Hypertension and smoking during pregnancy have been linked to various adverse maternal and fetal outcomes. The objective of this work is to study how the smoking influences the development of hypertension, its effects on the pregnant woman, and on the newborn.
Materials and methodsAn observational study in two phases was carried out: the descriptive first phase allows characterization of the sample and the analytical second phase is a case–control nested in a retrospective cohort corresponding to pregnancy.
ResultsA total of 712 women were included in the study. Of the 672 (94.4%) non-hypertensive women, 533 (79.3%) were non-smoking and 139 (20.7%) smoking. For the 40 (5.6%) hypertensive women, 30 (75.0%) were non-smoking and 10 (25.0%) smoking. The prevalence of hypertension was of 5.6%. Women who quit smoking before pregnancy saw a reduced risk of hypertension. For women who smoke during pregnancy, those of younger ages, with a normal body mass index, who are primiparous, employed and with a low-medium level of education have higher risk of hypertension. The risk of hypertension according to the level of physical activity during leisure time follows a “U” shape, with those who perform light physical activity at the lowest risk of hypertension. Hypertensive women have a higher risk of small for gestational age newborns. Smoking does not pose an additional risk for adverse outcomes once hypertension is diagnosed.
ConclusionsFuture studies should aim to determine the role of smoking habit in the appearance of hypertension in pregnancy in order to establish adequate intervention guidelines that may aid in reducing the prevalence of hypertension.
La hipertensión y el tabaquismo durante el embarazo se han relacionado con diversos resultados adversos maternos y fetales. El objetivo de este trabajo es estudiar cómo influye el tabaquismo en el desarrollo de la hipertensión, sus efectos sobre la gestante y sobre el recién nacido.
Materiales y métodosSe realizó un estudio observacional en 2 fases: la primera, descriptiva, que permite caracterizar la muestra, y la segunda, analítica, que es un caso-control anidado en una cohorte retrospectiva correspondiente al embarazo.
ResultadosSe incluyó en el estudio a un total de 712 mujeres. De las 672 (94,4%) mujeres no hipertensas, 533 (79,3%) eran no fumadoras y 139 (20,7%) fumadoras. De las 40 (5,6%) mujeres hipertensas, 30 (75,0%) eran no fumadoras y 10 (25,0%) fumadoras. La prevalencia de hipertensión fue del 5,6%. Las mujeres que dejaron de fumar antes del embarazo vieron reducido el riesgo de hipertensión. En el caso de las mujeres que fuman durante el embarazo, las de menor edad, con un índice de masa corporal normal, primíparas, empleadas y con un nivel de estudios medio-bajo tienen mayor riesgo de hipertensión. El riesgo de hipertensión según el nivel de actividad física durante el tiempo de ocio sigue una forma de «U», siendo las que realizan una actividad física ligera las que tienen menor riesgo de hipertensión. Las mujeres hipertensas tienen un mayor riesgo de recién nacidos pequeños para la edad gestacional. El tabaquismo no supone un riesgo adicional de resultados adversos una vez diagnosticada la hipertensión.
ConclusionesFuturos estudios deben ir encaminados a determinar el papel del hábito tabáquico en la aparición de hipertensión en el embarazo para establecer pautas de intervención adecuadas que puedan ayudar a reducir la prevalencia de hipertensión.
In the Valencian Community there is the phenomenon commonly known as the Spanish or Mediterranean paradox,1 the prevalence of cardiovascular (CV) mortality2 is not explained by the Mediterranean diet followed mainly in the region, considered especially heart-healthy, nor by the smoking habit nor is it justified by the known classic associated risk factors.
Due to the structural and hemodynamic changes that occur during pregnancy, the CV system suffers a significant burden.3 There are significant increases in cardiac output,4 decrease in systemic vascular resistance,5 the renin–angiotensin–aldosterone system is significantly activated,6 heart and vasculature undergo remodeling7 and insulin resistance occurs as a result of the secretion by the placenta of hormones such as growth hormone (GH), placental lactogen, progesterone and cortisol.6
Hypertensive disorders occur in 2–10% of pregnancies.8 So far, hypertension (HTN) during pregnancy has been linked to premature delivery,9 intrauterine growth restriction,10 placental abruption,11 preeclampsia and eclampsia,12 neonatal intensive care unit admission, as well as fetal death13 and maternal mortality.14 Hypertensive disorders of pregnancy produce endothelial damage that generates alterations in the short term; in the medium term it generates a metabolic syndrome; and in the long term it leads to cardiovascular disease (CVD).8 In fact, hypertensive disorders of pregnancy double the risk of CVD in women who have had these complications compared to the rest.8 HTN is the main risk factor for having a stroke or suffering from ischemic heart disease15 and other risk factors include hypercholesterolemia and smoking.16
The importance of smoking is that it is the main modifiable risk factor to avoid CV disease.17 Much has been studied about HTN and smoking18 but there are hardly any studies on pregnant women whose only pathology is HTN,16 since other pathologies such as gestational diabetes,19 anemia,20 urine infections,21 depression22 are usually present, acting as modifying factors.
Smoking during pregnancy is associated with an increased risk of adverse effects such as low birth weight,23 spontaneous abortion,24 placental abruption,25 premature delivery26 and intrauterine growth restriction.27 It also produces persistent metabolic effects in the newborns of smoking mothers.20 Despite the evidence of the adverse effects that tobacco produces during pregnancy, many women continue to smoke during pregnancy.28
Tobacco smoke contains more than 4000 different chemical substances, of which more than 40 are carcinogenic.29 The effects of exposure to smoke in the placenta are critical with COHb contributing to the appearance of fetal hypoxia.30 Nicotine produces vasoconstriction,31 a decrease in prostaglandin synthesis,32 increases vascular resistance, and decreases fetal blood flow.33,34 All this implies a deficient oxygenation of the tissues, thus increasing the risk of suffering from CV diseases.35
The main objective of this work is to study how the smoking habit influences the development of HTN, its effects on the pregnant woman, and on the newborn.
Material and methodsThis is an observational study developed in two phases: the descriptive first phase allows characterization of the sample and the analytical second phase is a case–control nested in a retrospective cohort corresponding to pregnancy.
An a priori power analysis was performed to determine the sample size required to test the study hypothesis: that smoking during pregnancy may be related to HTN, which in Spanish pregnant women has been estimated to be between 2 and 10%.8 The sample size calculation was made using a confidence level of 95%, a margin of error of 5%, an estimated prevalence of HTN during pregnancy of 5%, an estimated prevalence of smoking during pregnancy of 25% and an unlimited population size. The required sample size yielded was of a total of 323 women.
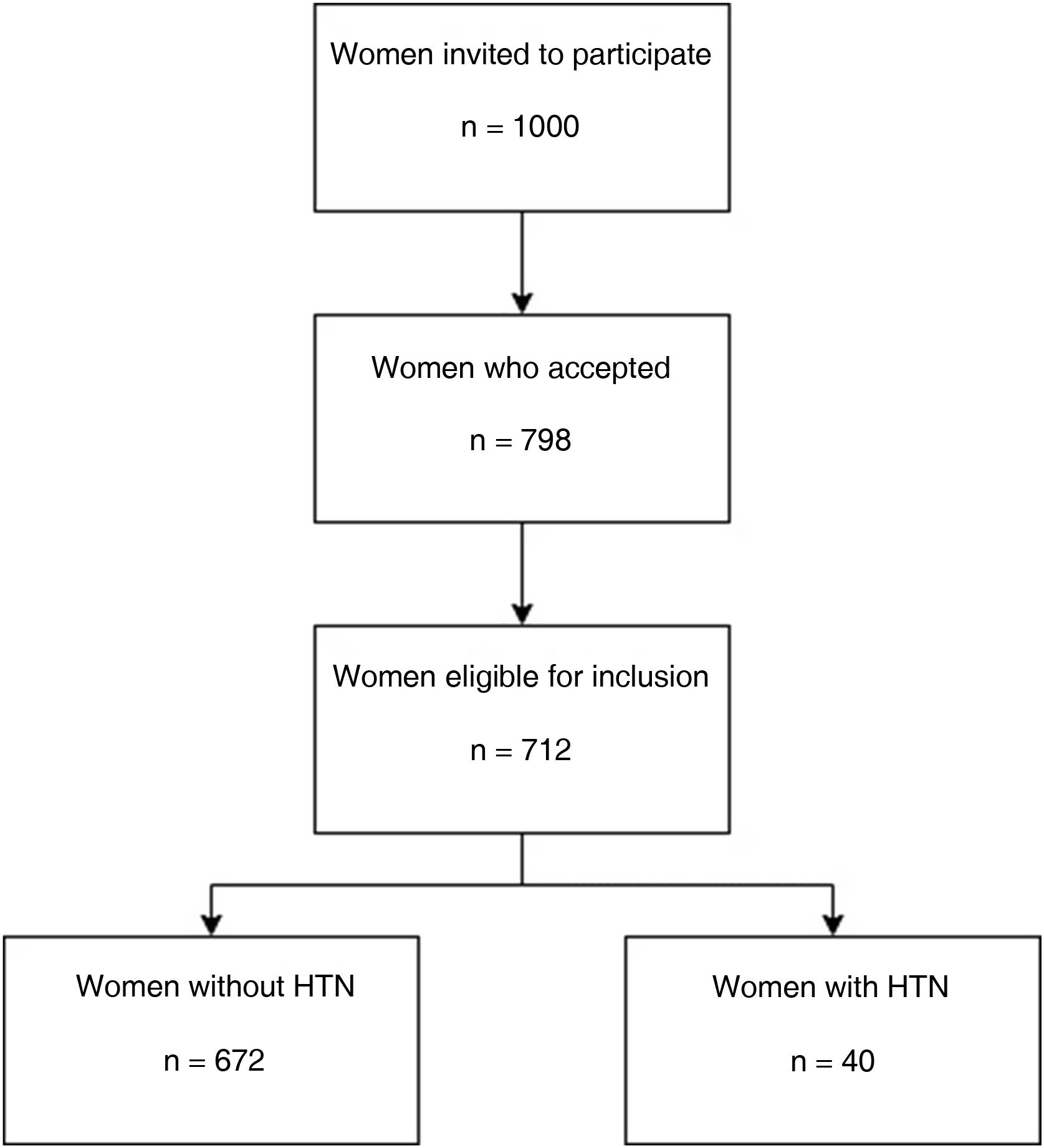
All mother–child pairings admitted after delivery to the Obstetrics Ward of University and Polytechnic Hospital La Fe in Valencia and belonging to the health coverage area of this hospital were considered for the study population and 1000 were randomly selected to be invited to participate. Of the invited women, 798 accepted to be included and 712 were finally eligible for inclusion (Fig. 1).
We have considered the mother's smoking habit during pregnancy as the exposure and clinically diagnosed HTN as the outcome, evaluating the possible results that such exposure may have on the mother and the newborn. The effects on the newborn were collected and evaluated at the time of delivery and using the electronic newborn health-card, while the characteristics of the mother were also collected at the time of birth but were evaluated retrospectively using the electronic clinical history of the whole pregnancy and the pregnancy health-card.
In the descriptive part of our study, the personal, socioeconomic, anthropometric characteristics of the women, clinical and obstetric history were analyzed, as well as data related to pregnancy and childbirth, including the state of the newborn. In the analytical part of the study, these data were related to smoking and HTN in the mother, as well as other possible effects on the health of the mother and newborn.
Thus, considering smoking, determined as cigarettes per day, as the exposure variable and having HTN as the outcome, those pregnant women with HTN free of other pathologies were identified as cases, while the controls would be those pregnant women without HTN or other pathologies. Forming in total four groups (smoking and non-smoking non-hypertensive patients (NHTNP), and smoking and non-smoking hypertensive patients (HTNP)). Blood pressure was taken manually with the patient sitting down with the arm at the level of the heart, using an appropriate cuff that covers 1.5 times the circumference of the arm.
There are different hypertensive disorders during pregnancy:
- -
Chronic HTN: blood pressure ≥140/90mmHg that is diagnosed before the 20th week of gestation.
- -
Gestational HTN: the appearance of HTN (blood pressure ≥140/90mmHg) without proteinuria after 20 weeks of gestation.
- -
Preeclampsia: a HTN that appears after 20 weeks of gestation and is accompanied by proteinuria (≥300mg protein/24h).
- -
Eclampsia: is the appearance in a pregnant woman with preeclampsia, of seizures of great type not badly attributable to other causes.
In our study, chronic HTN was not differentiated from gestational HTN and both were considered HTNP. HTN diagnosis required a measured blood pressure ≥140mmHg of systolic and/or 90mmHg of diastolic recorded in two different measurements separated by at least 6h over the course of a week. The diagnosis of HTN could have been made during any of the prenatal visits, therefore, at the time of admission for delivery the women considered as HTNP already had a previous diagnosis of HTN.
The inclusion criteria included:
- •
informed consent of the participating mother for the inclusion of her and her child in the study and
- •
required data on both mother and child was available and reliable.
Thus, those mother–child couples in which:
- •
the mother did not give consent for participation
- •
data corresponding to the characteristics of the mother and/or newborn were not available for consultation and collection or did not appear reliable and/or
- •
the responses of the mothers during the personal interview were inconsistent, or incomplete
were excluded from the study. Those mother–child pairs in which the mother was admitted to the Intensive Care Unit after birth were also excluded.
The mothers included in the study signed a consent evaluated and accepted by the Clinical Research Ethics Committee of the University and Polytechnic Hospital La Fe, which included an agreement of confidentiality of the data collected according to Organic Law 15/1999, of December 13, on Protection of Official Data.
The technique used to collect the information in the present study was the review of the corresponding medical records of both the mother and the newborn in addition to the pregnancy and newborn health cards and a direct and personal interview with the mother performed by a trained professional. The design of this interview was structured, using a questionnaire that contained different sections.
The information collected in the study was both qualitative and quantitative, a fact that was considered when coding and analyzing it statistically. All this information was entered into a database in SAV format using the IBM SPSS Statistics 22 program.
To calculate whether the newborn was adequate for gestational age (AGA), small for gestational age (SGA) or large for gestational age (LGA), the gestational calculator V2021.2 (Hospital Universitari Clínic de Barcelona/BCNatal), was used. This calculator allows for a quick and easy determination of the percentile according to the weight for gestational age and sex curves and is the method used to establish adequacy of weight for gestational age at University and Polytechnic Hospital La Fe.
ResultsOf the women in our study (n=712), 672 corresponded to the control group or NHTNP, 533 non-smoking and 139 smoking, while 40 women were part of the case group or HTNP, 30 non-smoking and 10 smoking. The prevalence of HTN without other pathologies in the sample studied was of 5.6%. In the control group, 20.7% were smokers while in the case group 25.0% were smokers. The relative risk of HTN for smokers was RR=1.28 (95% CI: 0.61–2.68) with an attributable fraction of 21.8% (0–62.7%).
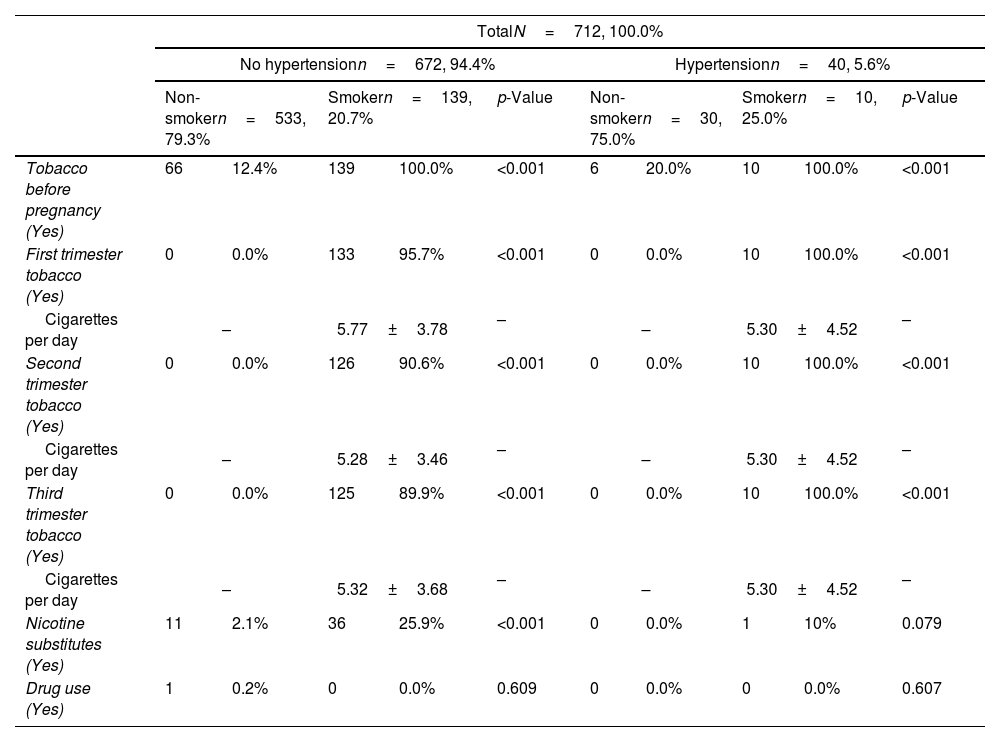
In the control group, 66 women (12.4%) quit smoking before pregnancy while, of the 139 smokers, as the pregnancy progressed, 36 (25.9%) used nicotine substitutes to quit smoking with 14 women being able to quit smoking (10.1%). In the group of women with HTN, 6 (20%) quit tobacco before pregnancy while only 1 (10.0%) used nicotine substitutes to try to quit smoking with none of the 10 smoking HTNP managing to quit smoking during pregnancy. Pregnant women who were able to quit smoking before pregnancy have risk of HTN of OR=0.79 (95% CI: 0.28–2.27). The details pertaining to smoking habit can be seen in Table 1.
Use of tobacco and drugs of pregnant women, according to the diagnosis of hypertension and their smoking habit.
| TotalN=712, 100.0% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No hypertensionn=672, 94.4% | Hypertensionn=40, 5.6% | |||||||||
| Non-smokern=533, 79.3% | Smokern=139, 20.7% | p-Value | Non-smokern=30, 75.0% | Smokern=10, 25.0% | p-Value | |||||
| Tobacco before pregnancy (Yes) | 66 | 12.4% | 139 | 100.0% | <0.001 | 6 | 20.0% | 10 | 100.0% | <0.001 |
| First trimester tobacco (Yes) | 0 | 0.0% | 133 | 95.7% | <0.001 | 0 | 0.0% | 10 | 100.0% | <0.001 |
| Cigarettes per day | – | 5.77±3.78 | – | – | 5.30±4.52 | – | ||||
| Second trimester tobacco (Yes) | 0 | 0.0% | 126 | 90.6% | <0.001 | 0 | 0.0% | 10 | 100.0% | <0.001 |
| Cigarettes per day | – | 5.28±3.46 | – | – | 5.30±4.52 | – | ||||
| Third trimester tobacco (Yes) | 0 | 0.0% | 125 | 89.9% | <0.001 | 0 | 0.0% | 10 | 100.0% | <0.001 |
| Cigarettes per day | – | 5.32±3.68 | – | – | 5.30±4.52 | – | ||||
| Nicotine substitutes (Yes) | 11 | 2.1% | 36 | 25.9% | <0.001 | 0 | 0.0% | 1 | 10% | 0.079 |
| Drug use (Yes) | 1 | 0.2% | 0 | 0.0% | 0.609 | 0 | 0.0% | 0 | 0.0% | 0.607 |
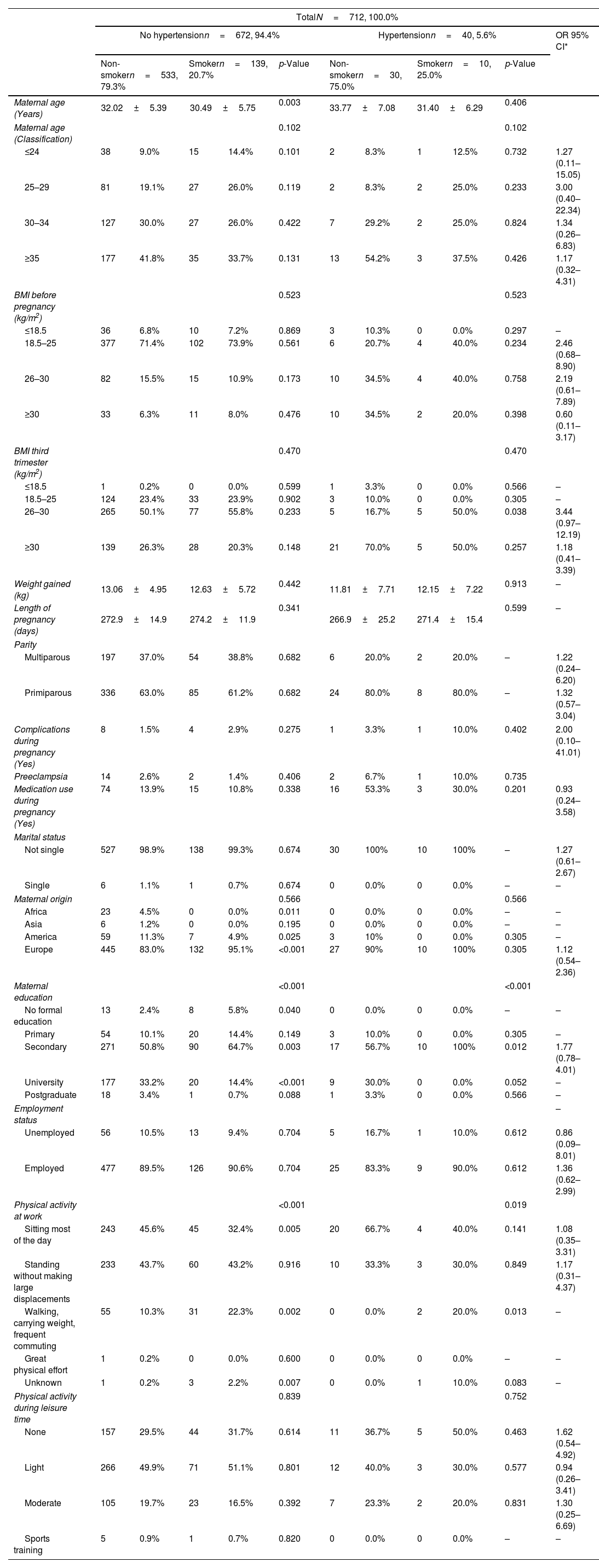
The personal characteristics of the pregnant women according to their HTN and smoking status are shown in Table 2. There are significant differences in the ages of the NHTNP with the average age of smokers being 30.49±5.75 years, and 32.02±5.39 years in non-smokers. No differences are observed in HTNP. The risks of HTN due to smoking in each age group have been calculated. The highest risk is found in 25–29-year olds, with OR=3.00 (95% CI: 0.40–22.34).
Characteristics of pregnant women based on the diagnosis of hypertension and their smoking habit.
| TotalN=712, 100.0% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No hypertensionn=672, 94.4% | Hypertensionn=40, 5.6% | OR 95% CI* | |||||||||
| Non-smokern=533, 79.3% | Smokern=139, 20.7% | p-Value | Non-smokern=30, 75.0% | Smokern=10, 25.0% | p-Value | ||||||
| Maternal age (Years) | 32.02±5.39 | 30.49±5.75 | 0.003 | 33.77±7.08 | 31.40±6.29 | 0.406 | |||||
| Maternal age (Classification) | 0.102 | 0.102 | |||||||||
| ≤24 | 38 | 9.0% | 15 | 14.4% | 0.101 | 2 | 8.3% | 1 | 12.5% | 0.732 | 1.27 (0.11–15.05) |
| 25–29 | 81 | 19.1% | 27 | 26.0% | 0.119 | 2 | 8.3% | 2 | 25.0% | 0.233 | 3.00 (0.40–22.34) |
| 30–34 | 127 | 30.0% | 27 | 26.0% | 0.422 | 7 | 29.2% | 2 | 25.0% | 0.824 | 1.34 (0.26–6.83) |
| ≥35 | 177 | 41.8% | 35 | 33.7% | 0.131 | 13 | 54.2% | 3 | 37.5% | 0.426 | 1.17 (0.32–4.31) |
| BMI before pregnancy (kg/m2) | 0.523 | 0.523 | |||||||||
| ≤18.5 | 36 | 6.8% | 10 | 7.2% | 0.869 | 3 | 10.3% | 0 | 0.0% | 0.297 | – |
| 18.5–25 | 377 | 71.4% | 102 | 73.9% | 0.561 | 6 | 20.7% | 4 | 40.0% | 0.234 | 2.46 (0.68–8.90) |
| 26–30 | 82 | 15.5% | 15 | 10.9% | 0.173 | 10 | 34.5% | 4 | 40.0% | 0.758 | 2.19 (0.61–7.89) |
| ≥30 | 33 | 6.3% | 11 | 8.0% | 0.476 | 10 | 34.5% | 2 | 20.0% | 0.398 | 0.60 (0.11–3.17) |
| BMI third trimester (kg/m2) | 0.470 | 0.470 | |||||||||
| ≤18.5 | 1 | 0.2% | 0 | 0.0% | 0.599 | 1 | 3.3% | 0 | 0.0% | 0.566 | – |
| 18.5–25 | 124 | 23.4% | 33 | 23.9% | 0.902 | 3 | 10.0% | 0 | 0.0% | 0.305 | – |
| 26–30 | 265 | 50.1% | 77 | 55.8% | 0.233 | 5 | 16.7% | 5 | 50.0% | 0.038 | 3.44 (0.97–12.19) |
| ≥30 | 139 | 26.3% | 28 | 20.3% | 0.148 | 21 | 70.0% | 5 | 50.0% | 0.257 | 1.18 (0.41–3.39) |
| Weight gained (kg) | 13.06±4.95 | 12.63±5.72 | 0.442 | 11.81±7.71 | 12.15±7.22 | 0.913 | – | ||||
| Length of pregnancy (days) | 272.9±14.9 | 274.2±11.9 | 0.341 | 266.9±25.2 | 271.4±15.4 | 0.599 | – | ||||
| Parity | |||||||||||
| Multiparous | 197 | 37.0% | 54 | 38.8% | 0.682 | 6 | 20.0% | 2 | 20.0% | – | 1.22 (0.24–6.20) |
| Primiparous | 336 | 63.0% | 85 | 61.2% | 0.682 | 24 | 80.0% | 8 | 80.0% | – | 1.32 (0.57–3.04) |
| Complications during pregnancy (Yes) | 8 | 1.5% | 4 | 2.9% | 0.275 | 1 | 3.3% | 1 | 10.0% | 0.402 | 2.00 (0.10–41.01) |
| Preeclampsia | 14 | 2.6% | 2 | 1.4% | 0.406 | 2 | 6.7% | 1 | 10.0% | 0.735 | |
| Medication use during pregnancy (Yes) | 74 | 13.9% | 15 | 10.8% | 0.338 | 16 | 53.3% | 3 | 30.0% | 0.201 | 0.93 (0.24–3.58) |
| Marital status | |||||||||||
| Not single | 527 | 98.9% | 138 | 99.3% | 0.674 | 30 | 100% | 10 | 100% | – | 1.27 (0.61–2.67) |
| Single | 6 | 1.1% | 1 | 0.7% | 0.674 | 0 | 0.0% | 0 | 0.0% | – | – |
| Maternal origin | 0.566 | 0.566 | |||||||||
| Africa | 23 | 4.5% | 0 | 0.0% | 0.011 | 0 | 0.0% | 0 | 0.0% | – | – |
| Asia | 6 | 1.2% | 0 | 0.0% | 0.195 | 0 | 0.0% | 0 | 0.0% | – | – |
| America | 59 | 11.3% | 7 | 4.9% | 0.025 | 3 | 10% | 0 | 0.0% | 0.305 | – |
| Europe | 445 | 83.0% | 132 | 95.1% | <0.001 | 27 | 90% | 10 | 100% | 0.305 | 1.12 (0.54–2.36) |
| Maternal education | <0.001 | <0.001 | |||||||||
| No formal education | 13 | 2.4% | 8 | 5.8% | 0.040 | 0 | 0.0% | 0 | 0.0% | – | – |
| Primary | 54 | 10.1% | 20 | 14.4% | 0.149 | 3 | 10.0% | 0 | 0.0% | 0.305 | – |
| Secondary | 271 | 50.8% | 90 | 64.7% | 0.003 | 17 | 56.7% | 10 | 100% | 0.012 | 1.77 (0.78–4.01) |
| University | 177 | 33.2% | 20 | 14.4% | <0.001 | 9 | 30.0% | 0 | 0.0% | 0.052 | – |
| Postgraduate | 18 | 3.4% | 1 | 0.7% | 0.088 | 1 | 3.3% | 0 | 0.0% | 0.566 | – |
| Employment status | – | ||||||||||
| Unemployed | 56 | 10.5% | 13 | 9.4% | 0.704 | 5 | 16.7% | 1 | 10.0% | 0.612 | 0.86 (0.09–8.01) |
| Employed | 477 | 89.5% | 126 | 90.6% | 0.704 | 25 | 83.3% | 9 | 90.0% | 0.612 | 1.36 (0.62–2.99) |
| Physical activity at work | <0.001 | 0.019 | |||||||||
| Sitting most of the day | 243 | 45.6% | 45 | 32.4% | 0.005 | 20 | 66.7% | 4 | 40.0% | 0.141 | 1.08 (0.35–3.31) |
| Standing without making large displacements | 233 | 43.7% | 60 | 43.2% | 0.916 | 10 | 33.3% | 3 | 30.0% | 0.849 | 1.17 (0.31–4.37) |
| Walking, carrying weight, frequent commuting | 55 | 10.3% | 31 | 22.3% | 0.002 | 0 | 0.0% | 2 | 20.0% | 0.013 | – |
| Great physical effort | 1 | 0.2% | 0 | 0.0% | 0.600 | 0 | 0.0% | 0 | 0.0% | – | – |
| Unknown | 1 | 0.2% | 3 | 2.2% | 0.007 | 0 | 0.0% | 1 | 10.0% | 0.083 | – |
| Physical activity during leisure time | 0.839 | 0.752 | |||||||||
| None | 157 | 29.5% | 44 | 31.7% | 0.614 | 11 | 36.7% | 5 | 50.0% | 0.463 | 1.62 (0.54–4.92) |
| Light | 266 | 49.9% | 71 | 51.1% | 0.801 | 12 | 40.0% | 3 | 30.0% | 0.577 | 0.94 (0.26–3.41) |
| Moderate | 105 | 19.7% | 23 | 16.5% | 0.392 | 7 | 23.3% | 2 | 20.0% | 0.831 | 1.30 (0.25–6.69) |
| Sports training | 5 | 0.9% | 1 | 0.7% | 0.820 | 0 | 0.0% | 0 | 0.0% | – | – |
No significant differences are found in pre pregnancy BMI. The risk of HTN due to smoking decreases as BMI increases although without statistical significance.
There is no difference in weight gained between smokers and non-smokers in either the cases or controls. However, weight gained was less overall in the HTNP group, 11.81±7.71kg for non-smokers and 12.15±7.22kg for smokers versus 13.06±4.95kg for non-smokers and 12.63±5.72kg for smokers.
HTNP have a shorter average pregnancy duration (266.9±25.2 days for non-smokers and 271.4±15.4 days for smokers), being the length of pregnancy of the control group 272.9±14.9 days for non-smokers and 274.2±11.9 days for smokers. In both cases and controls, smokers had longer average pregnancy durations than non-smokers.
In the control group, smokers (2.9%) had almost twice as many complications during pregnancy as non-smokers (1.5%), while in the HTNP group, smokers (10.0%) had triple the complications during pregnancy than non-smokers (3.3%). A prevalence of preeclampsia for the control group of 2.6% for non-smokers and 1.4% for smokers was identified. No cases of eclampsia were detected.
When it comes to medication use during pregnancy, cases have much higher rates most likely due to the use of medication to treat their HTN but the number of HTNP who have not received medication (47% of non-smokers and 70% of smokers) is notable. However, no differences in medication use appear between smokers and non-smokers for either the cases or controls.
In regard to maternal origin, most of our participants are European (95.1%). It must be noted that none of the participating African or Asian woman smoked, all smokers were either American or European.
Most of the women included in the study have a secondary education. Significant differences are observed between smokers and non-smokers, with smokers having lower rates of higher education.
No significant differences were found for employment status. In the employed group risk of HTN due to tobacco presents an OR=1.36 (95% CI: 0.62–2.99) while in the unemployed group the OR=0.86 (95% CI: 0.09–8.01).
Significant differences in both controls and cases can be seen regarding physical activity in the home or workplace. In the control group, there are more than twice as many smokers (22.3%) doing work carrying weight with frequent displacements, compared to non-smokers (10.3%). While in the cases, 20% of smokers and 0% of non-smokers perform this activity. For physical activity during leisure time, the differences are not statistically significant and no clear pattern for the risk of HTN associated to smoking can be seen but those that do not do any sort of leisure exercise present the highest risk of HTN.
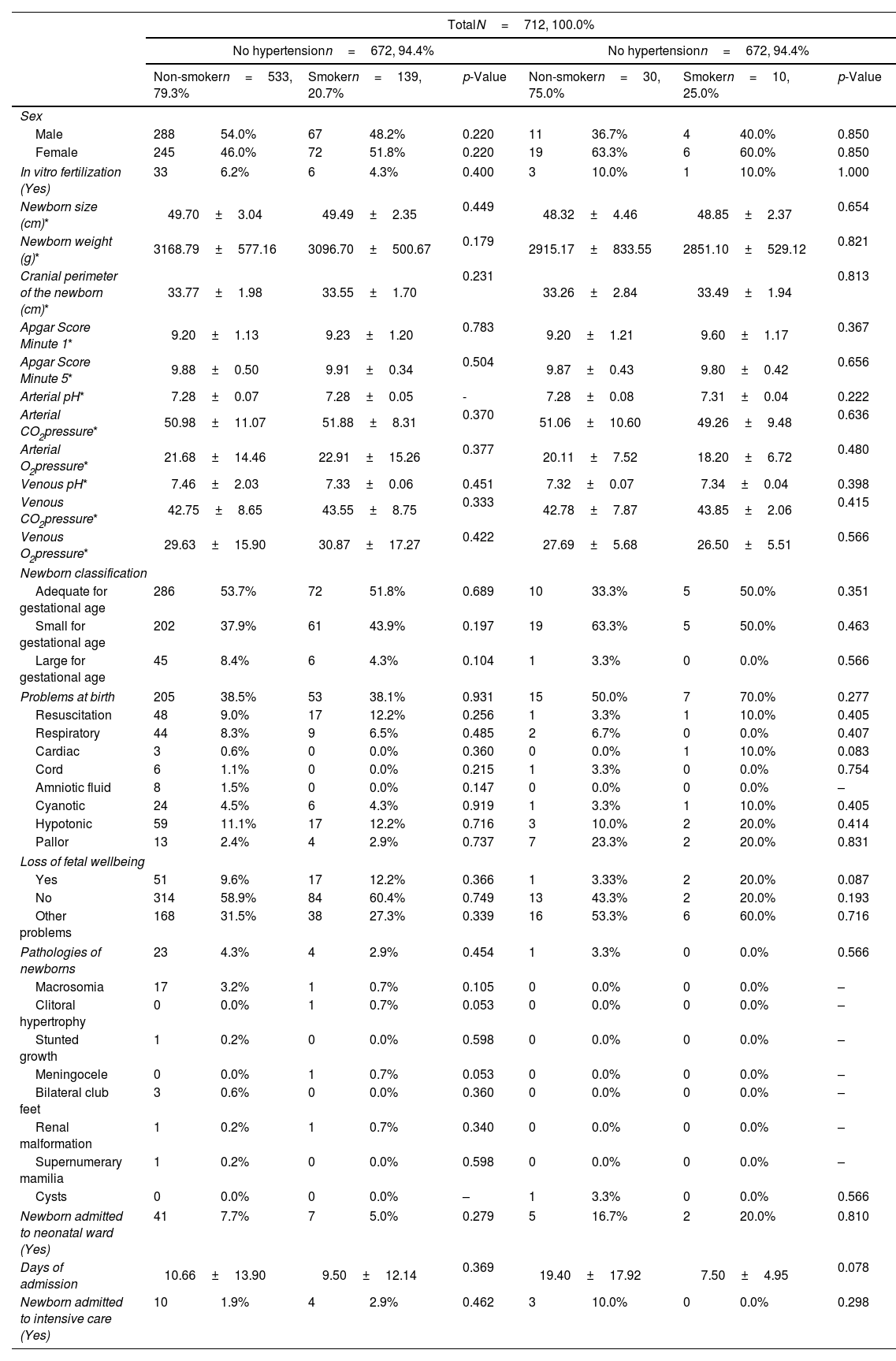
When it comes to the characteristics of the newborns which can be seen in Table 3, no statistically significant differences between smokers and non-smokers were found. In the control group, newborn sex is very even, however, more girls (63.3% to non-smokers and 60.0% to smokers) than boys were born in the group of women with HTN.
Characteristics of the newborn according to the diagnosis of hypertension and the mother's smoking habit.
| TotalN=712, 100.0% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No hypertensionn=672, 94.4% | No hypertensionn=672, 94.4% | |||||||||
| Non-smokern=533, 79.3% | Smokern=139, 20.7% | p-Value | Non-smokern=30, 75.0% | Smokern=10, 25.0% | p-Value | |||||
| Sex | ||||||||||
| Male | 288 | 54.0% | 67 | 48.2% | 0.220 | 11 | 36.7% | 4 | 40.0% | 0.850 |
| Female | 245 | 46.0% | 72 | 51.8% | 0.220 | 19 | 63.3% | 6 | 60.0% | 0.850 |
| In vitro fertilization (Yes) | 33 | 6.2% | 6 | 4.3% | 0.400 | 3 | 10.0% | 1 | 10.0% | 1.000 |
| Newborn size (cm)* | 49.70±3.04 | 49.49±2.35 | 0.449 | 48.32±4.46 | 48.85±2.37 | 0.654 | ||||
| Newborn weight (g)* | 3168.79±577.16 | 3096.70±500.67 | 0.179 | 2915.17±833.55 | 2851.10±529.12 | 0.821 | ||||
| Cranial perimeter of the newborn (cm)* | 33.77±1.98 | 33.55±1.70 | 0.231 | 33.26±2.84 | 33.49±1.94 | 0.813 | ||||
| Apgar Score Minute 1* | 9.20±1.13 | 9.23±1.20 | 0.783 | 9.20±1.21 | 9.60±1.17 | 0.367 | ||||
| Apgar Score Minute 5* | 9.88±0.50 | 9.91±0.34 | 0.504 | 9.87±0.43 | 9.80±0.42 | 0.656 | ||||
| Arterial pH* | 7.28±0.07 | 7.28±0.05 | - | 7.28±0.08 | 7.31±0.04 | 0.222 | ||||
| Arterial CO2pressure* | 50.98±11.07 | 51.88±8.31 | 0.370 | 51.06±10.60 | 49.26±9.48 | 0.636 | ||||
| Arterial O2pressure* | 21.68±14.46 | 22.91±15.26 | 0.377 | 20.11±7.52 | 18.20±6.72 | 0.480 | ||||
| Venous pH* | 7.46±2.03 | 7.33±0.06 | 0.451 | 7.32±0.07 | 7.34±0.04 | 0.398 | ||||
| Venous CO2pressure* | 42.75±8.65 | 43.55±8.75 | 0.333 | 42.78±7.87 | 43.85±2.06 | 0.415 | ||||
| Venous O2pressure* | 29.63±15.90 | 30.87±17.27 | 0.422 | 27.69±5.68 | 26.50±5.51 | 0.566 | ||||
| Newborn classification | ||||||||||
| Adequate for gestational age | 286 | 53.7% | 72 | 51.8% | 0.689 | 10 | 33.3% | 5 | 50.0% | 0.351 |
| Small for gestational age | 202 | 37.9% | 61 | 43.9% | 0.197 | 19 | 63.3% | 5 | 50.0% | 0.463 |
| Large for gestational age | 45 | 8.4% | 6 | 4.3% | 0.104 | 1 | 3.3% | 0 | 0.0% | 0.566 |
| Problems at birth | 205 | 38.5% | 53 | 38.1% | 0.931 | 15 | 50.0% | 7 | 70.0% | 0.277 |
| Resuscitation | 48 | 9.0% | 17 | 12.2% | 0.256 | 1 | 3.3% | 1 | 10.0% | 0.405 |
| Respiratory | 44 | 8.3% | 9 | 6.5% | 0.485 | 2 | 6.7% | 0 | 0.0% | 0.407 |
| Cardiac | 3 | 0.6% | 0 | 0.0% | 0.360 | 0 | 0.0% | 1 | 10.0% | 0.083 |
| Cord | 6 | 1.1% | 0 | 0.0% | 0.215 | 1 | 3.3% | 0 | 0.0% | 0.754 |
| Amniotic fluid | 8 | 1.5% | 0 | 0.0% | 0.147 | 0 | 0.0% | 0 | 0.0% | – |
| Cyanotic | 24 | 4.5% | 6 | 4.3% | 0.919 | 1 | 3.3% | 1 | 10.0% | 0.405 |
| Hypotonic | 59 | 11.1% | 17 | 12.2% | 0.716 | 3 | 10.0% | 2 | 20.0% | 0.414 |
| Pallor | 13 | 2.4% | 4 | 2.9% | 0.737 | 7 | 23.3% | 2 | 20.0% | 0.831 |
| Loss of fetal wellbeing | ||||||||||
| Yes | 51 | 9.6% | 17 | 12.2% | 0.366 | 1 | 3.33% | 2 | 20.0% | 0.087 |
| No | 314 | 58.9% | 84 | 60.4% | 0.749 | 13 | 43.3% | 2 | 20.0% | 0.193 |
| Other problems | 168 | 31.5% | 38 | 27.3% | 0.339 | 16 | 53.3% | 6 | 60.0% | 0.716 |
| Pathologies of newborns | 23 | 4.3% | 4 | 2.9% | 0.454 | 1 | 3.3% | 0 | 0.0% | 0.566 |
| Macrosomia | 17 | 3.2% | 1 | 0.7% | 0.105 | 0 | 0.0% | 0 | 0.0% | – |
| Clitoral hypertrophy | 0 | 0.0% | 1 | 0.7% | 0.053 | 0 | 0.0% | 0 | 0.0% | – |
| Stunted growth | 1 | 0.2% | 0 | 0.0% | 0.598 | 0 | 0.0% | 0 | 0.0% | – |
| Meningocele | 0 | 0.0% | 1 | 0.7% | 0.053 | 0 | 0.0% | 0 | 0.0% | – |
| Bilateral club feet | 3 | 0.6% | 0 | 0.0% | 0.360 | 0 | 0.0% | 0 | 0.0% | – |
| Renal malformation | 1 | 0.2% | 1 | 0.7% | 0.340 | 0 | 0.0% | 0 | 0.0% | – |
| Supernumerary mamilia | 1 | 0.2% | 0 | 0.0% | 0.598 | 0 | 0.0% | 0 | 0.0% | – |
| Cysts | 0 | 0.0% | 0 | 0.0% | – | 1 | 3.3% | 0 | 0.0% | 0.566 |
| Newborn admitted to neonatal ward (Yes) | 41 | 7.7% | 7 | 5.0% | 0.279 | 5 | 16.7% | 2 | 20.0% | 0.810 |
| Days of admission | 10.66±13.90 | 9.50±12.14 | 0.369 | 19.40±17.92 | 7.50±4.95 | 0.078 | ||||
| Newborn admitted to intensive care (Yes) | 10 | 1.9% | 4 | 2.9% | 0.462 | 3 | 10.0% | 0 | 0.0% | 0.298 |
The HTNP group had smaller newborns (height and weight) compared to the control group and while not significant, smokers of both groups had lower weight newborns compared to the non-smokers of their respective groups. There are no notable variations in cranial perimeter, Apgar Score Minute 1, Apgar Score Minute 5, blood pH or blood gas values. The newborns of non-smoking HTNP spent more days in the neonatal intensive care unit (19.40±17.92 days) than all other groups.
In the classification of the newborn, it can be observed that in the control group, newborns that are adequate for gestational age (AGA), represent 53.7%, however, in the group of HTNP only 33.3% are AGA. In pregnant women in the control group, 37.9% of their newborns are small for gestational age (SGA), while this percentage rises to 63.3% in the HTNP group. With regard to newborns that are large for gestational age (LGA), they account for 8.4% of newborns in the control group and 3.3% in the HTNP group.
When it comes to complications or pathologies present in the newborn, there are no statistically significant differences between smoker and non-smokers in either the NHTNP or HTNP groups. The low incidence of these complications and the small number of women in the HTNP do not allow for a very detailed analysis of the results.
DiscussionIn this study, 5.6% of pregnant women have been identified as having HTN as their only pathology. According to the Spanish Cardiology Society between 68% of pregnant women suffer from hypertensive disorders during pregnancy36 and another study has identified that up to 10% of pregnant women may suffer from HTN.37
A prevalence of 2.6% of preeclampsia has been identified for the NHTN for non-smokers, and 1.4% for smokers. This result is similar to a study carried out in the nearby city of Crevillent in 2015,38 which obtained a prevalence of preeclampsia of 2.35%. This separation between HTN and preeclampsia is important, given that while smoking increases the risk of HTN,39 numerous studies have found it decreases the risk of developing preeclampsia.40,41 It is believed that CO could prevent the development of preeclampsia,42,43 and a previous study demonstrated an inverse relationship between CO in the environment and preeclampsia.44 It should be remembered that any interpretations must take into account that the pathogenesis of preeclampsia is complex, since it also seems to involve genetic, immunological and environmental factors.45
The profile of pregnant women with HTN in this study is that they are older, and this delay in having children is a known factor that can increase the risk of HTN.46,47 HTNP are more likely to be overweight or obese than NHTNP but they have gained less weight during pregnancy and have also had shorter pregnancies, coinciding with the results of other studies.48–50 HTNP had a higher number of small for gestational age (SGA) newborns as also seen in previous studies,51,52 and newborns were more likely to need hospitalization in the neonatal and/or intensive care wards than the NHTNP group.
A smoking habit prevalence of 20% for the control group and 25% for the HTNP was found, with this prevalence being higher than in another study conducted in Spain,28 where a prevalence of 15.6% was found. It is also possible that overall exposure to tobacco smoke is underestimated since there may be passive exposure in both groups that has not been accounted for here.
The profile of the smokers in our study is that these women are younger, which agrees with the smoking habit in today's Spanish society, in which now there are more female smokers, especially at young ages. Smokers also have a lower level of education than non-smokers which is in line with previous studies.53 This group should receive targeted health education campaigns that emphasize the importance of smoking cessation.
Pregnant women in our study who quit smoking before pregnancy had a decreased the risk of HTN, coinciding with other studies.54 This highlights one of the possible benefits of smoking cessation programs during pre-conception and pregnancy. In general, these results suggest that one of the strategies for the prevention of cardiovascular diseases (particularly HTN) would be to avoid smoking during the period of conception and pregnancy.
The number of HTNP who have not received medication during pregnancy is striking. In non-smokers it is 47%, and for smokers 70%. This could be because mild and moderate HTN is not automatically treated pharmacologically in all pregnant women, but it is studied individually. It is true, however, that in mild and moderate HTN, the risk of developing severe HTN is reduced by half with antihypertensive medication,55 although it does not prevent the risk of preeclampsia, or perinatal complications. In addition, antihypertensive medication can reduce uterine-placental flow and compromise fetal health. Therefore, it is not advisable to reduce diastolic blood pressure to less than 80mmHg but with blood pressure levels greater than 160/110mmHg, antihypertensive therapy should be initiated with the aim of reducing maternal complications such as encephalopathy and cerebral hemorrhage.56
The result of our study is important in which HTNP have had (60%) of their small newborns for their gestational age (SGA), while the control group has had a result of (39.1%) of (SGA), this result being statistically significant (p≤0.05). Confirming like other studies57–59 that HTN is a risk factor for having small newborns for their gestational age. We identified a strong association between HTN, which is a risk factor for having small newborns for gestational age. Being very important to have blood pressure controlled during pregnancy, to reduce the CV risk of the pregnant woman and have more children suitable for gestational age.
It is observed that the risk of having HTN due to smoking is inversely proportional to the BMI. Pregnant women who have normal weight (BMI of 18.5–25), are at a higher risk of having HTN due to smoking than those who are obese (BMI≥30). This may be due to the fact that the majority of tobacco smoke pollutants are lipophilic,60 and when pregnant women have higher BMIs fat percentage is also normally higher. There are studies61,62 that relate lipophilic contaminants and obesity and although due to the sample size we have not been able to calculate the OR for the low weight group (BMI≤18.5), it is expected that it will be the group that presents the highest risk of HTN if it has been exposed to smoking as the same amount of pollutants will in these group give way to a higher concentration of pollutants.
When it comes to the level of maternal education, the group of non-smokers were three times more likely to have university degrees than to only have a primary level education while smokers were just as likely to have university degrees as to have a primary education. This is in line with previous studies that have linked educational level with smoking habit in women.63
Although the history of the relationship between physical exercise and pregnancy has been contradictory, there is currently scientific evidence that supports the inclusion of this healthy habit among pregnant women.64,65 It has been shown that light physical activity is beneficial for the health of the pregnant woman and the newborn.66 This is confirmed in this study within the pregnant women who smoke, as the risk of HTN is reduced when there is light physical activity during pregnancy, while not exercising and moderate physical activity increase the risk of HTN.
After delivery, it is very important to establish from the primary care office, and in collaboration with obstetricians, midwives, cardiologists, and other specialists as needed, the re-evaluation and follow-up of women who have developed HT during pregnancy, with respect to the future risk of developing CVD.8 The growing evidence of the impact of pregnancy on a woman's future cardiovascular health and increased awareness on the part of clinicians and women should also lead to a paradigm shift in the coming years in the early detection of these risk factors in pregnancy and their follow-up and treatment after delivery to reduce potential complications.8
LimitationsThis study presents several limitations that warrant mentioning. First, while meeting the required sample size, it is still a small sample of women, and this could affect thar results and their interpretation. Given the way participants were recruited, no selection bias would be expected. There is the possibility of an analysis bias given that confounding factors that could alter the observed relationship between HTN and smoking may not have been considered. Among these, two of the more relevant could be HTN medication and lifestyle related factors such as stress or diet. The use of appropriate treatment for HTN could mask or hide the possible consequences on the mother or child associated with smoking. HTN can be of multifactorial origin and lifestyle related factors like stress, which can also lead to more smoking, and inadequate salt and/potassium intake could play a role in the etiology of HTN. In order to correctly account for these possible confounders, specific data on them and a larger sample would be needed.
ConclusionsThe prevalence of smoking in pregnant women in our study is higher than in other studies conducted in the Spanish population. The group with HTN smokes an additional 5%, raising the risk of suffering a CV accident. If there is exposure to smoking, women of young ages, with a normal body mass index, and with a level of education low-medium have higher risk of HTN. The risk of HTN according to the level of physical activity forms a “U” shaped graph, placing women who perform light physical activity at lower risk of HTN. Women in our study who quit smoking before pregnancy saw a reduced risk of HTN. Hypertensive women have a higher risk of having SGA newborns.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.