High-impact viral infections have become more relevant in recent years, with arthropods as a vector of particular interest. Among these, the most important are those caused by the Dengue virus (DENV), the Chikungunya virus (CHIKV) and the Zika virus (ZIKV). The objective of this review is to make known the immune response of the organism to these infections. When clinical characteristics are similar to each other, it is difficult to differentiate them clinically, which is why the role of the laboratory plays a crucial role in its diagnosis. At present, there is no specific treatment for these infections, so the role of health services should be focused on prevention campaigns, with critical importance placed on the reduction and control of new cases.
In the last decade, high-impact viral infectious have become more relevant, especially those transmitted by arthropods to humans, the most important of these infections being DENV, CHIKV, and ZIKV, the latter presenting a rise in fetal mortality.1 These viruses share transmission through the same vectors, these being Aedes aegypti or Aedes albopictus.2 Infection by the DENV virus is caused by one of these four serological types: DENV-1, DENV-2, DENV-3, and DENV-4.3 It is usually asymptomatic, but can manifest a wide range of clinical manifestations. Today, estimations suggest that there are 390 million infections by the DENV virus a year worldwide, significantly impacting public health programs across the world.4 In the case of CHIKV, its name derives from an African language and means “the one who folds” because of its manifested clinical characteristics. Among the multiple symptoms presented by the CHIKV virus infection, joint and muscle pain are particularly relevant.
The ZIKV virus kindles a particular interest due to the alterations caused by the products of infected mothers.1 Currently, there is an outbreak caused by this infection in America, the Caribbean, and the Pacific, and it had been declared a public health emergency of international interest by the WHO. Clinical manifestations caused by this virus are seen in approximately 20% of infected patients, with various signs and symptoms.6
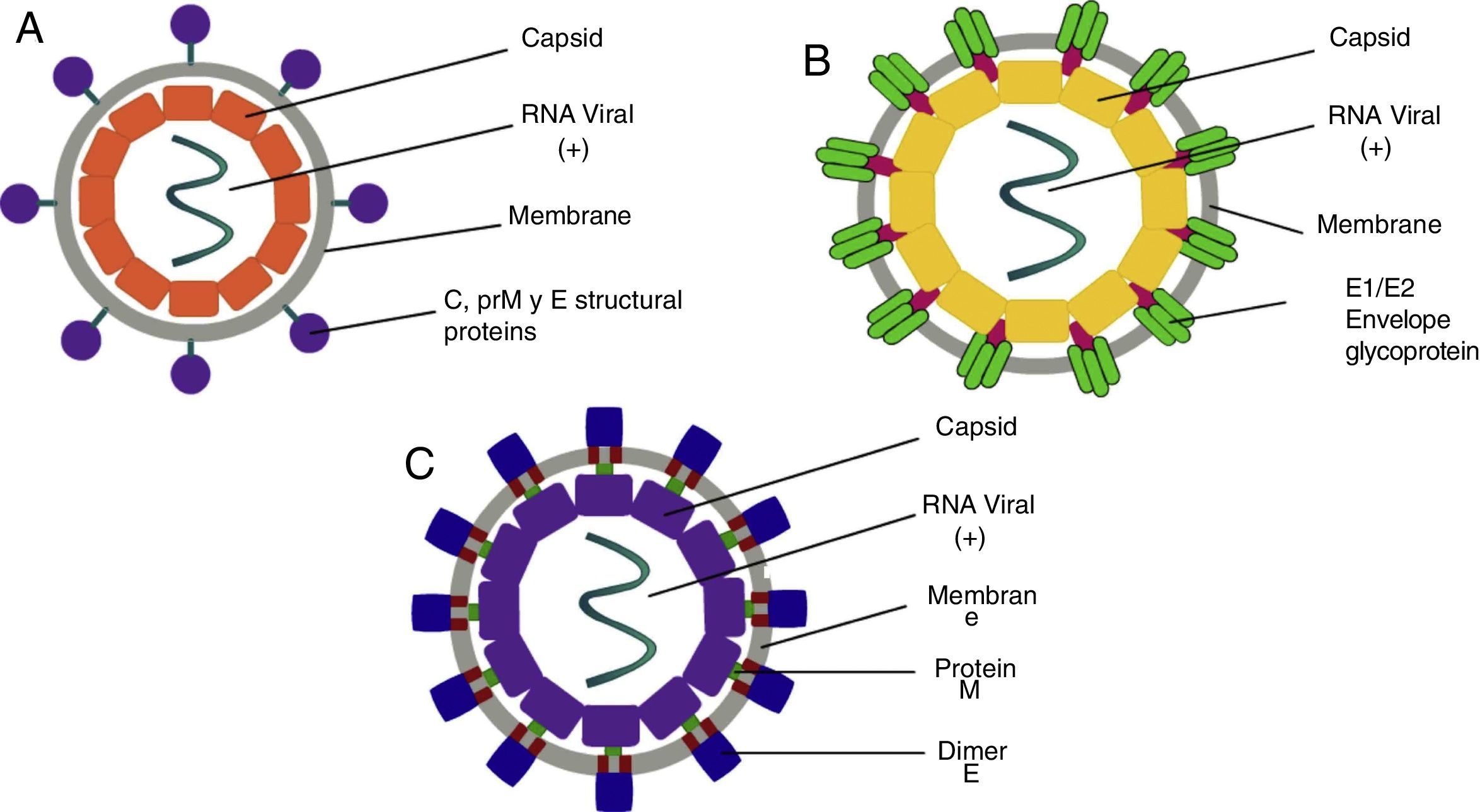
ClassificationThe word Arbovirus is an acronym of Arthropod-Borne Viruses, with particular interest attached to the Flaviviridae family, of the Flavivirus genre, where the DENV and ZIKV viruses belong, while the CHIKV virus is classified within the Togaviridae family, of the Alphavirus genre.7 The DENV virus is of the positive-stranded RNA type, with four serotypes, which are composed of a genome of 11,000bp (base pairs), coded for seven non-structural proteins and three structural proteins.8 On the other hand, the CHIKV is a positive-stranded RNA type virus with 11,600bp, which codifies four non-structural and three structural proteins.9 Lastly, the ZIKV virus consists of an RNA positive-strand with a genome of 10,749bp, all distributed in a polyprotein which is coded for three structural and seven non-structural proteins, giving them their biological characteristics.10
EpidemiologyDengue (DENV)Currently, there are about 2.5 billion people (40% of the world's population) living in hazardous areas at risk of becoming infected by dengue.4 This infection is endemic in at least 100 countries in Asia, the Pacific, America, Africa, and the Caribbean. The World Health Organization estimates that between 50 and 100 new cases are reported each year.4 In Mexico, there were 17,795 confirmed cases reported in 2016 (including dengue with and without severe or significant alert symptoms),11 and there have been 541 confirmed cases in the first ten weeks of 2017; out of these 541, 76 are from Nuevo Leon.12
Chikungunya (CHIKV)Up until 2013, there had been outbreaks identified in Africa, Asia, Europe and the Pacific and Indian oceans.5 By the end of that same year, the first infection by local transmission in America was identified in the Caribbean countries, spreading to 45 countries all throughout the continent.13 In Mexico, there were 168 registered confirmed cases during 2016, and eight confirmed cases up to week 9 of 2017, from which only one case was reported in our state, Nuevo Leon.14
Zika (ZIKV)Initially identified in Africa during the 1940s-1950s with few reports of infection in humans up until recent years. In the year 2007, an outbreak was detected in the Pacific's Yap island, in Micronesia, resulting in the infection of 70% of their population in 3 years. Between 2013 and 2014, there were reports of 4 countries in the Pacific islands where outbreaks of this infection were detected. By the year 2015, the arrival of the Zika virus to the Americas was confirmed by the Brazilian Health Ministry.15 This led to a dissemination throughout the entire continent and was linked to an increase in the number of newborns with microcephalia, as well as an increase in cases of Guillian-Barré syndrome. In Mexico, there are 8033 confirmed cases of ZIKV, and 40,607 confirmed autoctonous ZIKV cases in pregnant women between 2015 and 2016. During the year 2017, by epidemiological week 9, there have been 94 confirmed cases reported, and 43 confirmed autoctonous ZIKV cases in pregnant women, 6 of which are in Nuevo Leon.16
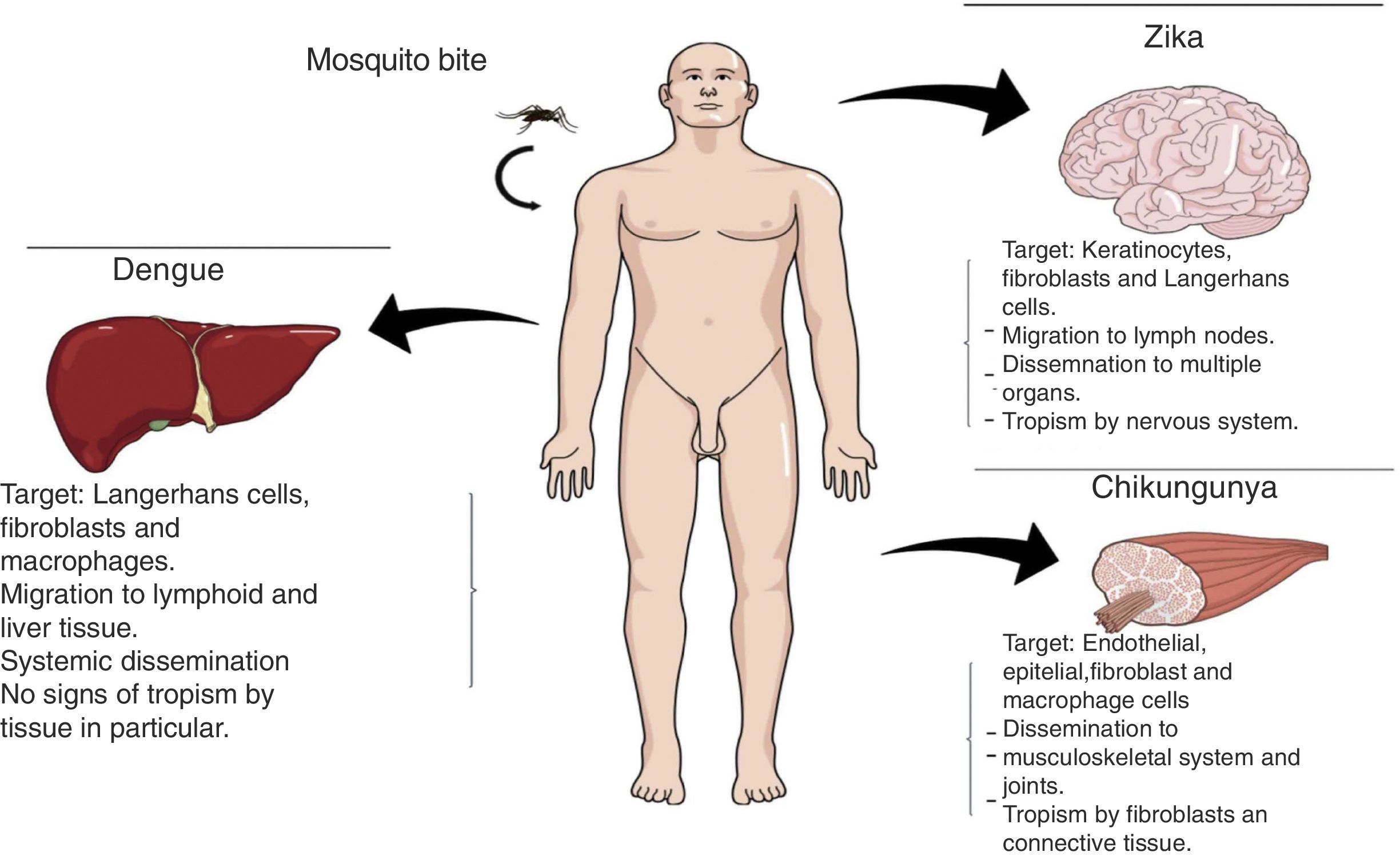
PathophysiologyDengue (DENV)The DENV virus contains a single positive-stranded RNA. For the incorporation of the virions to the cells to occur, the interaction of the primary glycoprotein (E) with the receptors on the cellular surface is critical; these include heparan sulfate or lectins (DC SIGN and GLEC5A). Moreover, they can also bind to immunoglobin-type surface receptors in the presence of antibodies against glycoprotein-E or membrane precursor protein (pre-M)8 (Fig. 1). After the fusion of the viral and cell membranes through acidified endocytic vesicles, the viral RNA enters the cytoplasm along the other viral proteins. Non-structural NS5 proteins are the polymerase of RNA dependent of RNA, which fits viral proteins as well as cell proteins to form the replication complex, transcribing viral RNA to produce the RNA template, beginning the replication of viral genetic material. The DENV virus enters the organism through a mosquito bite, infecting the Langerhans cells and fibroblasts.17 The viremia starts three days after the vector bite, is detectable 6–18hours before the onset of the signs and symptoms, and concludes at the moment of solving the fever.18 The immunological response of the organism to the virus is complex, with elevated levels of interferon (IFN) α and β, which are part of the type-I IFN group contributing to the elimination of viral particles. However, viral proteins are capable of inhibiting the production of interferons as well as the infected cells’ antiviral function.19 The antibody answer is mainly directed at specific determinants of each serotype, from these, the primary targets are protein E, membrane precursor protein (pre-M) and NS1 protein. When there is a primary infection with one of the four serotypes of the DENV virus, it will generally provide long-term immunity to infections with viruses of the same serotype. Nevertheless, it does not work for a virus of a different serotype, which may result in a secondary infection.
Once an immunological answer is assembled, this will cause most of the symptoms in the patient, hence the presence of the capillary leak syndrome, which is caused by the actions of FNT-α IL-8, IFN-γ, IL-2, and some fractions of the serum complement like C3a and C5a. Thus, the intensity of the immunological response will be determined by the magnitude of the viral infection.20,21
Chikungunya (CHIKV)It is a single-strand RNA virus composed of 11,600 nucleotides coding four nonstructural proteins (nsP1-nsP4) and three structural proteins (C, E1, E3). The latter are the capsid and two envelope glycoproteins (E1 and E2)22 (Fig. 1). This virus replicates in multiple adherent cells, including endothelial and epithelial cells, fibroblasts and macrophages. After the mosquito bites, the virus will infect the cell needing an endocytic pat dependent on pH.23 Its replication is inhibited by IFN-I and II, which are controlled by the innate immunological response, thus contributing to this rapid decrease of its viremia during the acute phase of the disease. After infection, the fibroblasts will synthesize IFN-α and IFN-β to try to contain the virus. Usually, during the acute phase, the virus occurs in skeletal muscle and joints, causing symptoms. Also, during this phase, we are able to observe the elevation of IL-6, IL-1β, MIG, IP-10 y MIG.22,24,25 The virus presents tropisms by fibroblasts, myocytes, and hepatocytes.
Zika (ZIKV)ZIKV carries a compound genome made up of a single-strand RNA with a size of 10,749 nucleotides, which codify 3419 amino acids. It contains two non-codifying regions (5′ AND 3′ NCR), and a capsid, in addition to a membrane precursor protein, envelope, and nonstructural proteins15 (Fig. 1). E protein is a surface protein which is involved in receptor recognition and membrane fusion, and its domain III contains an antigenic epitope panel which is the target of serological essays, neutralizing antibodies and vaccines. It has been proposed that the loss of the E protein at the N154 glycosylation site is linked with the adaptation of the mosquito, thus facilitating its transmission.26 When an infected mosquito bites an infected patient, it ingests blood containing the Zika virus, which is replicated in the mosquito's intestinal epithelial cells and subsequently in the salivary gland cells. After 5 to 10 days, this can be found in the mosquito's saliva, infecting humans. When it comes in contact with the skin, the virus can infect keratinocytes, fibroblasts and Langerhans cells, which contain AXL, Tyro3, TIM-1 (keratinocytes and fibroblasts) and DC-SIGN (Langerhans) cells, which are possible receptors of the virus.27 The infection of fibroblasts on the skin is linked with the regulation of the TLR3 mRNA expression and RIG-I and MDA transcription, which are innate immunological answers that the organism activates in response to the infection caused by the virus. This is followed by a rise in IFN-α and IFN-β expressions, both linked to the suppression of intracellular viral particles.28 Also, the replication of this virus by inducing autophagia in the infected cells has been proposed. Following replication of the virus at a local level, the viral particles will disseminate amongst the lymphatic region and other organs, including the central nervous system, myocardial and musculoskeletal system, and the placenta in pregnant women.
This virus presents tropism by cells of the nervous system, particularly neuronal progenitor cells, altering proliferation, migration, and differentiation, creating as a consequence a loss in cerebral development and alternating its viability, which can partially explain the microcephalia and associated neurological complications.29–32
Transmission methodsInfections by DENV, CHIKV, and ZIKV can occur through multiple paths of transmission, the most important being the mosquito bite. Once the virus has entered the organism, it causes tissue tropism (Fig. 2).
The infection transmission through these viruses is mainly caused by:
- •
Mosquito bite: The primary vector is Aedes aegypti, which feeds itself during the day, transmitting the virus. This vector is found in tropical and subtropical areas (latitude: 45° north to 35° south), presenting a wide distribution.33 Another vector is Aedes albopictus, which is more tolerant to cold conditions and presents a global distribution much broader than Aedes aegypti. However, its bite is less frequent in humans.
- •
Nosocomial transmission: this section includes transmission by blood components, by accidents with sharp objects and splatters in mucocutaneous areas. However, these last two mentioned do not present evidence of an efficient transmission through these pathways and has only been documented as an anecdote.
- •
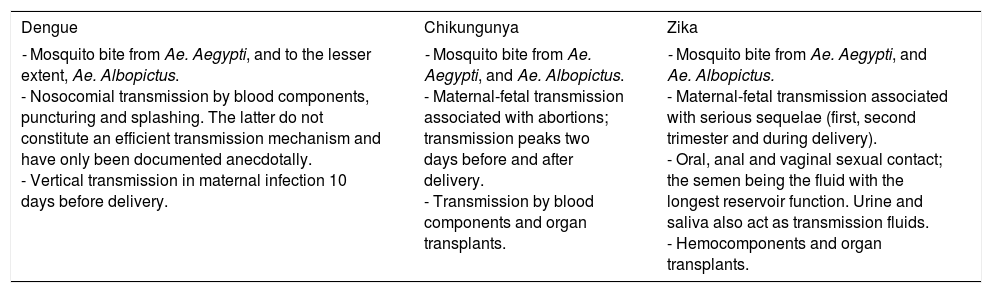
Vertical transmission: Depending on the virus, this is the preferred method of transmission (Table 1).
Modes of DENV, CHIKV and ZIKV transmission.
| Dengue | Chikungunya | Zika |
|---|---|---|
| - Mosquito bite from Ae. Aegypti, and to the lesser extent, Ae. Albopictus. - Nosocomial transmission by blood components, puncturing and splashing. The latter do not constitute an efficient transmission mechanism and have only been documented anecdotally. - Vertical transmission in maternal infection 10 days before delivery. | - Mosquito bite from Ae. Aegypti, and Ae. Albopictus. - Maternal-fetal transmission associated with abortions; transmission peaks two days before and after delivery. - Transmission by blood components and organ transplants. | - Mosquito bite from Ae. Aegypti, and Ae. Albopictus. - Maternal-fetal transmission associated with serious sequelae (first, second trimester and during delivery). - Oral, anal and vaginal sexual contact; the semen being the fluid with the longest reservoir function. Urine and saliva also act as transmission fluids. - Hemocomponents and organ transplants. |
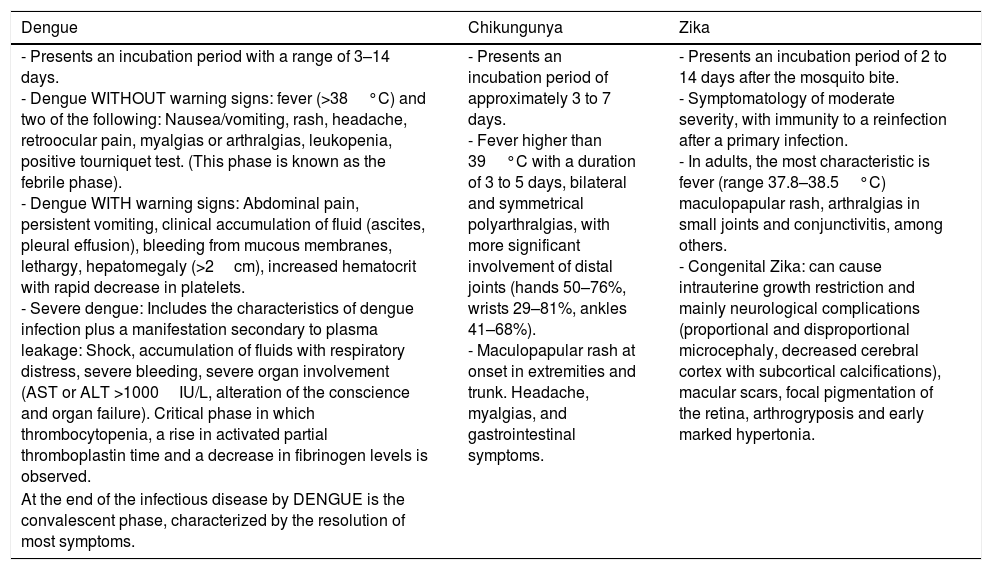
Clinical characteristics caused by these viruses tend to be similar. Nevertheless, we ought to keep in mind the signs and symptoms which predominate in each one, directing us to the proper diagnosis (Table 2).
Clinical manifestations of DENV, CHIKV, and ZIKV.
| Dengue | Chikungunya | Zika |
|---|---|---|
| - Presents an incubation period with a range of 3–14 days. - Dengue WITHOUT warning signs: fever (>38°C) and two of the following: Nausea/vomiting, rash, headache, retroocular pain, myalgias or arthralgias, leukopenia, positive tourniquet test. (This phase is known as the febrile phase). - Dengue WITH warning signs: Abdominal pain, persistent vomiting, clinical accumulation of fluid (ascites, pleural effusion), bleeding from mucous membranes, lethargy, hepatomegaly (>2cm), increased hematocrit with rapid decrease in platelets. - Severe dengue: Includes the characteristics of dengue infection plus a manifestation secondary to plasma leakage: Shock, accumulation of fluids with respiratory distress, severe bleeding, severe organ involvement (AST or ALT >1000IU/L, alteration of the conscience and organ failure). Critical phase in which thrombocytopenia, a rise in activated partial thromboplastin time and a decrease in fibrinogen levels is observed. | - Presents an incubation period of approximately 3 to 7 days. - Fever higher than 39°C with a duration of 3 to 5 days, bilateral and symmetrical polyarthralgias, with more significant involvement of distal joints (hands 50–76%, wrists 29–81%, ankles 41–68%). - Maculopapular rash at onset in extremities and trunk. Headache, myalgias, and gastrointestinal symptoms. | - Presents an incubation period of 2 to 14 days after the mosquito bite. - Symptomatology of moderate severity, with immunity to a reinfection after a primary infection. - In adults, the most characteristic is fever (range 37.8–38.5°C) maculopapular rash, arthralgias in small joints and conjunctivitis, among others. - Congenital Zika: can cause intrauterine growth restriction and mainly neurological complications (proportional and disproportional microcephaly, decreased cerebral cortex with subcortical calcifications), macular scars, focal pigmentation of the retina, arthrogryposis and early marked hypertonia. |
| At the end of the infectious disease by DENGUE is the convalescent phase, characterized by the resolution of most symptoms. |
- •
Innate response: After the insect bite, the virus infects the target cells (Langerhans cells, interstitial dendritic cells, and mononuclear phagocytic cells)19,35 thanks to the non-integrin adhesion molecule-3 (DC-SIGN)36 and the mannose receptor (CD206),37 allowing Rab5-mediated endocytosis. Pattern recognition receptors (PRRs) in these cells are responsible for detecting viral particles or nucleic acids. Most immunocomplexes formed by antibodies and antigens clear from circulation through the reticuloendothelial system.38,39 Moreover, viral antigens expressed on the surface of infected cells may be recognized by NK cells and eliminated through cellular cytotoxicity. Subsequently, a viral replication occurs in the spleen, lymph nodes, bone marrow and muscle and from there, disseminate into systemic circulation.
- •
Cellular immune response (T lymphocytes): Infection by DENV of the dendritic cells stimulates T lymphocytes CD4+ and T CD8+, producing pro-inflammatory cytokines [TNF-α (Tumor Necrosis Factor Alpha) and IFN-γ (Interferon-gamma)], that is, in the case of the Th1 cells, the production of IFN-γ, TNF-α, IL-2 (Interleucina-2) and CCL4 (C-C Motif Chemokine Ligand 4) and for Th2 cells, the liberation of IL-4 and IL-13.40–42 A more significant activity of the T CD8+ cells during secondary infection has been proven,43 as well as crossed recognition in the case of severe dengue with a decrease of cytolytic and cytotoxic activity without altering the production of cytokines.41 During secondary infection, a response mediated by specific T CD8+ cells occurs, which is characterized by the activation of markers, active proliferation, high apoptosis levels and low avidity by the virus causative of the primoinfection. A response of the T cells which correlates with the severity of the disease has been observed.
- •
Humoral immune response (Lymphocytes B/antibodies): An early response has been observed to be mediated by IgM, subsequently, a response by IgG (predominated by the IgG1 and IgG3 subclasses) occurs, recognizing antigens E, NS3 and NS5 in the primoinfection by DENV, while there is a broader recognition in reinfection (E, C, prM, NS1, NS3, and NS5), presenting a more significant response against E protein.44 Lymphocytes B activity is stimulated during secondary infection as a result of the memory generated in primoinfection. In the case of heterotopic infection by DENV, high amounts of antibodies are produced, enhancing the infection rather than having a neutralizing effect on the virus. This response is called antibody-dependent enhancement (ADE), which acts by connecting viruses with their target cells, mainly monocytes, and macrophages, through receptors to the Fc portion of antibodies (FcyR). FcyRIa and FcyRIIa are the mediators of this ineffectiveness by DENV, which bind IgG.45 Non-neutralizing heterological antibodies recognize viral epitopes and stimulate the dependent ineffectiveness of Fc.46,47
- •
Chemokines: Chemokine receptors are expressed on the leukocyte surface, are linked to G protein and contain seven transmembrane domains.48 CC-type chemokines play an important role in the recruitment of leukocytes, with a greater importance placed on CCL4/MIP-1b and CCL3/MIP-1a, both ligands to the CCR1 receptor, and CCL2/MCP-1 for the ligand CCR2. The CCR1 receptor has been described to not play a significant role in the pathogenesis of DENV. Moreover, the relevance of the CCR4 receptor has been observed in hepatic damage and inflammation.
- •
Other immunoregulator factors: Immunoregulation of the organism facing infection by DENV occurs through the response of mediated signaling by STAT1 and STAT2. In the case of platelets, we observed the increased IL-1b expression after DENV exposure, freeing microparticles through mechanisms dependent on NLRP3 and IL-1B, a secretion dependent on caspase-1 by the platelets.49
- •
Interleukin profiles as a response to viral infection: Induction of Th17 cells is through TCR activation in the presence of cytokines, which activate STAT3, including IL-6, IL-21, and IL-23. Its polarization is characterized by the expression of the CC6 chemokines receptor and its Cc120 ligand, producing IL-17a, IL-17F, IL-21, and IL-22. The receptor of the IL-22 is expressed in non-hematopoietic cells, which helps regulation mediation by IL-22 of the response of local tissue during the infection and/or inflammation, having a protective and regenerative effect.50,51 On the other hand, IL-17 has an inflammation-inductor effect.52 Elevated levels of TNF-α, IL-6, IL-8, CCL2, CCL3, CXCL10 and IFN-γ have been proven in primary and secondary infections by DENV in humans,53 in addition to reporting protective activity by IFN-γ, which stimulates cellular resistance to primary infection by DENV, controlling nitric oxide synthase two production.54 In the case of TNF-α, it has been linked to a rise in its production during episodes of severe dengue.19 Inflammatory mediators act on the endothelium, altering its permeability, resulting in the extravasation of fluid to extracellular space.
- •
TLRs: Toll-like receptors and the cytoplasmic receptor of the RNA helicase box DExD/H family (Gen 5 associated with the differentiation of melanoma (MDA5) are the two most important sensor families present in the cells of mammals to detect nucleic acids, which, once they recognize the virus, activate two critical families of transcriptional factors: Interferon regulating factors and NF-kB, both producing IFN-α/β and inflammatory cytokines. TLR3 has been observed to recognize and restrict DENV replication in multiple cellular lineages, just like TLR7 has been linked to induced production of IFN-1 by DENV in dendric cells.55
- •
Original antigenic sin: The immune system's inability to gather a response against the same microorganism, but with antigenic variations. In the case of reinfection by DENV, it always involves different viral serotypes, triggering a different immunological response to the primoinfection, altering B and T cell reactivation, not having an optimal avidity for the viral epitopes corresponding to the new infection.47,56,57
- •
Innate response: The main target cells for CHIKV are fibroblasts, endothelial cells, monocytes, and macrophages.25 The association of MCP-1 with the acute phase of the disease has been observed, attracting monocytes to the inflammation site, thus infecting them. During the acute phase of the infection by CHIKV, we are able to observe a response of INF-α produced by leukocytes, causing a protective effect, as well as a response of IFN-β, produced by fibroblasts. The production of type-I IFNs is stimulated by the pattern-recognition receptors (PRRs)58,59 detecting molecular patterns linked to pathogens (PAMPS). The organism's first line of defense against infection by CHIKV are natural killer cells (NK), activating the CD94/NKG2C receptor and producing a cytotoxic effect60 as well as T lymphocyte recruitment through the production of Th1-dependent cytokines. The role of monocytes and macrophages seem to be that of a viral reservoir, in addition to participating in the control of apoptosis and signaling by IFN-α.61
- •
LT response: There is a decrease in T cell count during acute infection by CHIKV.60 The response by CD8+ occurs during the first stages of the infection, while infection by CD4+ is manifested during the late stages. However, there is a conversion during the final phases of the infection, presenting a response by IFN-γ mediated by CD8+.62
- •
LB response: Anti-CHIKV antibodies are capable of promoting viral dissemination protection and control through direct neutralization and activation of the complement. Anti-CHIKV antibodies of the IgM and IgG type have been detected, the latter with persistent titles for years. Neutralizing antibodies are predominated by the IgG3 subclass.
- •
Interleukins: IL-12 elevation has been reported during the acute phase, stimulating NK cell activity, as well as the elevation of IL-1β, IL-6, and TNF-α. Type-I IFN production is carried out by infected fibroblasts,63 which is regulated by CARDIF, having a regulating effect on MDA5 and RIG-I.
- •
Interleukin profiles as a response to viral infection: The response to Th1 releases pro-inflammatory cytokines (TNF-α, IL-1B and IL-8), which are poorly expressed in the acute phase of the infection, while the response to Th2 releases cytokines (Il-4, IL-6, IL-10) in a few patients.
- •
TLRs: Two types of PRRs have been associated with the recognition of viral PAMPs; TLRs and RIG-I receptors
- •
Innate response: The virus infects white cells, fibroblasts, keratinocytes and immature dendritic cells, and enters thanks to adhesion factors DC-SIGN, AXL, Tyro, and TIM-1. When presented with a ZIKV infection, fibroblasts express pathogen recognition receptors (PPR's), along with TLR3, RIG-I, and MDA-5, which triggers the expression of IFN type I, including OAS2, ISG15, MX1, and inflammatory chemokines.27 It seems that both groups of IFN (type I and type II) have regulatory effects on the virus, and their replication in fibroblasts is inhibited.
- •
LT response: This response is activated during the acute phase of infection by ZIKV (Th1, Th2, Th9, and Th17),64 marking an increase in the production of cytokines and pro-inflammatory factors.
- •
LB response: The role of B cells is critical for disease control when releasing IgG, IgM and neutralizing antibodies, providing partial protection against infection.
- •
Interleukins: In the acute phase, elevation of IL-1α, IL-2, IL-4, IL-6, IL-9, IL-10, IL-13, and IL-17, with a decrease in IL-8, have been observed.28,64 In the recovery phase, IL-1α, IL-8 and IL-10 rise even more than in the acute phase. No “cytokine storm” effect has been observed, unlike infection by other arboviruses.
- •
Effects on the fetus and placenta: ZIKV has been isolated from fetal nervous tissue. Also, IgM has been detected in the CSF of neonates, with microcephaly compatible with congenital infection by ZIKV.65,68,70 The vertical transmission of ZIKV has been observed, as has its effects on neurological development, presenting radial glial cells as a possible white cell.29 There is also a probable inhibitory effect on the proliferation and differentiation of cortical neural progenitor cells, as well as alteration in the transition from glial radial cells to intermediate progenitor cells, as well as a cytopathic effect activating caspase-3. In the case of the placenta, the replication of ZIKV is inefficient in primary trophoblasts of placentas in terminal phases of pregnancy, its main defense mechanism being the release of interferons. In vitro, the production of IFN type III by syncytiotrophoblasts has been observed.26,66 We have a theory of ZIKV access by placenta, evading the IFN type I and III response through the degradation of STAT2 by NS5.64,65
- •
Interleukin profiles in response to viral infection: They are associated with the profile Th1 (Il-2 and IFN-□), Th2 (IL-4, IL-13), Th17 (IL-17) and Th9 (IL-9).26 However, a large part of the immunoregulatory unchained mechanisms are not known.
- •
TLRs: The Zika virus activates TLR2 in embryonic cells, causing the deregulation of genes involved in neurogenesis and apoptotic pathways.
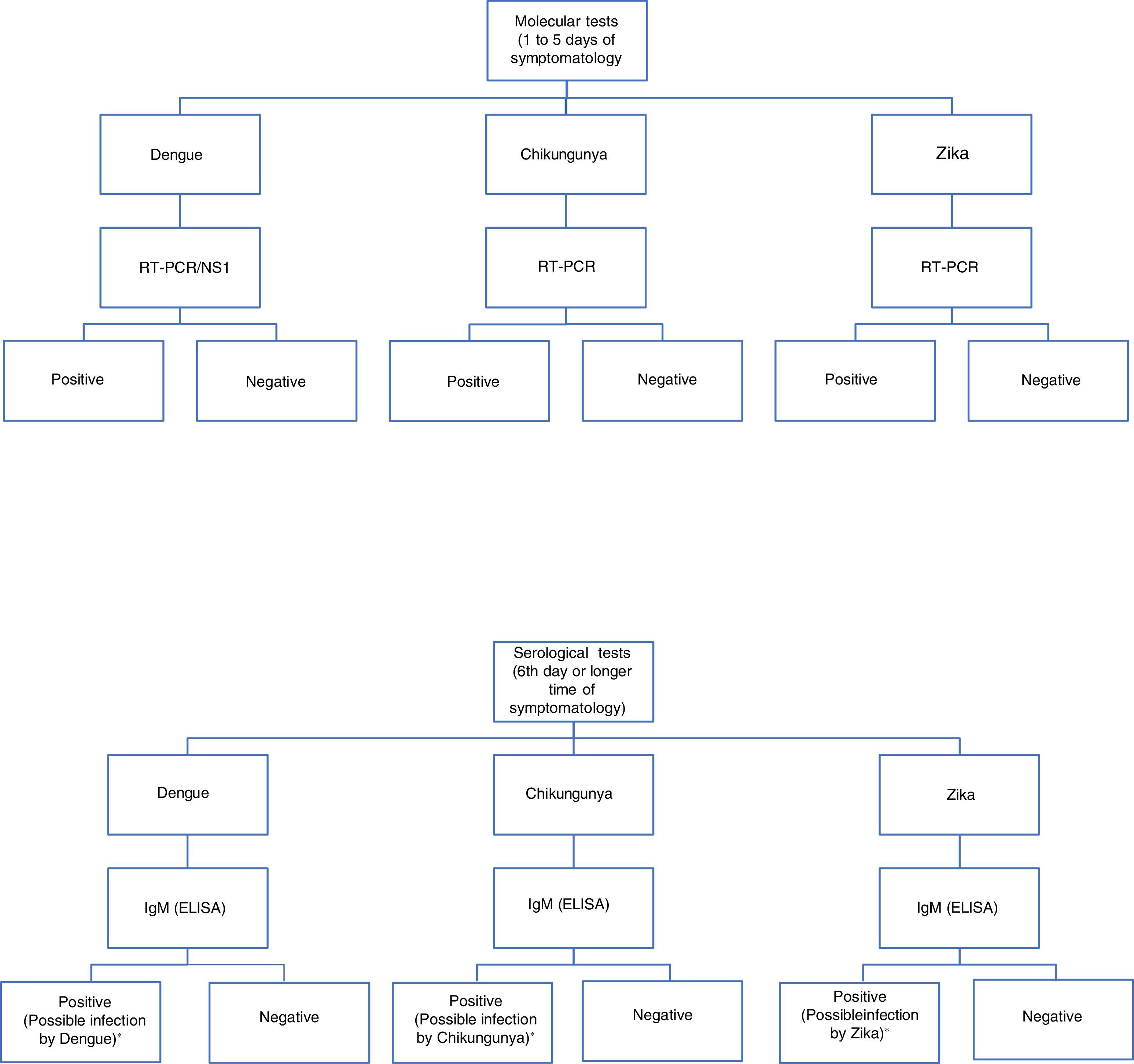
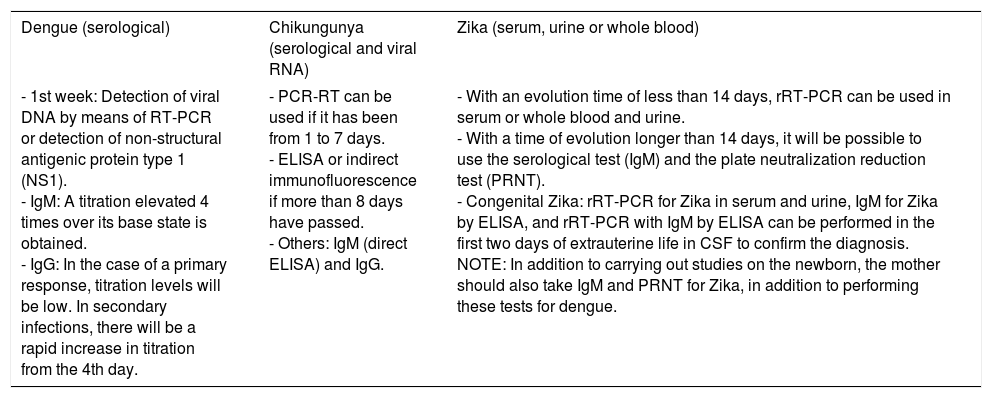
For diagnosis, early detection of viral components in serological samples ought to be documented, either directly or indirectly.20,67,69 However, the type of test used for detection will depend on the stage of the disease (Table 3 and Fig. 3).
Diagnosis of infections by DENV, CHIKV, and ZKV.
| Dengue (serological) | Chikungunya (serological and viral RNA) | Zika (serum, urine or whole blood) |
|---|---|---|
| - 1st week: Detection of viral DNA by means of RT-PCR or detection of non-structural antigenic protein type 1 (NS1). - IgM: A titration elevated 4 times over its base state is obtained. - IgG: In the case of a primary response, titration levels will be low. In secondary infections, there will be a rapid increase in titration from the 4th day. | - PCR-RT can be used if it has been from 1 to 7 days. - ELISA or indirect immunofluorescence if more than 8 days have passed. - Others: IgM (direct ELISA) and IgG. | - With an evolution time of less than 14 days, rRT-PCR can be used in serum or whole blood and urine. - With a time of evolution longer than 14 days, it will be possible to use the serological test (IgM) and the plate neutralization reduction test (PRNT). - Congenital Zika: rRT-PCR for Zika in serum and urine, IgM for Zika by ELISA, and rRT-PCR with IgM by ELISA can be performed in the first two days of extrauterine life in CSF to confirm the diagnosis. NOTE: In addition to carrying out studies on the newborn, the mother should also take IgM and PRNT for Zika, in addition to performing these tests for dengue. |
Algorithm for the detection of arboviruses in cases where there is a suspicion of infection by Dengue, Chikungunya or Zika. ELISA: Enzyme-Linked ImmunoSorbent Assay; IgM: Immunoglobulin M; RT-PCR: real-time polymerase chain reaction; NS1: Non-structural antigenic protein type 1. Adaptation of the algorithm proposed by the *Pan-American Health Organization.71
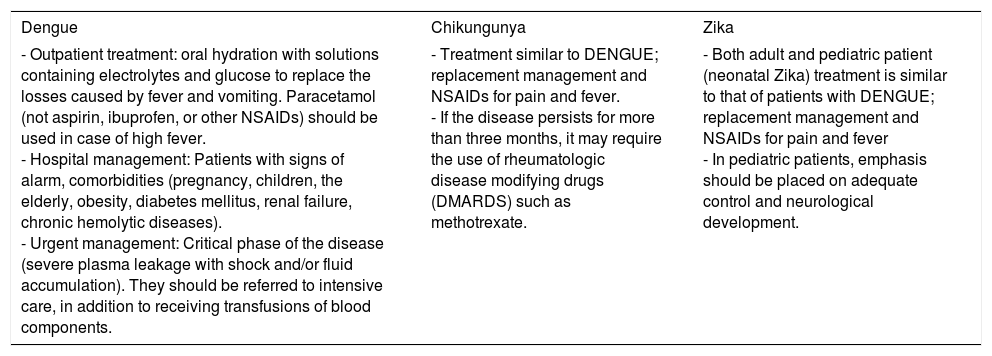
There is no specific treatment for each one of these infections.23,28,34 Management is symptomatic, depending on the patients’ needs and on the current stage of their disease. (Table 4) Today, multiple vaccines for infections caused by arboviruses are being developed; in the case of DENV, there is one vaccine (CYD-TDV) which consists of attenuated virus (recombinants), which is tetravalent and consists of single or multiple doses (5 doses). These vaccines induce the production of neutralizing antibodies against all 4 DENV serotypes, as well as a response from the T cells against structural antigens. Currently, its efficiency in the first two years after the application of the first dose has been reported.72 The vaccine has been approved by the WHO in over 10 countries (Mexico, the Philippines, Brazil, El Salvador, Costa Rica, Paraguay, Guatemala, Peru, Indonesia, Thailand and Singapore).
Management of infections by DENV, CHIKV, and ZKV.
| Dengue | Chikungunya | Zika |
|---|---|---|
| - Outpatient treatment: oral hydration with solutions containing electrolytes and glucose to replace the losses caused by fever and vomiting. Paracetamol (not aspirin, ibuprofen, or other NSAIDs) should be used in case of high fever. - Hospital management: Patients with signs of alarm, comorbidities (pregnancy, children, the elderly, obesity, diabetes mellitus, renal failure, chronic hemolytic diseases). - Urgent management: Critical phase of the disease (severe plasma leakage with shock and/or fluid accumulation). They should be referred to intensive care, in addition to receiving transfusions of blood components. | - Treatment similar to DENGUE; replacement management and NSAIDs for pain and fever. - If the disease persists for more than three months, it may require the use of rheumatologic disease modifying drugs (DMARDS) such as methotrexate. | - Both adult and pediatric patient (neonatal Zika) treatment is similar to that of patients with DENGUE; replacement management and NSAIDs for pain and fever - In pediatric patients, emphasis should be placed on adequate control and neurological development. |
Infections caused by DENV, CHIKV, and ZIKV trigger diverse and complex immuno-pathophysiological mechanisms. We attempted to review each one to learn the interactions between the host and the virus, presenting relevance not only clinically but also at diagnostic and molecular levels. Today, most of these mechanisms are known. However, many remain undiscovered, which in the future will be crucial for the understanding of these three emergent viral infections.
Conflict of interestThe authors have no conflicts of interest to declare.