The first case of coronavirus disease 2019 (COVID-19) infection in Spain was reported on 31 January 2020. By 23 March, 14000 cases had been detected, 5000 of which were in the Community of Madrid.
Given the emergency situation in the social-health care centres, the Primary Health Care Directorate of the northwest area of Madrid organised Nursing Home Support Units (UAR, for its acronym in Spanish) consisting of a doctor, a nurse and a driver, whose task was to provide health care to residents, support for the sectorisation of the centre and the isolation of infected patients and their close contacts.
The vaccination campaign against severe acute respiratory syndrome type 2 coronavirus (SARS-CoV-2) started on 27 December 2020 in all European Union countries. People living in social-health care centres are particularly vulnerable to severe SARS-CoV-2 infections and experience high rates of severe illness and mortality, so they were the first to be targeted in the vaccination programme.
The UAR vaccinated a total of 4512 patients (residents and workers) in social-health care centres with Pfizer’s BNT162b2 vaccine between 28 December 2020 and 18 February 2021.
Our aim is to report the finding of eyelid erythema observed in a series of 10 cases after administration of the vaccine.
All patients aged ≥16 years, institutionalised in social-health care centres for the disabled or nursing homes for the elderly, and their workers, who signed the informed consent form, were included in the vaccination programme. Patients with a previous history of severe adverse reaction or anaphylaxis to any of the components of the vaccine, acute SARS-CoV-2 infection, hospital admission or convalescence period from any disease were excluded.
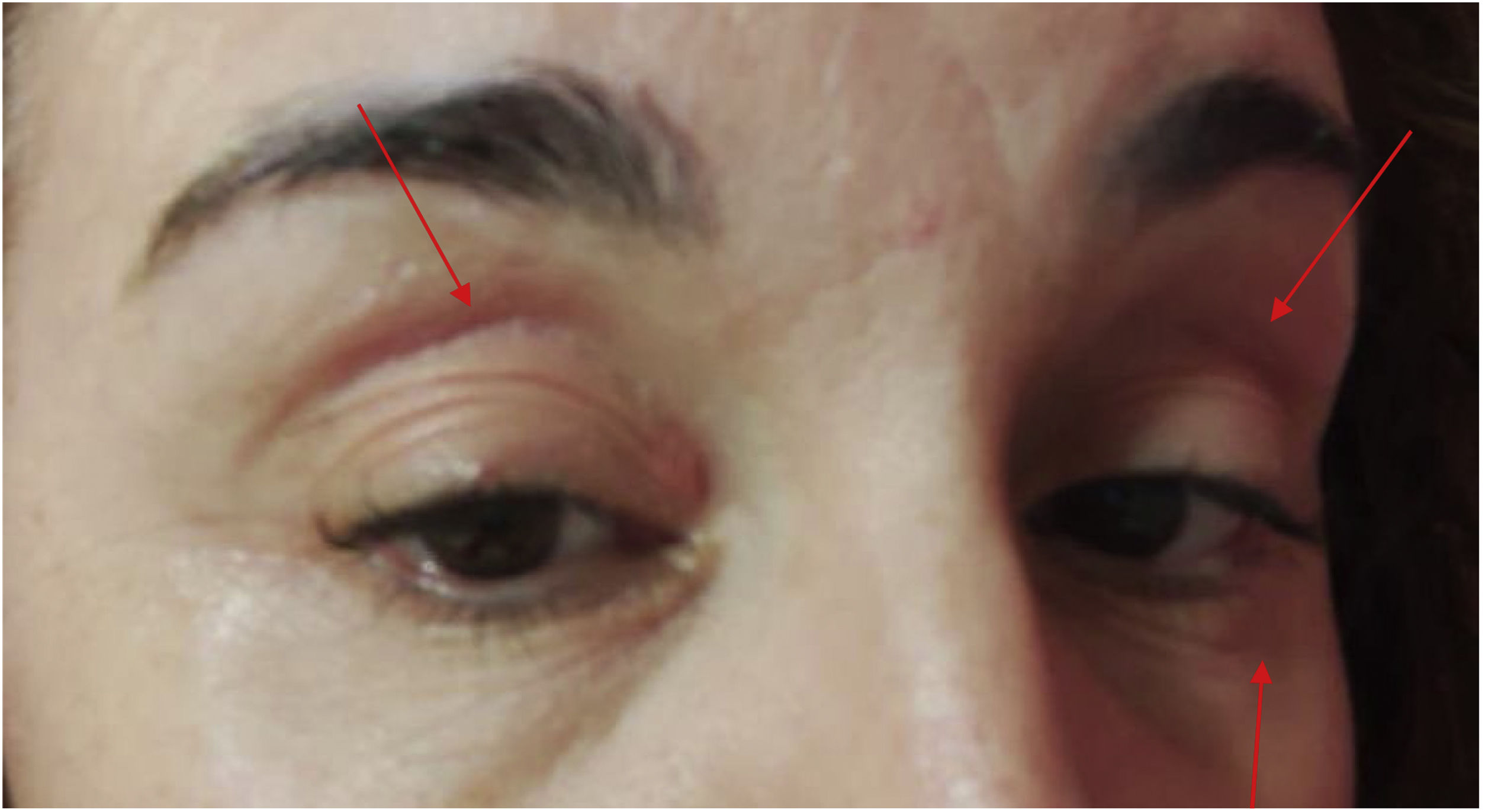
Ten patients (0.22%) aged 47–99 years out of 4512 vaccinated had red-violet eyelid erythema (Fig. 1), on the upper and lower eyelids, non-scaling, non-pruritic, irritative, with a burning sensation, after administration of the vaccine.
Six of them had suffered from a COVID-19 infection prior to vaccination. None of them had previously suffered from a similar condition, they had no history of dermatological disease or eyelid surgery.
In one patient the condition developed at 24 h after the first dose, in seven of them 24 h after the second dose, and in only two of them did it recur after the first and second doses, at 24 and 8 h respectively. It was not associated with any other side effects.
In all cases the duration was 4 days, in 4 patients it improved with topical corticosteroid treatment and in the other 6 it cleared spontaneously.
Side effects reported so far refer to general symptoms (the most common are fever, headache, chills, fatigue, myalgia and joint pain), or local symptoms at the puncture site (pain, swelling and redness) and were more common after receiving the second dose of vaccine.1–4 To date, we have not found any case in the literature of palpebral erythema secondary to vaccination with Pfizer’s BNT162b2, nor is it included in its SmPC.
The pattern of onset and healing with respect to the day the vaccine was administered, and the type of skin lesion suggests a cause-effect relationship in all cases observed. The occurrence of this clinical sign following the administration of Pfizer’s BNT162b2 vaccine, or any other vaccine, should be assessed in larger series of patients, together with its temporal and pathophysiological relationship with the administration of the vaccine.
All patients gave their consent for the publication of the cases and images.
FundingThis work has not received any funding.
Please cite this article as: Bárcena-Dahl A, Bonivento-Martínez V, Rey-Pérez de Pipaón M. Eritema palpebral tras vacunación frente a la infección por coronavirus. Med Clin (Barc). 2022;158:395–396.