Complex regional pain syndrome is a chronic and painful condition that affects the quality of life of patients. It is usually triggered by a traumatic event of the soft tissues involving the nervous tissue. Although the factors that cause the syndrome are varied and not well known, different etiopathologic concepts have been proposed to explain the presence of this syndrome, such as autonomic dysfunction and changes in CNS plasticity, among others. The patient characteristically presents pain, sensory abnormalities, vasomotor disturbances in the skin, edema, changes in sweating, and motor alterations. The pain is associated with changes in the autonomic nervous system and has a distal predominance. Since there is no definitive diagnostic test, diagnosis is mainly based on a complete medical history and physical examination. Treatment is multidisciplinary and based on pain relief. Although in most cases evolution is favorable, rapid diagnosis and treatment are recommended to avoid dystrophic stage as much as possible.
The first description of complex regional pain syndrome (CRPS) dates from the seventeenth century and was reported by the French surgeon Ambroise Paré to describe persistent pain and contractures of the arm suffered by King Charles IX after the bloodletting to which he was subjected. During the American Civil War, Mitchell described cases in which the soldiers suffered from burning pain secondary to gunshot wounds. This was described as causalgia. In 1900 Sudeck described traumatic complications in the extremities characterized by intractable pain, edema, and limitations in motor function. Lerich in 1916, suggested that causalgia was caused by excessive activity of the sympathetic nervous system. It was not until 1946 when Evans proposed reflex sympathetic dystrophy.1
In 1979 the International Association for the Study of Pain (IASP) defined causalgia as “a syndrome of sustained, burning pain after a traumatic nerve injury, combined with vasomotor and sudomotor and later trophic changes” and reflex sympathetic dystrophy as “similar, but from other causes.”1
The term proposed by the IASP in 1994, which differentiates complex regional pain syndrome into type 1 and 2, is currently used with the dissimilarity that type 1 is caused by an injury or trauma to tissue and in type 2 there is prior and obvious neurological damage.
Since the characteristics of these two types of disease are essentially the same and treatment is not different, the rest of this text will not distinguish between them with respect to pathophysiology, diagnosis or treatment.
EpidemiologyWorldwide, the incidence and prevalence of CRPS is unknown. Some studies have reported an incidence rate that ranges from 5.46 to 26.2 per 100,000 persons year.2,3 In addition, the prevalence subsequent to trauma ranges from 0.03 to 37%, based on retrospective studies. In 40% of cases it is associated with a fracture or surgery, with compression of the median nerve being the most common, although it can also appear after a sprain (10%), root lesions (9%), lesions of the spinal cord (6%), and spontaneously (5–10%). It was found that it more frequently affects women (2–3:1) with a peak between 50 and 70 years of age, with a predominance in the arms.4
It is noteworthy that the severity of the original injury is not correlated with the severity of the symptoms of CRPS, although psychological factors such as stress are risk factors that worsen symptomatology.5
CRPS is also associated with other diseases and conditions such as stroke, mastectomy, pregnancy, and the use of drugs such as phenobarbital and isoniazid. There are predisposing factors for the development of this syndrome in addition to trauma and diabetes mellitus.6
The main feature of the history is a fracture, and immobilization has been proposed as a possible predisposing factor for CRPS. Immobilization studies in animals have found increased sensitivity to stimuli, and changes at the spinal level. In humans it was found that plaster splint immobilization results in an increase in cerebral blood flow in areas related to sensory, motor, and emotional processing.7
It is believed that psychogenic or hysterical factors, mainly associated with depressive symptoms, may contribute to CRPS. Any psychological factor can interact with catecholamine release and thus interfere with the pathophysiological mechanisms mentioned; however this is only a hypothesis.8 The success of psychotherapy and occupational and cognitive therapy in CRPS patients shows that the symptoms of dystonia and myoclonus are of a psychogenic origin in some patients. It is not always easy to distinguish these symptoms from simulation.9
CRPS often occurs in several family members and at younger ages, which may indicate a genetic predisposition. Accordingly, HLA has been proposed to have a role in CRPS. Genetic studies have also identified polymorphisms in the TNF-α gene and the angiotensin converting enzyme gene, but no contrasting results have been found with the latter.10
Studies have shown that the use of ACE inhibitors at the time when the causal trauma is suffered, as well as a medical history of asthma or migraines, is associated with an increased risk of developing CRPS. It is noteworthy that these factors imply underlying inflammation, since ACE inhibitors increase the availability of substance P, and both migraines and asthma share neurogenic inflammation mechanisms.10
PathophysiologyCRPS is a chronic pain condition that usually arises after a traumatic event of the soft tissues. The “definitive” cause still remains unknown, although different pathogenetic concepts have been proposed; three of the most studied are: autonomic dysfunction, neurogenic inflammation, and changes in CNS neuroplasticity, all of which are still in dispute. However, current evidence shows that this problem could have a multifactorial origin.
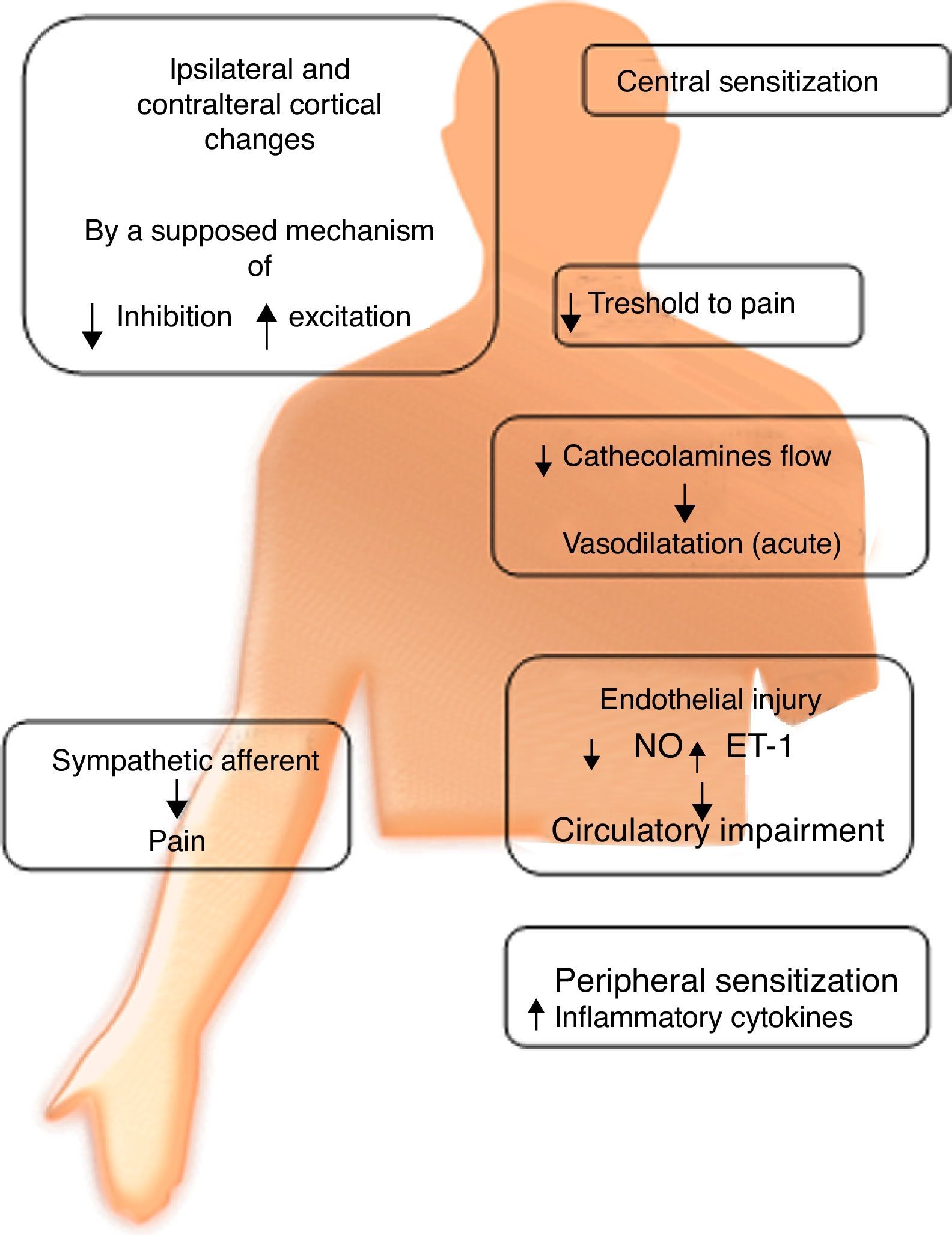
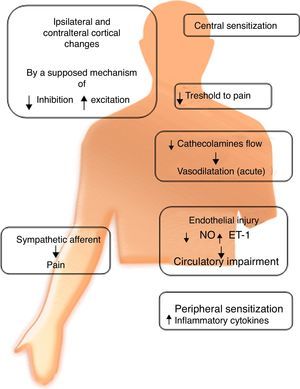
Autonomic dysfunctionRefers to an alteration of the sympathetic nervous system. It has been suggested that its degree depends on the stage in which the syndrome is found. This suggests the existence of inhibition of sympathetic vasoconstrictor neurons, expressing lower levels of norepinephrine in the affected limb compared to its counterpart. This triggers vasodilation, and chronicity of this condition allows vasoconstriction. This chronicity contributes to a redistribution of blood flow through arterioles, causing inadequate capillary nutrition, which results in hypoxemia and acidosis. These alterations can produce free radicals, which cause histopathological changes by oxidative stress.
There is evidence of an increase in the number of α-adrenergic receptors in the skin of patients with CRPS. Their activation would trigger an increase in noradrenaline release, which in turn produces hyperstimulation of nociceptive fibers causing pain and hyperalgesia, even in sympathectomized patients.
Cutaneous injection of norepinephrine induces pain via these adrenoreceptors in patients who respond to sympathetic blockade, whereas there is no reaction in patients who showed no response to the blockade. These data imply that CRPS may involve abnormal adrenoreceptors expressed in nociceptors which, when stimulated by circulating catecholamines, are activated and cause hyperalgesia and possibly alodinia.11
Another group of researchers found that in CRPS II, nerve damage causes an upward regulation of catecholamine receptors (Fig. 1).
Catecholamine levelsPlasma norepinephrine levels were lower in the affected limb compared to its healthy counterpart. However adrenaline levels were similar in both extremities.6
InflammationRecent studies suggest the existence of two different sources of inflammation: Acute, tissue damage mediated by classical inflammation mechanisms (IL-1, IL-6, TNF-alpha, CD4, macrophages, neutrophils) and neurogenic, mediated by proinflammatory cytokines and neuropeptides released directly by nociceptors in response to various causal factors.8
The following are related substances:
Not sympathetic neurotransmittersSubstance P, one of the principal pain mediators and a vasodilator, triggers mast cell degranulation and activates osteoblasts.
The calcitonin gene-related peptide (CGRP) is a vasodilator that plays a role in glandular secretion, is involved in sensory transmission, and stimulates endothelial cell growth. Bradykinin has also been associated.
Sympathetic neurotransmittersVasoactive intestinal peptide is located in the bones and promotes bone resorption, in addition to being a vasodilator and stimulating sweat gland secretion. Neuropeptide Y is a potent vasoconstrictor that enhances the effects of adrenaline. It is the most abundant peptide in the CNS and its periphery.6,8
The nociceptive stimulus not only causes an inflammatory response, but also a low pain threshold. Recent research has shown that some patients respond to treatment with immunoglobulins, and of those, most carry serum IgG autoantibodies directed against autonomic receptors.12
SensitizationPeripheral: After tissue injury, local primary afferent fibers release various substances, which sensitize nociceptive nerve endings to other substances such as histamine and bradykinin, contributing to the development of hyperalgesia and allodynia.8 Sympathetic denervation causes an increase in the sensitivity of blood vessels to catecholamines, produced by an increase in the number or sensitivity of adrenoceptors. This increase may be responsible for the decrease in blood flow to the skin in chronic conditions. It is hypothesized that sympathectomy causes sensitization in the long-term, which could explain why some patients have transient benefits.
Central: It has been found that N-methyl d-aspartate (NMDA) receptors play an important role in central sensitization; two controlled clinical trials showed that low doses of ketamine (an NMDA antagonist) dramatically reduce pain in patients with CRPS.13
Moreover, electrical stimulation of nociceptive mechanical-insensitive fibers (CMiHI), characterized by a large electrical excitation threshold and innervation of wide areas, can be the cause of secondary hyperalgesia. In studies, low-frequency stimulation caused mechanical hyperalgesia and hypoesthesia, whereas high frequency stimulation generated hypoalgesia and hypoesthesia. It was also found that pain itself can trigger inhibitory mechanisms which develop hypoalgesia; this type of pain relief is known as “counter irritation”.14
Microvascular pathology of soft tissuesThis hypothesis suggests an underlying cause in muscle, bone and perineural microvasculature that cuases ischemia and subsequent inflammation originating persistent abnormal pain, creating central sensitization. Coderre et al., in 2004, developed a mouse model that they called CPIP (chronic post-ischemia pain), which involved a period of ischemia-reperfusion produced by placing a tourniquet on the hind leg of a rat, then withdrawing it and recording their findings. They observed a reduction in the density of sensory fibers and capillaries, spontaneous afferent discharge, decreased blood flow, elevated malondialdehyde (free radical product of lipid peroxidation) then, when attenuated by antioxidants, there was a dose-dependent improvement in allodynia in the animal. They also observed an increase in the production of proinflammatory cytokines, an increase in lactate levels in the limb subjected to ischemia-reperfusion and hypersensitivity to norepinephrine; symptoms similar to some patients with CRPS type I.15
This steady state of inflammation due to partial or intermittent ischemia ended up causing endothelial dysfunction, which could explain the increase in constriction, tissue hypoxia, metabolic acidosis, and increased permeability to macromolecules. Chronic ischemia can also lead to a state of capillary “no-reflow” where the decrease in vessel lumen is not only functional, but also physical; this could also explain why some patients, after undergoing sympathetic blockade, do not improve.7
Central changesNeuroplasticity: Janin and Baron were the first to suggest a central origin in the pathophysiology of CRPS. It is currently known that chronic pain can create a change in the cortical representation of the affected area, in particular, the representation of the affected area or limb in the somatosensory cortex (S1) which is relatively small compared to the healthy limb.8
Spinal neurons may increase their sensitivity in response to nociceptive bombardment caused by autonomic changes. A reorganization of the primary somatosensory area can be generated in the supraspinal space, as in amputee patients, demonstrated by MRI; due to this, it is said that peripheral, spinal, and supraspinal nociceptive cortical processing scales are involved in the genesis of CRPS.5
It is recognized that this neuronal plasticity, induced by pain, causes hyperalgesia but it can also cause hypoalgesia and hypoesthesia. Several studies show that neuronal plasticity may be a decline effect in response to pain.14
Altered functional connectivity in the resting stateIn recent years, there have been several studies regarding an alteration in the interconnections of different brain regions in patients with CRPS. This is based on previous research of chronic pain that has demonstrated a spatiotemporal disruption in functional connectivity at rest, also known as DMN (default mode network), which shows an increase in diffuse interconnections, unlike control groups. These areas show a proportional correlation to the intensity of pain experienced by patients.16
Dysfunction in the motor cortexBecause pain can interfere with the processing of afferent signals that contribute to the sense of positioning, and the mental image of the affected limb is distorted in patients with CRPS, proprioception could be significantly affected. There are observations that these patients need to carefully look at their affected limb to control movements, making it possible for them to compensate.17 However, more and better studies are required to have consistent evidence, since the only significant fact that has been found is an area of spatial representation of S1 lower on the side of the affected limb, unlike its healthy counterpart or in control groups.18
Clinical manifestationsThe onset of clinical manifestations may be hours or even months after the noxious event, characteristically they includes a triad of autonomic, sensory and motor abnormalities.
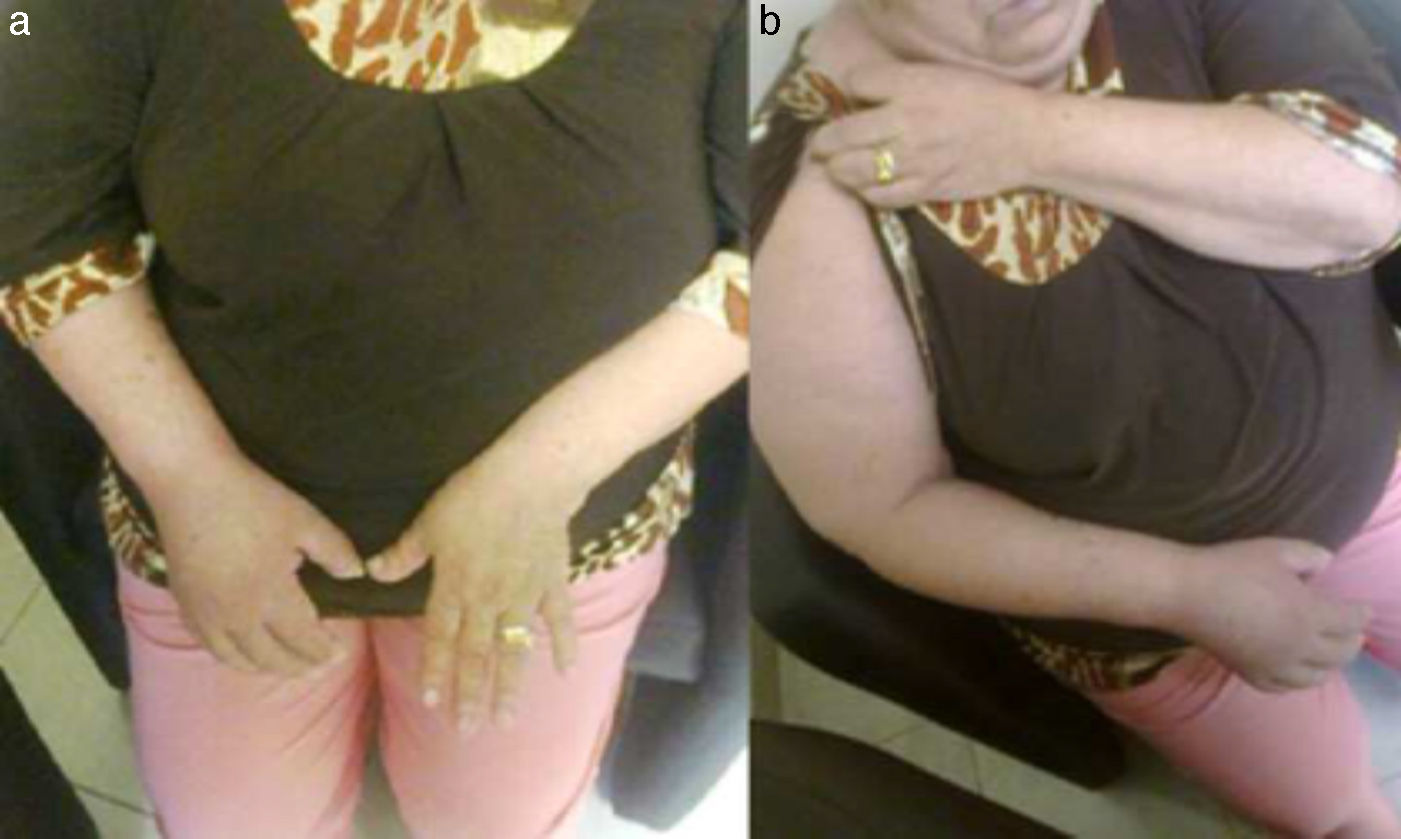
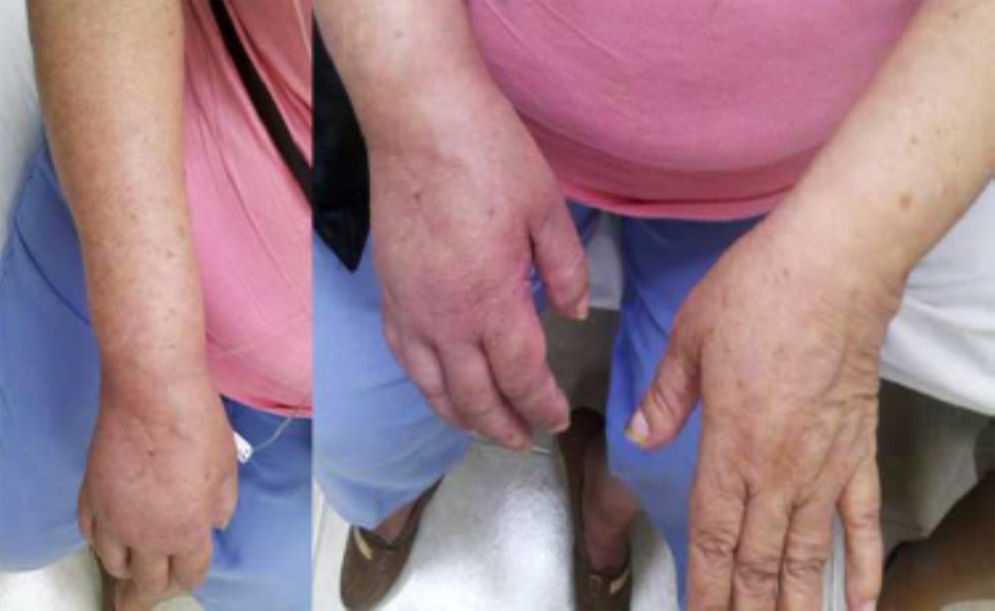
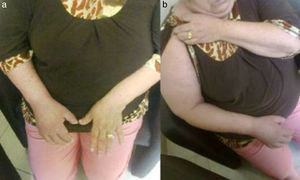
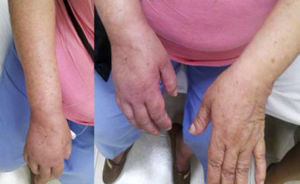
Autonomic alterationsAmong these alteration we can find early onset distal edema (in its soft and congestive form) in up to 80% of cases, as well as changes in skin color and temperature, which is reddish and hyperemic (≥1°C in comparison to the other limb), usually in the early stages; however, in 40% of cases it can progress with decreased skin temperature and pallor. Sudomotor phenomena, such as hypohidrosis or hyperhidrosis (the latter being the most common) are also seen; trophic changes, which can present as excessive hair growth, thin nails, and skin atrophy, evidenced by the appearance of “glowing” skin, thinning of the epidermis and muscle atrophy, as well as contractures, are also found. Finally, another alteration that is present in some cases is bone atrophy, which can be associated with osteoporosis (Figs. 2 and 3).
(a) Female patient with right upper limb CRPS affected before treatment. (b) Same patient with hyperemic, shiny bow and decreased range of motion secondary to the presence of edema, during initiation of treatment with infusion pump (Ropivacaine 2%/20 days22).
Female with upper member CRPS affected, posterior entitled to 20 days of completing treatment with infusion pump to the infraclavicular brachial plexus (Ropivacaine 2%22).
The main and most common symptom (90%) is pain, which is described as burning or stinging. It is usually felt as deep (68%) rather than superficial (32%) pain. It can be exacerbated after temperature changes, exercise or episodes of stress and/or anxiety, and there have been cases where it increases at night. Pain is often accompanied by other phenomena such as hyperalgesia and allodynia. Of these the most important is muscle weakness; other manifestations in this category are essential tremors in the affected limb, myoclonus and dystonia, which is most frequently observed in patients with type II CRPS.
It is important to remember that the manifestations may change depending on the location of the condition. Proximal and deep joints undergo a reduction in function.19 This differs from other neuropathic syndromes due to the presence of edema, vasomotor and sudomotor changes, in addition to an orthostatic component which is reduced in intensity when the limb is raised, and increased when it is held down.5 It was thought that CRPS could have a temporal progression of symptoms; however, this idea was rejected by the International Association for the Study of Pain (IASP).1
DiagnosisThere are no pathognomonic signs or symptoms and there is no definitive diagnostic test. Diagnosis is based on a complete medical history that includes the severity and duration of symptoms and signs, fracture type and severity of the injury and physical examination of the affected limb.20
The IASP published a review of the clinical diagnostic criteria in 2007 called the “Budapest Criteria”, which has a sensitivity of 85% and a specificity of 69% (Table 1). There must be regional pain that exceeds a dermatome or a single nerve territory, continuous or evoked pain of an intensity and/or duration disproportionate to the trauma or injury that may have caused it, and which is associated with a range of symptoms and signs of sensory, motor, vasomotor, sudomotor and trophic disturbances. Symptoms and signs can be variable depending on the time of evolution of the syndrome.4 There are other approaches to the diagnosis, such as the diagnostic criteria of Kozin (Table 2) and Veldman (Table 3).
IASP Diagnostic criteria for complex regional pain Syndrome (2007 Budapest criteria).
| 1. Continuous pain disproportionate to the event that caused it |
| 2. Symptoms (must meet at least one symptom in three of the four categories) |
| Sensory: hyperesthesia and/or allodynia |
| Vasomotor: asymmetry in skin temperature and/or asymmetry of skin color and/or changes in skin color |
| Sudomotor: edema and/or sweating changes and/or asymmetric sweating |
| Motor: decreased range of motion and/or motor dysfunction (tremor, dystonia, weakness) and/or trophic changes (skin, hair, nails) |
| 3. Signs (must meet at least one sign in two or more of the four categories) |
| Sensory: evidence of hyperalgesia (to puncture) and/or allodynia (touch/temperature/deep pressure/joint movement) |
| Vasomotor: asymmetry in skin temperature >1°C and/or asymmetry of skin color and/or changes in skin color |
| Sudomotor: evidence of edema and/or sweating changes and/or asymmetric sweating |
| Motor: evidence of decreased range of motion and/or motor dysfunction (tremor, dystonia, weakness) and/or trophic changes (skin, hair, nails) |
| 4. Rule out other conditions that may explain the previous symptoms and signs. |
Kozin diagnostic criteria.
| 1. Pain and tenderness of a limb |
| 2. Symptoms or signs of unsteadiness |
| Raynaud's Phenomenon |
| Cold, pale skin |
| Hot or erythematous skin |
| Hyperhidrosis |
| 3. Swelling of limb |
| Edema with or without fovea |
| 4. Trophic skin changes |
| Atrophy |
| Desquamation |
| Hypertrichosis |
| Hair loss |
| Nail changes |
| Thickening of the palmar aponeurosis |
Defined: meets 4 criteria; probable: meets criteria 1, 2, and 3; possible: meets criteria 1 and 2.
Veldman diagnostic criteria.
| 1 The presence of 4–5 of the following: |
| Unexplained diffuse pain |
| A difference in skin color in relation to another extremity. |
| Diffuse edema |
| A difference in skin temperature relative to another extremity. |
| Limited range of active motion. |
| 2. Occurrence or increase in the above signs and symptoms after use. |
| 3. The above signs and symptoms are present in a larger area than the primary area of injury or operation and includes the area distal to the primary lesion. |
An ill-defined subchondral heterogeneous “mottled” demineralization of varying intensity is observed with a sensitivity of 73% and a specificity of 57%, maintaining a regional character in later stages of the disease.20
GammagraphyThis study is recommended in stages I and II with a sensitivity of 97% and a specificity of 86%. Its main use is for the early diagnosis of CRPS. In the initial stages, intense and early bone hyper-uptake that exceeds the limits of the affected joint is seen.20
Magnetic resonance imagingThis method provides a differential diagnosis with osteonecrosis, especially of the hip. It also provides information about bone marrow edema, alteration of soft tissue, and the presence of joint effusion.6
Skin fluximetry by the laser doppler techniqueThis is one of the most precise techniques that are currently available for the early diagnosis of CRPS I. It provides information of changes in flow, volume and velocity of cutaneous microvascularity in CRPS I in stages I and II.
ThermographyAn increase in local temperature, especially in the first weeks of development, is found, although this is not a consistent finding (sensitivity 45%).21
Differential diagnosisThe differential diagnosis will depend on the stage of evolution. In the initial phase, infectious arthritis, rheumatic arthritis, inflammatory joint disease, peripheral arterial disease and deep vein thrombosis should be considered. In the chronic phase, Dupuytren's disease, scleroderma, and plantar fasciitis should be taken into account. In hip conditions, osteonecrosis and coxitis should be ruled out. If there is bone demineralization, it would be recommended to rule out osteoporosis and bone tumors (Table 4).20
TreatmentEarly treatment is necessary to achieve complete recovery and prevent damage. Treatment of CRPS requires a multidisciplinary approach to pain management which is also aimed at functional recovery of the affected limb.
PharmacotherapyNonsteroidal antiinflammatory drugs (NSAIDs), corticosteroids, cyclooxygenase (COX) 2 inhibitors and free radical scavengers are used with the intention of treating pain in addition to the inflammatory process in CRPS. However, inflammation in CRPS may be largely neurogenic (initiated by inflammatory mediators of the terminals of afferent nociceptors) and no drug has been studied for this type of inflammation.22
NSAIDs represent first-line treatment, especially in the early stages and at non-specialized units, although their efficacy specifically for CRPS is unproven, and they are not prescribed in the treatment of neuropathic pain. In initial phases they can be prescribed for treatment of inflammation.23
Oral corticosteroids are the only anti-inflammatory drugs for which there is direct evidence from CRPS clinical trials (evidence level 1). Most trials included acute cases, when inflammation is more common, and it is unknown if corticosteroids offer a similar benefit for chronic CRPS, where levels of proinflammatory cytokines are lower, or in CRPS cases with only mild inflammation. A short course of steroids may be indicated early in CRPS with prominent inflammation, but in longer courses there are contraindications to chronic use of steroids.22
Both minor (tramadol) and major (morphine, oxycodone, fentanyl, hydromorphone, buprenorphine) opioids have their place in moderate-severe pain that is difficult to control, and they have demonstrated efficacy in neuropathic pain.4
Antiepileptics (gabapentin) and tricyclic antidepressants (amitriptyline) and pregabalin have been used as adjuvants in the treatment of CRPS. There are no controlled trials of CRPS type I with these drugs, but their efficacy has been demonstrated in the treatment of neuropathic pain. These act by inhibiting pain pathways and neuronal plasticity, and are prescribed in acute phases with nerve injury, nerve ectopic activity or chronic phases.4
Free radical scavengers have shown some efficacy in the prevention and treatment of CRPS, although in acute phases and with moderate involvement. Vitamin C at doses of 500mg/day and N-acetyl cysteine at 600mg/day have shown efficacy in the prevention of CRPS in patients who have suffered wrist fractures. Dimethyl sulfoxide 50% cream seems to reduce pain and inflammation of the limb in the acute phase.4
There is evidence that several bisphosphonates have an acceptable safety profile and can significantly relieve spontaneous pain and improve the functional status of patients with an early disease (duration of 6 months) and with an abnormal uptake on the 3-phase bone scan. There are indications that the doses necessary to achieve long-term remission are very high, i.e., neridronate at 100mg or pamidronate at 90mg, each given via IV four times over a period of 10 days. Bisphosphonates have analgesic properties that go beyond their effect on bone metabolism, and preclinical data suggest that they have antinociceptive effects in animal models of neuropathic pain. Therefore, their efficacy cannot be limited to patients with CRPS-related bone pain, but relevant clinical data are not yet available.9
The recent use of ketamine, a potent agonist of N-methyl-d-aspartate (NMDA), in the management of CRPS-I, is due to the phenomenon of central sensitization. This central sensitization is expressed mainly in the first relay of nociceptive information integration, where the synapses formed by the central ends of the Aδ and C fiber nociceptors to nociceptive neurons in the dorsal horn of the spinal cord are very active. Several ketamine dosing schemes have been tested, from transcutaneous application to coma induced by ketamine. Although lower doses seem to provide the best results, the lack of regulatory approval for this indication and various side effects limit the use of ketamine in current practice.8
Topical anesthetics such as lidocaine cream or transdermal absorption patches at 5%, can be suitable in cases of allodynia and/or hyperalgesia.22
Interventional techniquesSympathetic nerve block is a treatment option for patients who are refractory to pharmacological treatments, especially when performed early in the course of the disease.24 Nerve blockage improves short-term pain and joint mobility and its effectiveness is greater when performed in the early stages of the disease, although there are few reliable studies and a few controlled studies that have failed to demonstrate long-term efficacy. Blockage provides a pain-free period that improves limb mobility, allowing performance of intensive physiotherapy, especially when using continuous techniques, such as local anesthetic infusion via an auxiliary or lumbar epidural catheter.4
Some uncontrolled studies have shown initial improvement in pain after percutaneous sympathectomy, but there is a high risk of recurrence between 6 months and 2 years, with neuralgia and post-sympathectomy pain (up to 10%.).9
Placement of a cervical or lumbar spinal epidural neurostimulator for posterior column spinal cord stimulation may be an option in severe disabling pain in CRPS cases that have not responded to other treatments. A study of 36 cases showed efficacy in reducing pain and improving quality of life in both the short and long term.4
A retrospective study found that intramuscular injection of botulinum toxin A (BTXA) in waist muscles of the upper limb was beneficial for short-term relief of pain caused by CRPS. There was a 43% reduction in local pain scores 4 weeks after intramuscular injections of BTXA. Studies that prove its efficacy and support its use in the long-term treatment of CRPS are still lacking.25
RehabilitationEarly rehabilitation is essential to try to prevent muscle atrophy and contractures, which in extreme cases can be irreversible, although it requires the active participation of the patient and this is not always possible due to severe pain and associated psychological disorders.
Physical therapy can reduce pain and improve limb mobility, although the intensity of treatment varies depending on the severity of the syndrome. Lymphatic drainage can improve edema. Transcutaneous electrical stimulation (TENS) may improve pain, although its use is not recommended in patients with severe allodynia or hyperalgesia. Occupational therapy can also enhance limb function and coordination.
Some studies suggest that mirror therapy may have a role in the treatment of CRPS. The patient performs the exercises in front of a mirror, perpendicular to the midline, which only allows him/her to observe the unaffected limb during treatment; this creates a sense of normality of the affected limb, probably due to activation of the frontal cortex, and pain relief, especially in acute phases of the disease.26
ConclusionAlthough the exact causes of CRPS have not yet been discovered, the progress made in recent years in the understanding of the pathophysiological mechanisms involved in the disease allow us to foresee new treatment options targeting the etiology. Understanding the etiological factors will lead to an early diagnosis and a better implementation of treatment.
Conflict of interestThe authors have no conflicts of interest to declare.