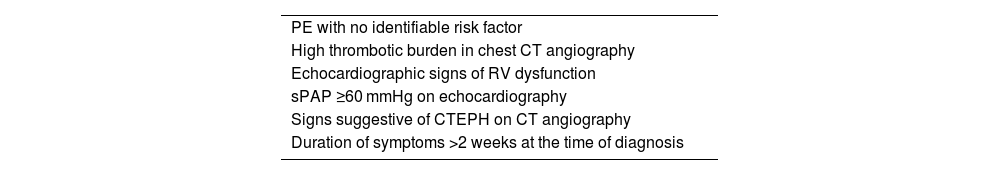
Risk factorsSome features of the initial episode of PE have been identified as risk factors for the subsequent development of CTEPH (Table 1).6,14–17 Among them, the presence of radiological signs suggestive of CTEPH on chest CT angiography performed for the diagnosis of acute PE is noteworthy.18 The presence of one or more of these signs may raise suspicion of underlying CTEPH during the acute episode. These changes are:
- •
Eccentric thrombi adherent to the vessel wall, which may be calcified (different from the central filling defects characteristic of acute PE).
- •
Hypertrophy of the bronchial circulation.
- •
“Mosaic” pulmonary perfusion pattern.
- •
Fine-band stenosis with post-stenotic dilatation.
- •
Intravascular membranes or webs.
- •
Complete saccular vessel obstructions.
- •
Atelectasis bands, old infarction, cavitated lesions and peripheral linear opacities (indicative of parenchymal scarring).
Factors associated with the development of chronic thromboembolic pulmonary hypertension at the time of pulmonary embolism diagnosis.
| PE with no identifiable risk factor |
| High thrombotic burden in chest CT angiography |
| Echocardiographic signs of RV dysfunction |
| sPAP ≥60 mmHg on echocardiography |
| Signs suggestive of CTEPH on CT angiography |
| Duration of symptoms >2 weeks at the time of diagnosis |
CTEPH, chronic thromboembolic pulmonary hypertension; CT, computed tomography; PE, pulmonary embolism; sPAP, systolic pulmonary artery pressure; RV, right ventricle; RV, right ventricle.
In a recent study, the presence of three or more of the following signs on diagnostic CT angiography of acute PE was associated with subsequent diagnosis of CTEPH with a sensitivity of 70% (95% confidence interval [CI] 55%–82%) and a specificity of 96% (95% CI 86%–100%): pulmonary artery contraction or dilatation; hypertrophy of the bronchial circulation; RV hypertrophy; and flattening of the interventricular septum.19 The use of systemic fibrinolytic therapy does not seem to reduce the likelihood of developing CTEPH after an episode of acute PE.20
With regard to prothrombotic states, elevated factor VIII levels have been identified as a risk factor for CTEPH.21,22 Several studies have described an elevated frequency of antiphospholipid antibodies in patients with CTEPH, in some series as high as 20%.23,24 More recently, the ADAMTS13-von Willebrand factor axis has been reported to be dysregulated in subjects with CTEPH.25 A high prevalence of dysfibrinogenemia has been reported in these patients and a higher resistance of fibrinogen from CTEPH patients to lysis when compared to healthy controls.26,27 Pulmonary artery endothelial dysfunction has also been documented in people with CTEPH, which may be involved in the pathogenesis and progression of the disease.28
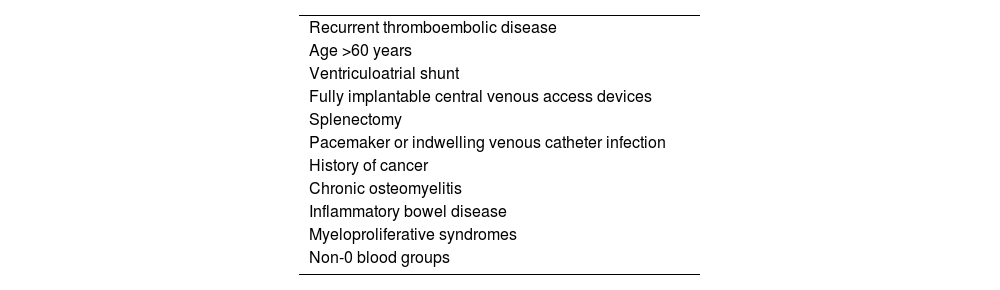
Several associated medical conditions have been identified that would increase the risk of developing CTEPH2,5,6,13–17,24,29–31 (Table 2). Of these, those most consistently associated with an increased risk of developing CTEPH are: recurrent venous thromboembolic disease (VTD) (odds ratio [OR] 15), splenectomy (OR 18), pacemaker or indwelling venous catheter infection (OR 76), history of cancer (OR 3.8) and hypothyroidism on replacement therapy (OR 6.1).5
Medical history and processes associated with the development of chronic thromboembolic pulmonary hypertension.
| Recurrent thromboembolic disease |
| Age >60 years |
| Ventriculoatrial shunt |
| Fully implantable central venous access devices |
| Splenectomy |
| Pacemaker or indwelling venous catheter infection |
| History of cancer |
| Chronic osteomyelitis |
| Inflammatory bowel disease |
| Myeloproliferative syndromes |
| Non-0 blood groups |
There are no specific signs or symptoms for CTEPH, which are mainly related to RV dysfunction and exertion in the early phase of the disease. As in other forms of PH, patients often report progressive exertional dyspnoea, fatigue, chest pain and palpitations.32 In more advanced stages, syncope, haemoptysis and signs of right heart failure may occur. Symptoms are the result of a combination of haemodynamic (failure to adequately increase cardiac output during exercise) and ventilatory (increased dead space ventilation) disturbances.33
Lack of symptom specificity often leads to delays in diagnosis. According to data from the International Registry, the average time from symptom onset to diagnosis of CTEPH is 14 months.2 Further complicating matters is the fact that a significant proportion of patients lack a history of symptomatic PE and that there is often an oligosymptomatic period after the PE episode.13 Recurrent thromboembolic disease and obesity are associated with delayed diagnosis of the disease, with a negative impact on the prognosis of these individuals.34
The World Health Organisation (WHO) functional class system is very useful in defining limitation in activities of daily living in patients with CTEPH and has prognostic implications. Objective measurement of exercise tolerance can be performed with simple (6 min walk test) or complex (cardiopulmonary exercise test) tests. The most commonly used is the former, the results of which correlate with prognosis, haemodynamic status and functional class. The cardiopulmonary exercise test shows a reduction in tolerated load, peak oxygen consumption, lactate threshold, oxygen pulse and ventilatory efficiency (elevation of ventilatory CO2 equivalent).1
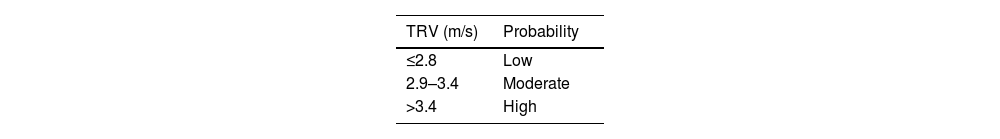
Transthoracic echocardiography (TTE) is essential as a screening tool to stratify the echocardiographic probability of PH. To determine this probability, the basic parameter is tricuspid regurgitation velocity (TRV), whose measurement requires tricuspid regurgitation (present in 80%–90% of the general population). TRV > 2.8 m/s is suggestive of PH, and together with other echocardiographic signs allows stratification of the probability of PH1 (Table 3). Three to six months after initiation of anticoagulation therapy, depending on the presence of dyspnoea and the presence of risk factors for the development of CTEPH, the indication for TTE should be assessed in patients with a history of PE in order to determine the echocardiographic probability of PH.15
Echocardiographic probability estimate of pulmonary hypertension.
| TRV (m/s) | Probability |
|---|---|
| ≤2.8 | Low |
| 2.9–3.4 | Moderate |
| >3.4 | High |
| Other echocardiographic signs of PHa | ||
|---|---|---|
| Ventricles | Pulmonary artery | IVC and RV |
| RV/LV > 1 | PAcT < 105 ms and/or meso-systolic notch | IVC diameter > 21 mm with reduced inspiratory collapse |
| Flattening of the IVS | Protodiastolic PRV > 2.2 m/s | Area RV > 18 cm2 |
| TAPSE/sPAP < 0.55 mm/mmHg | PA diameter > diameter AR | |
| PA diameter > 25 mm | ||
Adapted from Humbert et al.1
AR: aortic root; IVC: inferior vena cava; IVS: interventricular septum; LV: left ventricular diameter; PA: pulmonary artery; PH: pulmonary hypertension; PAcT: pulmonary acceleration time; PRV: pulmonary regurgitation velocity; RV: right ventricular diameter; sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular systolic excursion; TRV: tricuspid regurgitation velocity.
In patients with a high echocardiographic probability of PH and in those with intermediate probability who have elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) and/or risk factors for CTEPH, a V/Q lung scintigraphy is the technique of choice to rule out CTEPH. A normal or low probability V/Q lung scintigraphy in a patient with PH has a high specificity to rule out CTEPH. According to data from the International Registry, V/Q scintigraphy abnormalities are found in 98.7% of subjects.2 The most characteristic findings are segmental or larger perfusion defects with preserved ventilation. There is a growing trend in clinical practice to replace classical planar V/Q scintigraphy imaging with three-dimensional lung V/Q single photon emission tomography (SPECT) imaging. The addition of low-radiation computed tomography to V/Q SPECT (SPECT-CT) allows quantification of CTEPH severity and improves the specificity of the scan by allowing identification of concomitant lung parenchymal disease.35
Chest CT angiography is a complementary technique to V/Q scintigraphy in the study of these patients, allowing assessment of the degree of obstruction of the main, lobar and segmental pulmonary arteries, as well as the location of these lesions. Chest CT angiography is adequate for the diagnosis of proximal CTEPH, but a negative scan does not exclude CTEPH, as distal disease may be missed.5 Dual-energy CT provides lung perfusion mapping based on parenchymal iodine distribution, providing images similar to those obtained with scintigraphy, although its technical complexity, high cost and limited availability mean that it cannot replace scintigraphy as a screening tool.5 Magnetic resonance imaging (MRI) has the potential advantage of being able to provide both anatomical information on vascular obstruction (MRI angiography) and haemodynamic information (RV size and function). Although MRI angiography has numerous theoretical advantages over V/Q scintigraphy, it is technically more challenging and expensive, with limited availability and no multicentre validation, and therefore does not currently replace scintigraphy in routine clinical practice5.
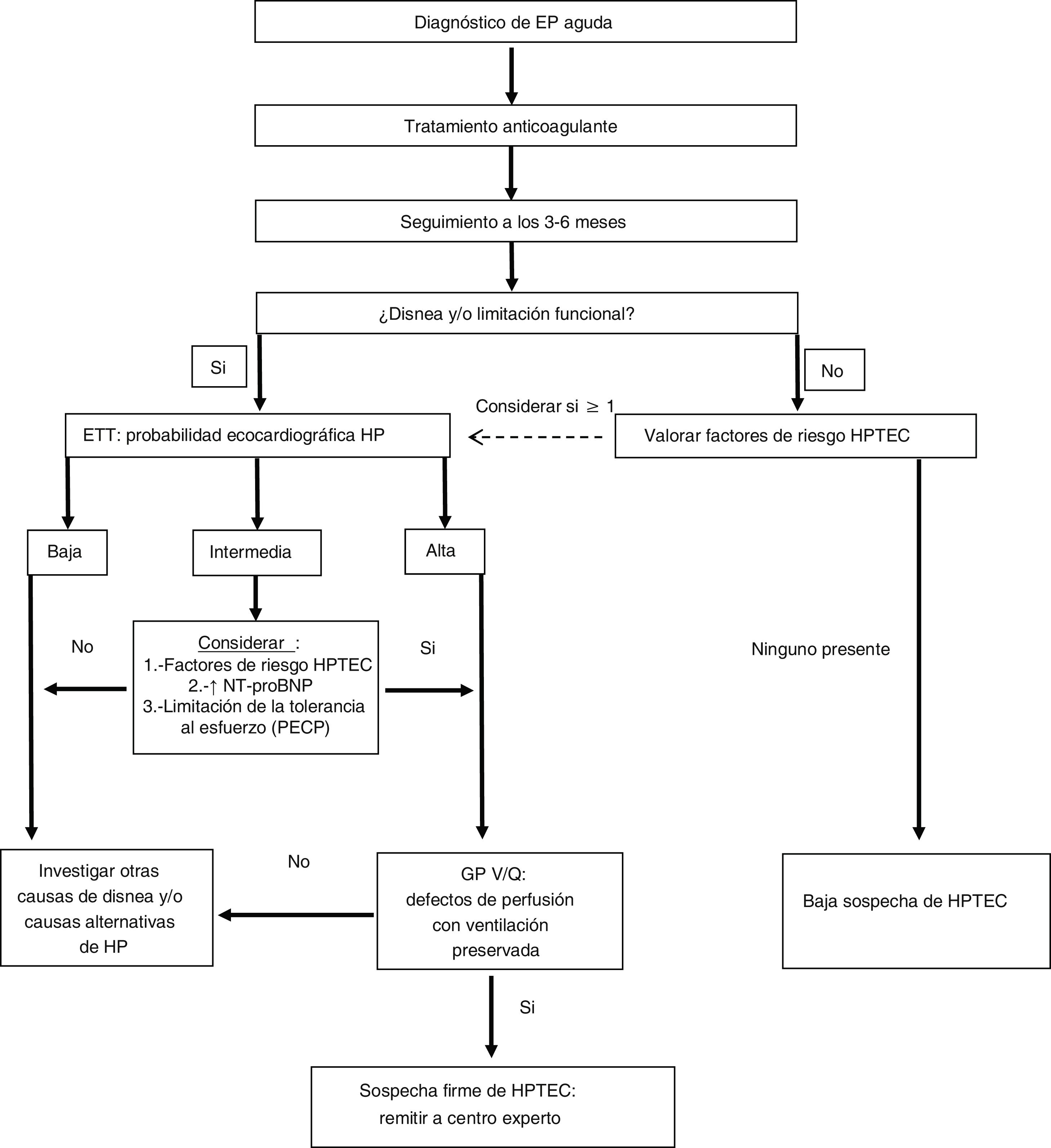
Right heart catheterisation and pulmonary arteriographyOnce the diagnostic suspicion of CTEPH is established based on clinical data (and after a minimum of three months of anticoagulation), a TTE with moderate to high probability of PH and an abnormal V/Q lung scintigraphy, the patient should be referred to an expert PH centre to complete the diagnostic process (Fig. 1). In this last phase, the existence of precapillary PH will be confirmed by right catheterisation and the surgical accessibility of the thrombotic lesions will be determined by pulmonary arteriography. Five angiographic patterns characteristic of CTEPH have been described36:
- •
Saccular endings of pulmonary arterial branches due to partially or totally obstructive thrombi.
- •
Existence of membranes or bands.
- •
Irregularities of the intima with a dentate appearance of the vascular wall.
- •
Abrupt narrowing of the larger pulmonary vessels.
- •
Complete vascular obstruction of the main, lobar or segmental pulmonary arteries at their point of origin.
Diagnostic algorithm for chronic thromboembolic pulmonary hypertension in patients with a history of pulmonary embolism.
Adapted from Konstantinides et al.15
CTEPH: chronic thromboembolic pulmonary hypertension; CPET: cardiopulmonary exercise test; PE: pulmonary embolism; PH: pulmonary hypertension; TTE: transthoracic echocardiography; V/Q LS: ventilation/perfusion pulmonary scintigraphy.
Two or more of these patterns are present in most patients with CTEPH, and typically the involvement is bilateral.33 The haemodynamic definition of PH has recently been modified, with precapillary PH being defined as the combination of: mean pulmonary artery pressure (PAP) > 20 mmHg + pulmonary capillary pressure ≤ 15 mmHg + PVR > two Wood units.1
TreatmentThe treatment of CTEPH is approached from a multimodal perspective, combining various therapeutic options aimed at treating the different anatomical lesions of this disease.1 Three treatments are available for CTEPH:
- •
Pulmonary endarterectomy
- •
Balloon pulmonary angioplasty (BPA)
- •
Pharmacological treatment
As a general measure, patients with CTEPH should receive long-term anticoagulation therapy to prevent in situ thrombosis of pulmonary circulation and recurrence of PE.1,5 Vitamin K antagonists (VKAs) are the anticoagulants that have traditionally been used in these patients, although direct-acting oral anticoagulants (DOACs) are increasingly used.5 However, as in all subjects without CTEPH, DOACs would not be recommended in those with antiphospholipid syndrome and triple-positive antibody testing.37 Despite the lack of clinical trials, an international registry including CTEPH patients treated with VKAs (n = 683) and DOACs (n = 198) showed similar bleeding rates in both groups, while the VTD recurrence rate in those treated with DOACs was slightly higher than in those treated with VKAs (3.0% and 2.2%, respectively).38 Supervised physical training is effective and safe in people with inoperable CTEPH or residual PH post-endarterectomy.39 The safety and feasibility of supervised physical training post-endarterectomy, with a programme initiated in hospital and continued on an outpatient basis after hospital discharge, has also been demonstrated.40 A supervised low-intensity rehabilitation programme is now considered standard of care after pulmonary endarterectomy.5
Pulmonary endarterectomyIf the patient is operable, pulmonary endarterectomy is the treatment of choice.1,5 This is a complex surgical technique requiring median sternotomy, extracorporeal circulation and periods of circulatory arrest with deep hypothermia, allowing the removal of the most proximal obstructive thrombotic material (down to a subsegmental vessel level).41 According to international registry data, 57%–59% of patients with CTEPH are treated by pulmonary endarterectomy.2,42 In Spain, according to data from the Spanish Registry of Patients with Pulmonary Arterial Hypertension (REHAP), the proportion of subjects treated with endarterectomy is lower (34%).43 The most common reasons for inoperability are: inaccessibility of thrombotic occlusions, poor correlation between haemodynamic severity and the number of accessible vascular occlusions (this data suggests prevalence of the microvasculopathy component), high-risk haemodynamic profile (PVR > 15 Wood units), existence of comorbidities or patient refusal.2,42 Age, as a sole criterion, does not exclude an individual from benefiting from this type of surgery.44 Perioperative mortality published in registries ranges from 3.5% to 4.7%.2,42,43
Some studies suggest that preoperative PVR > 12.5 Wood units would increase postoperative mortality.45 Consequently, the indication of pharmacological therapy to improve pulmonary haemodynamics prior to surgery in patients with high preoperative PVR is common practice, although there is no strong evidence to support it.1 In no case should the indication of pharmacological therapy prevent or delay potentially curative surgical treatment.
Published post-endarterectomy survival figures are 91%–93% at one year after the procedure and 89%–90% at three years.46,47 Factors associated with increased post-endarterectomy mortality are: functional class IV, elevated right atrial pressure, history of cancer, bridging therapy with PH drugs prior to surgery, postoperative PH, surgical complications and the need for additional cardiac procedures.46 Mean PAP ≥ 38 mmHg and PVR ≥ 5.3 Wood units during post-surgery follow-up are associated with lower long-term survival.47
Although the goal of pulmonary endarterectomy is restoring haemodynamic stability, residual PH after surgery is not uncommon.13 A meta-analysis of 4868 patients treated with endarterectomy describes residual PH in 25% of cases.45 In this study, endarterectomy resulted in a 21 mmHg decrease in mean PAP and a 65% decrease in PVR, with a significant increase in the distance covered in the 6 min walk test.45 Despite this haemodynamic improvement, some subjects still have limited exercise tolerance after endarterectomy. A study including 68 post-endarterectomy subjects describes a decreased exertional capacity of 66% six months after surgery, with the limitation being cardiovascular in 44% of them.48 It is noteworthy that 60% of patients (27/45) with limited exercise tolerance had no residual PH post-surgery.48
Although the universally accepted indication for pulmonary endarterectomy is CTEPH, very selected patients with CTEPH (without PH at rest), but symptomatic, with objective evidence of exercise tolerance limitation, can be successfully treated by pulmonary endarterectomy. The in-hospital mortality in a series of 42 subjects with CTEPH (mean PAP 21 mmHg, PVR two Wood units) treated with endarterectomy was 0%, with significant improvement in functional class (95% of patients in functional classes I–II at six months) and quality of life.49
Balloon pulmonary angioplastyOver the last decade, BPA has become an additional endovascular treatment option for selected patients with inoperable CTEPH or persistent/recurrent PH post-endarterectomy, improving haemodynamics, RV function and stress capacity.1 It does not replace endarterectomy or targeted drug therapy for CTEPH and may be considered in combination with either therapy as an adjunctive treatment, as it targets more distal lesions (subsegmental vessels 0.5–5 mm in diameter), not treatable with surgery or drugs.5 BPA is a staged procedure in which a limited number of lung segments are dilated in each session.1 The number of sessions required to obtain a good haemodynamic result varies, with four to five sessions per patient being the most common.42,43 According to data from an international registry, 19% of subjects with CTEPH were considered candidates for BPA.42 According to data from the REHAP registry, 9.6% of individuals were considered for BPA in Spain.43
In a prospective series, 56 patients with inoperable CTEPH were treated with BPA. Five procedures were performed per person, showing haemodynamic improvement (decreases in mean PAP of 18% and PVR of 26%), functional class and walking distance (+33 m). The majority (93%) received pharmacological treatment for PH prior to the procedure, which was maintained during the study. Procedure-related adverse events occurred in 32% of them, most of which were related to vascular perforation, including one (1.8%) who died of fatal pulmonary haemorrhage.50 In another series, 184 patients with inoperable CTEPH were treated with BPA (5.2 sessions per person). Improvements in haemodynamics (decreases in mean PAP of 26% and PVR of 43%), functional class and walking distance (+45 m) were observed. Adverse events related to the procedure occurred in 46% of them, with lung damage (9% of sessions), haemoptysis (7% of sessions) and pulmonary artery perforation (3% of sessions) being the most common. Three-year survival was excellent (95.1%).51
Pharmacological treatmentIt is not a curative treatment and its effect is usually modest, targeting the microvasculopathy component that can occur in CTEPH. Indications for drug therapy in CTEPH are: inoperable patients or patients with residual or recurrent PH after endarterectomy.1 A mean PAP threshold ≥30 mmHg post-endarterectomy has been suggested for the indication of pharmacological therapy.47 It may also be indicated in patients who refuse surgery and as pre-surgery conditioning therapy in subjects with a haemodynamic risk profile (PVR > 12.5 Wood units). The proportion of non-candidates for endarterectomy treated with PH drugs in registries ranges from 54% to 61%.42,43,46 The proportion of endarterectomy candidates treated with these drugs is clearly lower (26%–29%).42,46 Withdrawal of these drugs is generally considered after successful BPA and/or endarterectomy.5
The 2022 Guidelines on the diagnosis and treatment of PH recommend treatment with riociguat in patients with inoperable symptomatic CTEPH or with residual PH after endarterectomy (Class I recommendation, level of evidence B).1 Riociguat is a drug that targets the nitric oxide (NO) pathway and is administered orally. Its indication is based on a 16-week phase 3 clinical trial in 261 people with inoperable CTEPH or residual PH post-endarterectomy. Riociguat, compared to placebo, demonstrated a significant increase in walking distance in the gait test (primary endpoint). Improvements in functional class and significant decreases in PVR and NT-proBNP were also achieved.52 These beneficial effects were maintained in the one-year extension study.53 In more severe patients (functional class III-IV) with inoperable CTEPH or persistent PH post-endarterectomy, subcutaneous treprostinil may be considered (class IIb recommendation, level of evidence B).1,54 With a lower level of evidence (class IIb recommendation, level of evidence C), in those with inoperable CTEPH, combinations of drugs can be considered: soluble guanylate cyclase stimulator/phosphodiesterase 5 inhibitor, endothelin receptor antagonist (macitentan) or parenteral prostacyclin analogues.1,54,55
A recent clinical trial (RACE, Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension) compared the efficacy and safety of BPA versus riociguat in patients with inoperable CTEPH with no previous treatment. A total of 105 subjects were included (53 treated with riociguat; 52 with BPA). The primary endpoint was the change in PVR at 26 weeks of treatment. Symptomatic participants who at 26 weeks had a PVR > 4 Wood units benefited from an additional 26 weeks during which those initially treated with BPA received riociguat and those initially treated with riociguat were treated with BPA.56 After the first 26 weeks of treatment, the decline in PVR was significantly greater in patients on BPA (39.9% of baseline PVR) than in those on riociguat (66.7% of baseline PVR). Forty-two percent of those treated with BPA had severe treatment-related adverse events, while in those treated with riociguat this figure was only 9%. Notably, the incidence of BPA-related adverse events was lower in patients pre-treated with riociguat (14%) than in those first managed with BPA (42%), which may suggest a beneficial effect of this multimodality approach.56
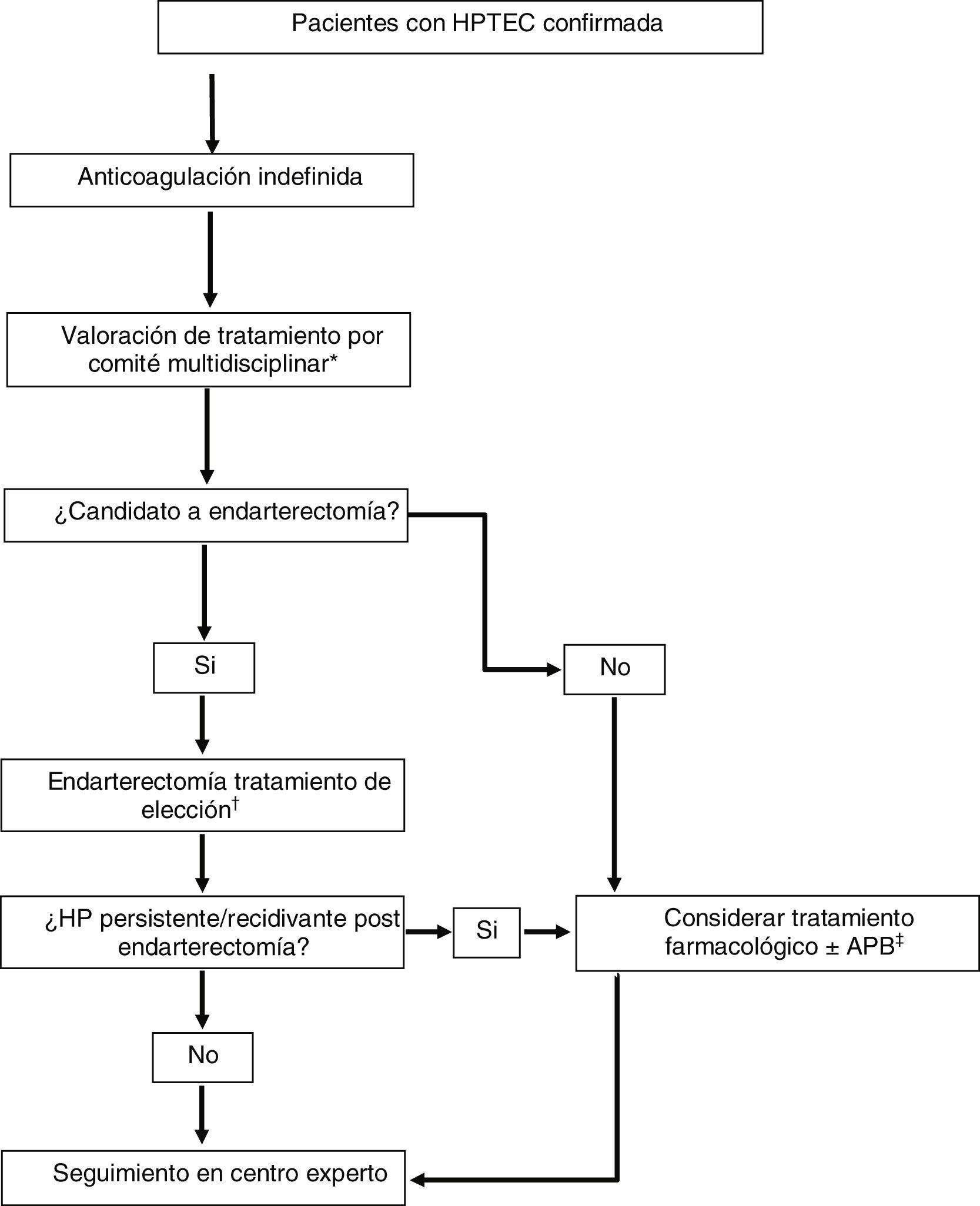
Multimodal treatment and lung transplantationMany patients with CTEPH have mixed anatomical lesions with involvement of main and lobar pulmonary arteries, segmental and sub-segmental arteries and microvasculopathy. The availability of three effective treatments targeting each of these anatomical lesions has shifted the treatment paradigm for this disease to a multimodality approach (Fig. 2).1,5 This approach involves the use of combinations of endarterectomy, BPA and drug therapy to target proximal lesions, more distal lesions and microvasculopathy, respectively. Currently, there are no clear guidelines establishing criteria for multimodality treatment, and patient selection is performed on a case-by-case basis in expert centres using a multidisciplinary approach.
Therapeutic strategy in patients with chronic thromboembolic pulmonary hypertension.
Adapted from Humbert et al.1
BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance.
*Consisting of: cardiovascular surgeon, interventional radiologist with expertise in BPA, PH specialist, thoracic radiologist and anaesthesiologist with experience in CTEPH.
†In patients who are candidates for endarterectomy with PVR > 12.5 Wood units, consider pre-procedure conditioning drug therapy (riociguat ± macitentan).
‡In patients who are candidates for BPA with PVR > 4 Wood units, consider treatment with riociguat before the procedure.
The different situations in which multimodal treatment can be considered are1:
- •
Pharmacological pre-endarterectomy conditioning therapy in patients with a haemodynamic risk profile. Some studies suggest that a preoperative PVR > 12.5 Wood units would increase postoperative mortality.45 In this patient profile, treatment with riociguat ± macitentan may be indicated to decrease PVR and improve the prognosis of surgery.
- •
Symptomatic patients with persistent/recurrent PH after endarterectomy. In these cases, pharmacological treatment and/or BPA may be considered.
- •
Patients with mixed anatomical lesions (surgically accessible lesions in one lung and inoperable lesions in the contralateral lung). In these cases, hybrid procedures can be planned: BPA of the non-surgical lesions in a first stage and endarterectomy of the operable lung in a second stage.
- •
Pharmacological treatment prior to BPA. Based on the results of the RACE clinical trial, treatment with riociguat prior to the procedure should be considered in patients with CTEPH that are candidates for BPA (conditional recommendation, very low quality of evidence) are.1,56
Due to the availability of these treatment options for CTEPH, the indication for lung transplantation is anecdotal. In the CTEPH registries, the proportion ranges from 0.15% to 0.7%.2,43 Bilateral lung transplantation for patients with CTEPH could be considered as a salvage option, only in very selected cases, as an alternative to failed pulmonary endarterectomy or as a potentially curative treatment in subjects with a significant prevalence of distal disease not amenable to surgical treatment or with BPA and no response to pharmacological treatment.33,57
FundingThis work has not received any funding.
Conflict of interestThe authors declare that they have no conflicts of interest.
Ethical considerationsThis work has not involved the use of human subjects and therefore no ethical considerations (obtaining informed consent) apply.
We wish to thank CERCA Programme/Generalitat de Catalunya for their institutional support.