Mild Cognitive Impairment (MCI) is a disease between normal cognitive ageing and dementia. In recent years the term MCI has been recognized as a pre-dementia state, raising an important subject for investigation in the prevention of dementia. There are various terms related to pre-dementia MCI, such as isolated memory complaint and pre-Alzheimer's disease; most of them do not comprise all the areas related to MCI. A central cholinergic deficit is present in amnestic MCI with neuronal loss in the Meynert basal nucleus. It is estimated that the rate of progression to dementia is about 10% every year. The prevalence of MCI is 10%-11% and the risk of progression to dementia is about 5%-16%. The continual development of pharmacologic approaches to modify and delay the natural history of progression of the disease motivates a great interest in an earlier diagnosis.
Introduction
Mild cognitive impairment (MCI) is an intermediate mental state between a normal cognitive state and dementia. In recent years, MCI has been recognized as a pre-dementia state, becoming an important subject for investigation in the prevention of dementia. There are several terms related to pre-dementia MCI, such as isolated memory complaint and pre-Alzheimer disease (AD); most of them do not comprise all the area related to MCI.1 MCI is a heterogeneous pathology in terms of clinical presentation, etiology, prognosis and prevalence; however, there is still controversy in its definition. Some studies have demonstrated that MCI can be reversed to a normal cognitive state. Prevalence is 3-19% in the elderly population, with an incidence between 8 to 58 per 1,000 people a year and a risk of developing into dementia of 11-33% within 2 years.2 Memory disorders represent a public health issue, with an average of 4.5 years until a patient is diagnosed with dementia, with major disabilities and economic loss for the family and the health sector.3,4 In Mexico there is no information about patients' survival.
Mild cognitive impairment subgroups
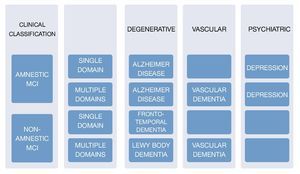
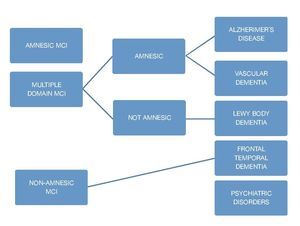
Amnestic MCI
It is considered to be AD's main predecessor; it is the most common subgroup with a 2:1 ratio compared with non-amnestic MCI. There are 2 classifications within this subgroup: single non-memory domain MCI and multi-domain MCI. The former refers to individuals who have a significant memory deficit (classified by the result of a battery of neuropsycho-logical tests) but do not meet the criteria for a dementia diagnosis; criteria for this classification are: presence of a cognitive complaint (confirmed by an informant), impaired memory determined objectively (correlated with age and educational level, and represented by an impairment greater than 1.5 standard deviations [SD] from the normal parameter), preserved general cognitive functions, the fact that everyday activities remain normal, and not meeting criteria for a dementia diagnosis. Amnestic multi-domain MCI includes those people who do not manifest a cognitive decline greater than 0.5-1 SD for their age and educational level. We have followed up on these patients and they generally do not display an evolution towards vascular dementia or AD.5
Within this MCI subgroup, the cognitive profile reflects normal aging; prognostic usefulness of this MCI is not clear. There is controversy among the different studies regarding this subgroup's conversion to dementia or the return to a normal cognitive state.6
Non-amnestic MCI
This type of MCI is characterized by an isolated impairment in a single non-memory domain, for instance an impairment of the executive function, language, or visuospatial skills. The non-amnestic MCI-single domain subgroup will progress, depending on the affected domain, to fronto-temporal dementia, primary progressive aphasia, Lewy body dementia, progressive supranuclear palsy, or corticobasal degeneration. Individuals in this group are considered to have a lower risk of conversion to dementia; however, evidence supporting this theory is limited. In regard to the non-amnestic multiple domains MCI subtype, it is considered that one of the main disorders is the development of neurofibrillary plaques caused by the proteins tau and alfa-synuclein.7
Epidemiology
There is a great variation between the reported prevalence rates, fluctuating between 2% and 20% in different series. Studies which have used different measurements such as age-related cognitive decline, cognitive impairment, no dementia, and minimal dementia estimate prevalence between 16% and 22% in adults older than 60. In several cohort-type studies, incidences between 14 and 77 for every 1,000 patients older than 60 years have been reported. Males have an odds ratio of 1:5 in community studies of patients between 70 and 89 years.2 The National Health and Aging Study in Mexico (ENASEM by its Spanish acronym) assessed the prevalence of cognitive impairment and its relationship with sociodemographic factors in the population, finding that just 7% had cognitive impairment and 3.3% had cognitive impairment and functional dependency.3
Pathology
Regarding histopathological findings, and MCI being a clinical expression of early symptoms of dementia, researchers detected intermediate findings between normality and advanced dementia. A central cholinergic deficit is present in amnestic MCI, related to neuronal loss in the basal nucleus of Meynert.8
In postmortem studies, evidence of a positive regulation in the acetyl transferase activity in the frontal cortex and hippo-campus has been found.9 There is evidence that cerebrovascular and neurodegenerative conditions contribute to MCI, especially white matter lesions and small lacunar infarcts.10
One of the main problems in the diagnosis of MCI is determining how much the memory processes are affected. Some criteria establish that in order to reach a diagnosis of MCI, the patient's performance carrying out memory tasks must be inferior to the average obtained by normal adults; however, other criteria establish that for a diagnosis of MCI, patients must be compared with subjects of the same age and educational level.
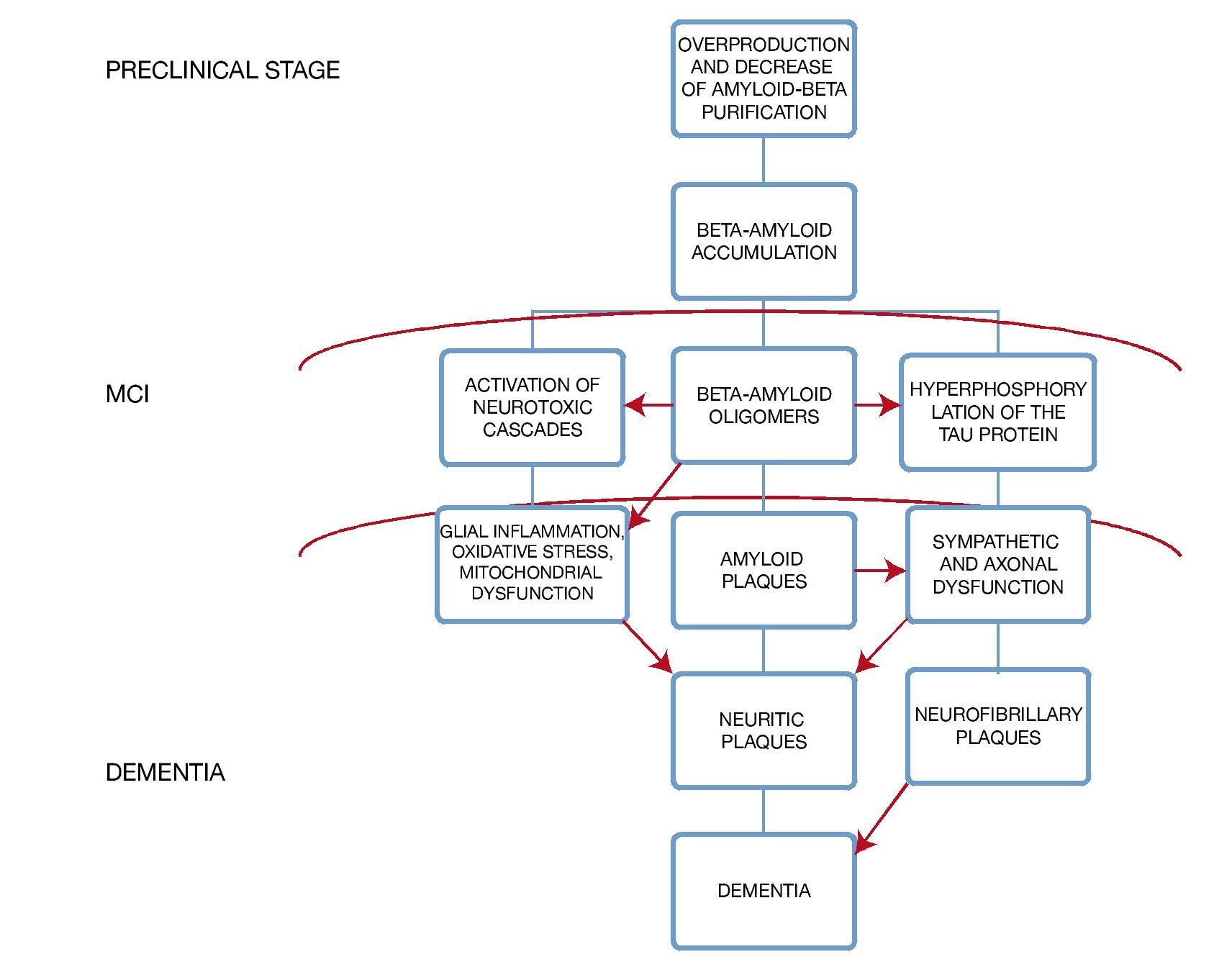
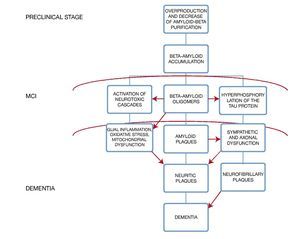
A very important point in the subgroup of patients with diabetes mellitus is to recognize that there is an exacerbated neurotoxicity caused by the beta amyloid plaques secondary to the advanced glycation end-products in their matrix; moreover, in animal models the existence of a diminished cholinergic transporter in patients with cognitive impairment and diabetes has been found4 (Fig. 1).
Figure 1 Histopathologic changes in the brain and its clinical correlation.
Hypertension has been studied as a risk factor for cognitive impairment because it affects cerebral vasculature through different mechanisms, including strokes, leukoaraiosis, atherosclerosis, etc.
In transversal studies, it has not been possible to find an evident association with arterial hypertension as a risk factor while in longitudinal studies such association has been demonstrated. Dyslipidemia has also been studied in different population samples finding a correlation between MCI and hyperlipidemia (especially hypercholesterolemia) in middle-aged patients. Furthermore, we are able to see in different studies that there is a protective association between the use of statins and MCI. Other risk factors which have been analyzed include chronic renal failure, vitamin B12 deficiency, vitamin D deficiency, hyperhomocysteinemia, testosterone deficiency, subclinical hypothyroidism, smoking, and excessive alcohol consumption.
Population studies
Many authors and institutions (The World Health Organization and The American Academy of Neurology, among others) have supported the term MCI. In recent years they have been trying to establish a greater delimitation of the term, mainly because different types of mild cognitive impairments have arisen (amnestic, non-amnestic, aphasia, apraxia or agnostic).
Most of the studies are directed at evaluating MCI, and are focused, mainly, in observing the performance in the memory processes in older people, considering there is well-founded evidence showing that cognitive complaints have a high prevalence in this population, suggesting a pre-clinical phase of a process of dementia; even though there are many longitudinal cohort studies of non-demented subjects, who have been developing cognitive impairment.
One of the most representative investigations about MCI, and one which has been taken as a reference, was carried out by the researchers at Mayo Clinic in the United States. The objective of the researchers was to clinically classify subjects with MCI through a transversal and longitudinal study. A sample of 76 subjects with MCI was compared to 234 control subjects and with 106 patients with mild AD from the clinic or from the community register of patients with AD in Rochester. All 3 groups were evaluated longitudinally using instruments which evaluate cognitive performance (MMSE, WAIS-R, WMS-R, Dementia Rating Scale, Free and Cued Selective Reminding Test and Rey's Auditory Verbal Learning Test, among others). Mild AD diagnosis was established by the DSM-III-R and the NINCDS-ADRDA. The results showed that in general intelligence measurements, subjects with MCI performance were more similar to that from control subjects than from patients with mild AD. In particular, in the complete WAIS-R scale, control subjects obtained an average IQ = 101.8 and subjects with MCI obtained an average IQ = 98, while patients with mild AD obtained an average IQ = 83.9. The results with the MMSE were very similar; control and MCI subjects obtained an average score of 28.3 and 26 respectively, while patients with mild AD obtained an average score of 22.6. On the other hand, subjects with MCI obtained a lower result than control subjects in the Controlled Oral Word Association Test, even though these results were within the normal expected range for their age, based on the community studies used for this research. However, performance carrying out memory tasks was very similar in subjects with MCI and patients with mild AD, yet the declining range of subjects with MCI performing these kinds of tasks was lower to the one showed by patients with mild AD but higher than that in control subjects. Researchers came to the conclusion that subjects considered under MCI criteria may be differentiated both from control subjects and patients with mild AD, thus considering MCI as a clinical entity characterized by a group of symptoms and amenable to of pharmacologically interactive treatment.5
Another study with great significance in MCI research was one carried out in St. Luis Missouri, US, where the local elderly population volunteered. The sample was of 454 subjects, who were classified according to the CDR as "cognitively healthy" (n = 177 y CDR = 0) and Lewy body dementia (LBD) (n = 277 and CDR = 0.5).
Based on the clinical suspicion that MCI represents an early stage of Alzheimer's, the MCI group with a score on the CDR = 0.5 was subdivided into three subgroups. The study had a follow-up of 9-and-a-half years, evaluating subjects with a neuropsychological battery and a neuropathological test for the subjects who passed away during research. The objective was to observe the progression of sub- jects towards a score in the CDR = 1, which characterizes "definite" mild Alzheimer's. The evaluation protocol included items from brief standardized cognitive assessment batteries, such as MMSE and the Short Blessed Test (Katzman et al. 1983), among others. In general, the results showed that all the subjects with CDR = 0-5 displayed memory impairment and a higher frequency of alelo e4 of the gene apoe compared to normal subjects CDR = 0. The second cognitive domain frequently more impaired was the judgment and problem solving skill. On the other hand, the results showed that 100% of the subjects of the group CDR=5-Alzheimer's progress to a severe dementia in a period of 9.5 years, of the rate of conversion of this group to Alzheimer's in 5 years 60.5% with an equal or superior score to 1 in the CDR. In the CDR = 0.5- incipient Alzheimer's, the range of conversion to Alzheimer's in 5 years was 35.7% and, for the CDR = 0.5- uncertain dementia, its range of conversion in the same period of time was 19.9%; However, in the control subjects (CDR = 0), the conversion range was only 6.8%. The conclusion was that MCI generally represents an early stage of Alzheimer's, due to the fact that progression to a severe dementia correlates with the base line of the degree of impairment, in other words, 96% of the subjects with CDR = 0.5 progressed to a neuropathological state of dementia, and out of these, 84% were an Alzheimer-type dementia1
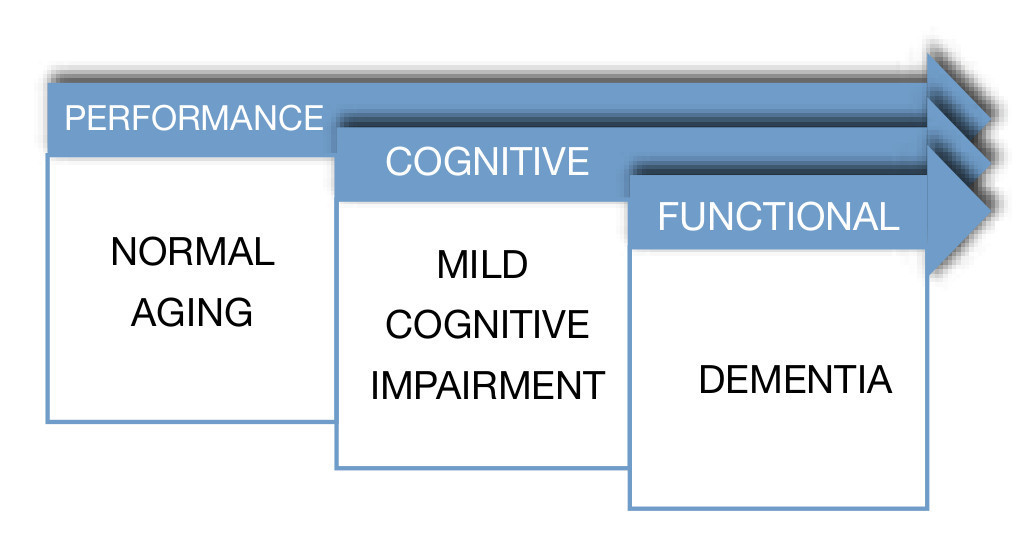
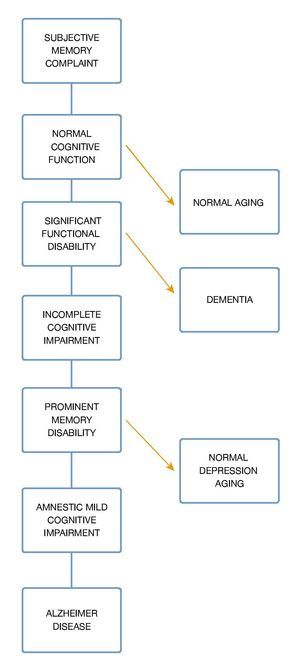
In a different study, Ritchie et al, with the objective of evaluating the predictive validity and temporal stability of the diagnosis criteria of MCI, analyzed a sample of 833 subjects with subclinical cognitive impairments, taken from a previous longitudinal study called the Eugeria Project, carried out in Montpellier, in the south of France. The sample was representative of the population and covered urban as well as rural populations. The study followed the cohort of subjects during a period of 3 years. During the first year, subjects were evaluated with an instrument used to screen cognitive functioning denominated Détérioration Cognitive Observée (DECO), which has demonstrated in diverse studies its high sensitivity to detect early changes in cognitive functioning due to different causes, observing the degree of change during the last year. Its estimation is similar to the one of a close person who has had continuous monthly contact with the patient during a period of three years. Using a neuropsychological computerized exam which covers most of the cognitive functions, six cognitive domains were selected; attention, primary memory, secondary memory, visuospacial skills, language and reasoning. In a second phase, MCI subjects were classified. The results showed that MCI prevalence in the general population was approximately 3.2%, and of cognitive impairment adjusted to their age of 19.3%. Thus, MCI turned out to be a poor background to a preclinical dementia phase in a period of three years, with a risk of conversion of 11.1% constituting, in general, an unstable group, because most of the subjects who belonged to this group, experimented category switches every year. On the contrary, the cognitive impairment adjusted group proved to be more homogenous and stable, with a conversion rate to dementia of 28.6% in a period of 3 years. The researchers came to the conclusion that criteria for MCI provide a poor performance when applied to a representative sample, given the fact that in most of the investigations which proposes MCI as a clinical diagnostic entity, samples have been obtained out of small selected clinical groups. Because cognitive complaints in older adults are the main symptom to establish MCI criteria, several clinical studies have been carried out. These studies have tried to establish the predictive power of cognitive complaints in the memory processes.6 Furthermore, it is important to emphasize "The Canadian Study of Health and Aging" for its importance in which they carried out a 5-year follow-up of 10,263 elderlies (over 65 years and up), representing the general population of older adults (residents of the community, as well as institutionalized). In this epidemiologic study about health care, dementia and functional state the term "nodementia cognitive impairment" surfaces, which represents a cognitive disorder accompanied by a mild neuro-cognitive disorder, also, it is attributable to a subjacent functional di- sorder. In this study they obtained results which indicate that non-dementia cognitive impairment prevalence is 16.8% in people older than 64 years. From this study on, several parallel studies utilized samples from this study7 (Fig. 2).
Figure 2 Dementia spectrum.
Clinical assessment
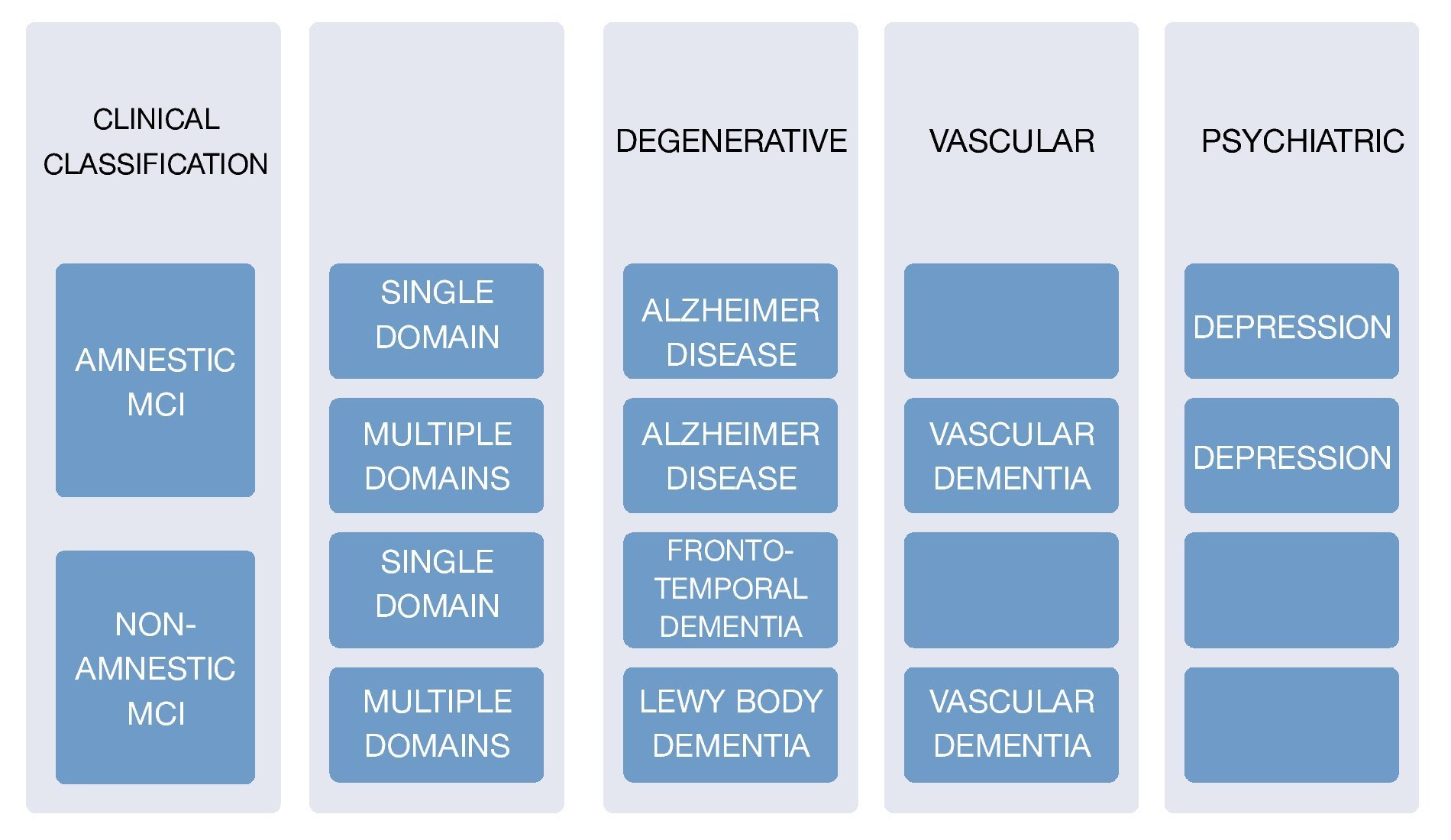
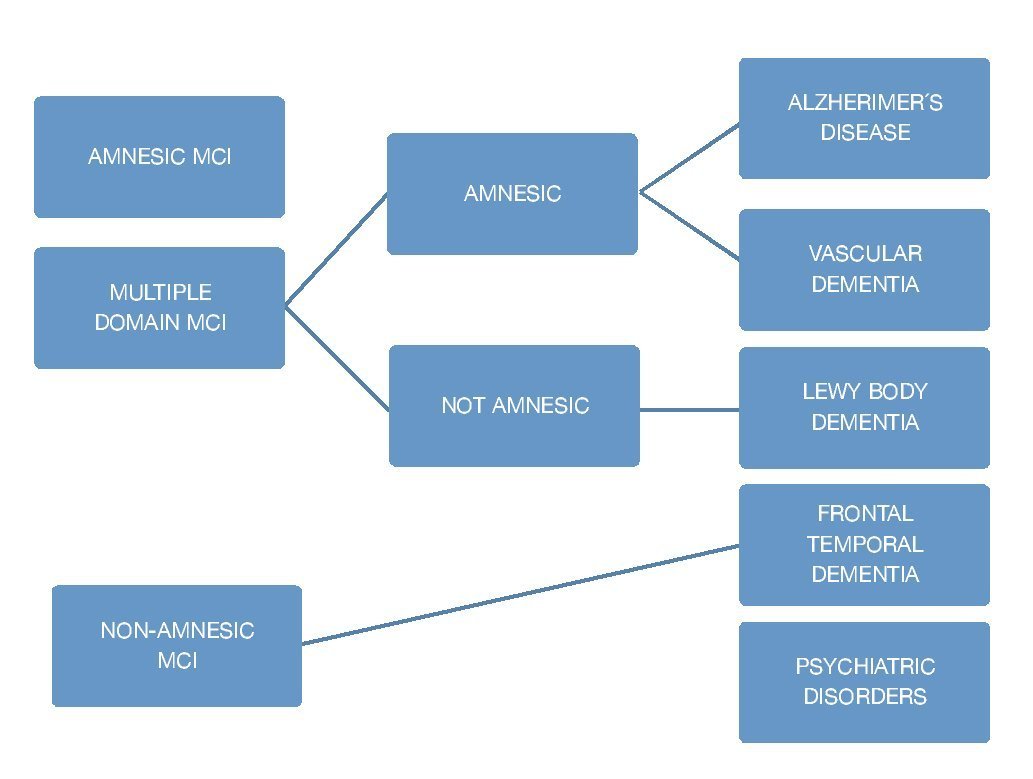
The corner stone of diagnosis consists of a detailed clinical interview, ideally with the presence of an informant. There are several scale systems, since 1982 there have been 2 main ones which are used to this day: Clinical Dementia Rating (CDR) which gives a point system of 0 for normal people, 1 for mild dementia, and 0.5 for mild cognitive deterioration, and The Global Deterioration Scale (GDS) which scores mild cognitive deterioration with a score of 3 where patients display cognitive deficits and suffer alterations in the executive function which affects social and occupational activities. It is important to keep in mind that the scales do not mean 100% MCI diagnosis, because it is necessary to check it with the clinical findings, and medical history. In order to evaluate the subjects' cognitive profile using the Folstein Mini-Mental State Examination Tool (MMSE), or via Montreal Cognitive Assessment (MOCA) taking into account their respective sensitivity and specificity. A medical and neurological examination must be carried out in order to identify non-degenerative cognitive alterations causes8,9 (Fig. 3).
Figure 3 Clinical classification and cause of MCI.
Emphasizing MMSE is one of the most utilized tests to evaluate these cognoscitive symptoms, it is very important to remember that this test assesses memory, orientation, language, spatial skills, attention, and calculus. It takes about 10 minutes to apply. Its score ranges from 0 to 30. A cut-off point of 23 is generally recommended as an indicator of cognitive impairment. The test has a reliability of 0.89. One of its limitations is the fact that it is influenced by several factors; for example, the level of education. Some authors even consider the test to be affected by the patient's ability to read. Other authors believe the test is affected by demographic factors such as gender and age. Therefore, differential cut-off points between subgroups have been suggested and alternative items have been generated (Galasko: the months of the year backwards).
There are certain considerations which must be taken into account for MCI diagnosis:
1. An international working group has formulated specific recommendations in MCI criteria: The individual is not demented nor fits in the normal category. There should be evidence of impairment in cognition, demonstrated by a decline in time, or subjective reports made by an informant. Everyday activities are preserved and complex functions are intact or minimally affected.10
2. It has been noted that neuropsychiatric symptoms including dysphoria, irritability, hallucinations, apathy, depression, agitation, and delusions are more frequent in MCI and Alzheimer dementia patients. The presence of neuropsychiatric symptoms involves a greater risk for MCI patients of progressing into AD. The Neuropsychiatric Inventory (Cummings et al., 1994) is the most used scale to assess neuropsychiatric alterations including 12 areas (agitation, delusion, hallucinations, depression, euphoria, abnormal motor activity, apathy, irritability, disinhibition, anxiety, drowsiness and hunger); a patient's relative who may have the information is questioned about these symptoms.11
3. Psychiatric conditions (depression), medication adverse events (anticholinergics, antihistamines), sleep disorders, infections, metabolic diseases (vitamin B12 deficiency, hypothyroidism, hyperglycemia, hypoglycemia, uremia, hepatopathy, sodium disorders), structural brain diseases.11 Due to a great diversity of pathologies, researchers have tried to find ideal biomarkers for a more accurate diagnosis; we must remember that markers may be positive in non-demented subjects. Some of these markers reflect key cognitive impairment processes in the cerebrospinal fluid, such as the levels of isoform of beta 42 amyloid and Tau protein. Other studied markers include amyloid deposits or neurofibrillary plaques like Pittsburgh B compound or the 18-FDDNP.12 There are other markers which reflect secondary processes like neuron dysfunction or neuronal loss either by electroencephalogram (EEG), nuclear magnetic resonance or positron emission tomography scan. As far as EEG goes, researchers have found that the rate between theta and alpha 1 waves is a reliable individual rate of cerebrovascular damage.13
4. Cerebral biopsy plays a very limited role in the diagnosis of dementia; its diagnostic capabilities are minimal, the procedure is invasive and involves risk for major complications. The biopsy is reserved for younger patients and for those with atypical clinical presentations with potentially treatable causes.
It is very important to promote the recognition and acknowledgement of this pathology as several studies have shown that between 29% and 76% of the cases may be identified in first contact care.14 Studies show that patients with MCI display a wide range of malnutrition. A study by The European Society for Clinical Nutrition and Metabolism found that from a sample of 623 patients, 18% had a normal nutrition status, 58% were at risk of malnutrition, and 24% showed clear malnutrition; thus, we must keep in mind that these types of patients display more alterations within the corporal economy in addition to neurological deficiencies.15
Within a pragmatic approach in the study of MCI, it is of great importance to cite several key questions for a proper assessment16 (Fig. 4):
Figure 4 Clinical approach to MCI.
1. What is the patient's basal functional status?
2. How is the symptomatology's evolution? (regarding time of onset)
3. What is the main characteristic of this deficit? (short-term or long term memory)
4. Are there any changes in behavior?
5. Is there a co-existence of depression in this patient?
6. Could the patient in question have a serious subclinical pathology?
Neuroimaging
Neuroimaging use is controversial; most guides do not recommend routine imaging studies, but they include rules to help identify patients who may have reversible causes of dementia, with factors that include being under 60 years old, showing focal neurological signs, and having been affected less than 2 years from the onset of the disease. However, their sensitivity and specificity are low. Magnetic resonance imaging (MRI) findings include focal and generalized atrophy, as well as white matter lesions; in general, these are unspecified findings. A tomography is important in those patients with acute unsaturation of the cognoscitive impairment and rapid neurological deterioration.17
A longitudinal cohort study was carried out in Rotterdam, The Netherlands. The main objective of this study was to determine whether or not the hippocampus and amygdala volume measured by MRI were useful for predicting the development of dementia in older people without cognitive impairment. The investigators monitored 511 patients between 60 and 90 years of age, for an average period of 6 years. Over this period of time, the atrophy of the hippo-campus and the amygdala measured by MRI predicted a time of development of dementia of 6 years, with a risk rate adjusted to age, gender, and educational level of 3 (95% confidence interval [CI] 2.0-4.6) for every unit of standard deviation for the hippocampus and of 2.1 (95% CI 1.5-2.9) for the amygdalin level. In patients with cerebral atrophy and leukoaraisis, the exact correlation degree and influence between imaging findings and the results of the neuropsychological tests are unknown. Therefore, it is hard to classify this type of patient, because there are cases with major pathological findings and imaging representing a preserved cognition.18
Transcranial Doppler ultrasound
Imaging techniques to analyze cerebral circulation are very diverse, there is no such thing as the ideal study. Transcranial Doppler ultrasound is a test performed with a portable device which allows measuring several blood flow parameters as well as the Circle of Willis, providing information about the velocity of blood flow (cm/s) through the brain's blood vessels, resistance rates, and pulsatility, as well as the brain's vasoreactivity following stimuli such as hyper-ventilation, induced hypercapnia, and even medications like acetazolamide. This study is performed with the patient in a decubitus position, after resting for 10 minutes, at room temperature, and comfortable, with the head at a 30 degree angle in a semi-Fowler position; a basal register is recorded and blood pressure registered every 5 minutes during the inhalation of 5%-7% carbon dioxide (CO2) for 5 minutes with an aesthetic mask covering both mouth and nose. The most utilized protocol is to register the velocity of systolic and diastolic flow, as well as the average resting flow of the middle cerebral artery and then again 5 minutes after inhaling CO2, assessing the responsive ability of cerebral arteries on entrance of carbon dioxide, numbers that adjust to age, gender, and changes in blood pressure. In patients with hypertension, after the vasodilator stimulus there is a decrease in the cerebral microvascular reactivity (explained by the decrease in the dilation ability to the vasodilator stimulus), which can be improved through the administration of antihypertensive medications.19
It has been shown that cerebral blood flow and cerebral vasoreactivity gradually decrease with age, and that this decrease is directly related to the presence of cerebral micro-strokes and impairment of cognitive function; in addition, vasoreactivity of the occipital is diminished even more in vascular dementia which is statistically significant compared to AD.20
Progression to dementia
The risk of dementia progression is estimated to be 10% per year; as for MCI patients, yearly progression rates fluctuate between 5% and 16%. Several long-term studies (over 5 years) have found that the risk of conversion decreases with time. Some of the factors associated with an increased risk of progression to dementia are socioeconomic variables and age while gender and level of education are not considered as factors. Age constitutes the main predictor of progression, risk increasing exponentially for every year people get older. The apolipoprotein E-epsilon4 (APOe4) genotype has been associated with an increased risk, as well as raised levels of the protein Thr phosphorylated tau 181, low levels of amyloid beta 42, rise in the activity of the beta-secretase, and of neprilysin (a degrading enzyme beta-amyloid peptide) activity diminution. An important rate of reversion of MCI to a normal cognitive state is observed (20%-25%).21
The American Academy of Neurology's evidence-based guidelines for early detection states that MCI patients should be detected early and followed long-term, because of their high risk of subsequent development of dementia.2
For dementia detection in individuals suspected of cognoscitive impairment, screening instruments used to assess progression are: Mini-Mental State Examination (score of 224 or less, specificity 57%-99%, sensitivity 82%-99%, positive predictive value 68%-91%, negative predictive value 96%-99%, evidence class I and II, the percentages vary because of the risk of dementia in each population, age classification, etc.), Kokmen short test of mental state (specificity 88%, sensitivity 86%), 7-minute screen, memory impairment screen (specificity 96%, sensitivity 87%, positive predictive value 54%-85%), Clock drawing test (specificity 96%, sensibility 92%), and Blessed dementia rating scale (specificity 46%, sensitivity 94%).
Moreover, different instruments should be applied for different aspects of the cognoscitive function, being more useful the ones which emphasize memory, for example the Mattis dementia rating scale (mainly focused on attention, initiative, perseverance, building, memory, and conceptualization, between 129/144, sensitivity 98%, specificity 97%).
It is necessary to apply neuropsychological batteries on the risk population, like the IQCODE (Informant Questionnaire on Cognitive Decline in the Elderly) or the CDR which
is a scale with 6 categories, and is based in orientation, judgment, memory, personal care, performance within the house and hobbies, with a score of over 1, a specificity of 94% was demonstrated and a sensitivity of 92%.
Diabetic patients have an odds ratio of 1.2-1.5 to obtain lower scores than those who are non-diabetics, regarding cognitive function; measured by Mini-Mental and the digital symbol test.22
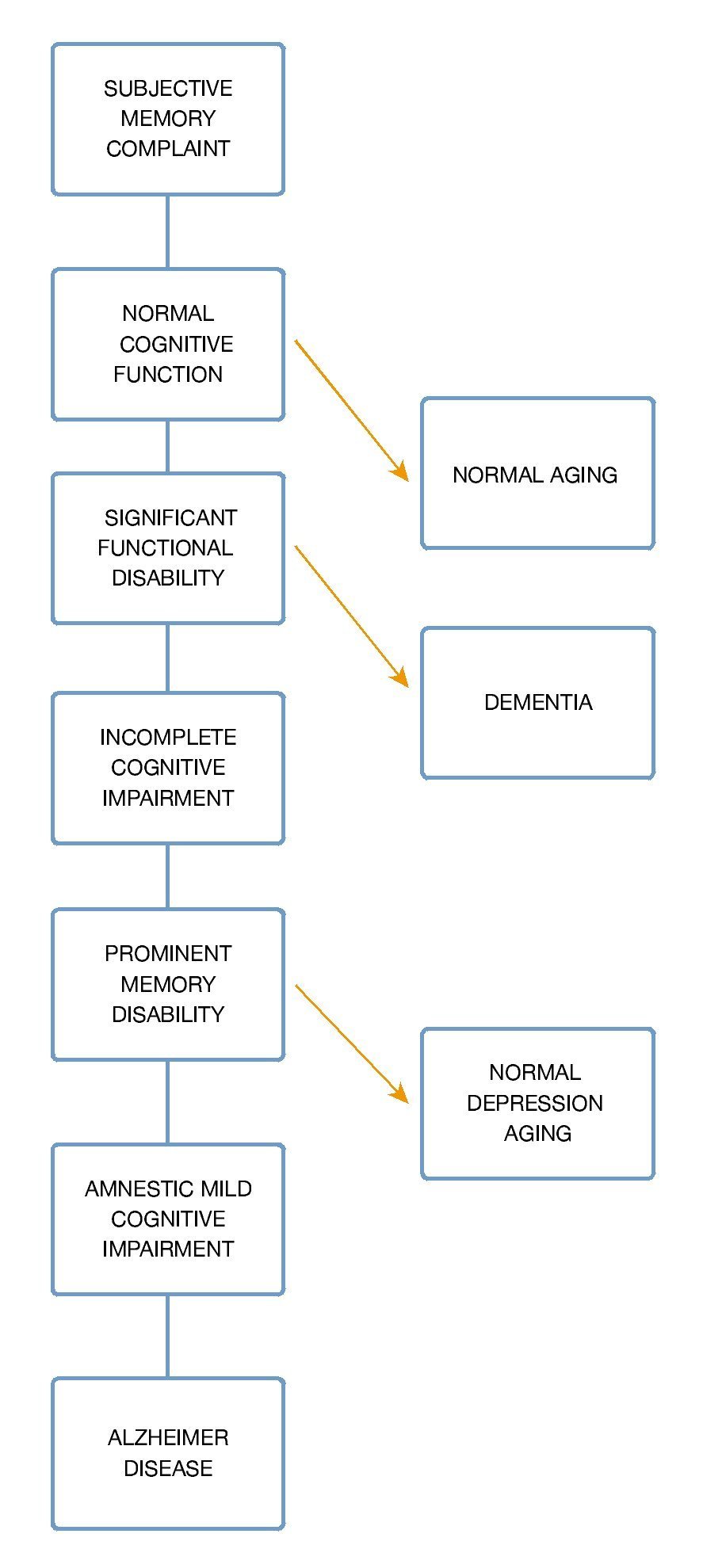
An adequate conversion to dementia prediction must be made on those patients with mild cognitive impairment already established, there are several models; one of the most important is the "Five-Predictor Combination" with a sensitivity of 85.2% and 90% specificity based on age, Mini-Mental result, "Selective Reminding Test", Functional activities questionnaire and University of Pennsylvania smell identification test23 (Fig. 5).
Figure 5 Progression of mild cognitive impairment.
Importane of the subject
The constant emergence of pharmacological and non-pharmacological preventive treatments to slow down or modify the course in AD and the other types of dementia, boost a great interest of reaching an early diagnosis in order to start acting as soon as possible with these patients. MCI is an adequate state for new therapies, thus its differential diagnosis is crucial, and a thing that nowadays is controversial, with great debates regarding its delimitation and acceptance as a new diagnostic entity. The development of specific instruments for MCI diagnosis is one of the most urgent tasks, given the fact that the current instruments have a significant tendency to only detect risk factors. Different studies agree that an adherence to the Mediterranean-style diet (hazard ratio [HR] = 0.60, CI 0.42-0.87) and high physical activity (HR = 0.67, CI 0.47-0.95) are independently associated with a reduced risk of cognitive impairment.24 Knowledge of an adequate control of systemic hypertension reduces the risk of developing vascular dementia, dementia with Lewy bodies, and frontotemporal dementia; nevertheless, it does not significantly decrease the development of MCI (RR = 0.97, CI 0.92-1.03) or AD.25 Currently, there is little evidence of the effectiveness and specificity of interventions that improve memory in the healthy elderly and those with MCI; studies show that after a systemic training the results improved in immediate and delayed verbal memory. Information to recommend MCI screening in asymptomatic individuals is lacking. Therefore, there is ongoing research and population analysis in order to determine which subjects will benefit the most from the search of this pathology.26
Treatment
Adherence to a Mediterranean-style diet has been marginally associated with the reduction of progression of MCI to AD (HR = 0.55, 95% CI 0.34-0.9).27
According to the Cochrane Collaboration, there are no studies assessing the role that carbohydrates play in improving the performance of the evaluation in older people with MCI.28
High-intensity aerobic exercise may be beneficial for patients with MCI; results of studies carried out with small randomized samples to exercise 40 to 60 minutes 4 days a week for a period of 6 months, have shown that exercise improved the levels of the factor similar to the growth hormone, plasma insulin reduction when fasting, cortisol, and executive function.29
Cholinesterase inhibitors slow down the conversion to full dementia for up to 3 years, but it has been associated with adverse effects in adults with MCI (diarrhea, nausea, vomiting, cramps, insomnia, headaches, syncope or vertigo); the most studied are donepezil, galantamine, and rivastigmine, the relative risks of progression to dementia are 0.69 (95% CI 0.47-1) within 1 year, 0.67 (95% CI 0.55-0.83) within 2 years, and 0.84 (95% CI 0.7-1.02) within 3 years.30which is frequently updated from the major healthcare databases (MEDLINE, EMBASE, CINAHL, PsycINFO and Lilacs
Galantamine is not recommended because of the mortality risk increase, with a number needed to harm (NNH) of 100;31 in addition, it has not really been associated with a diminution in the progression to dementia.32
In regards to donepezil, it may slow down the progression of AD; this is based on randomized studies of patients who received this medication at 5 mg a day for 6 weeks, which was titrated to 10 mg a day for 3 years. In general, the progression to dementia was seen in 16% of the 769 patients, with a significant improvement at 6, 12 and 24 months; among patients who were carriers of APOe4, the improvement in executive function was notable up to 3 years of follow-up.33 The most common adverse effects were diarrhea (NNH 10), cramps (NNH 7), insomnia (NNH 11), nausea (8.4%), vomiting (6%), and arthritis (2.5%).34
There are other diverse therapies for which the net clinical benefit provided is unknown; these include nicotine patch,35 gingko biloba,36 vitamin E,11 vitamins B637 and B12,38 folic acid,39 procaine,40 piracetam,41 and huperzine A.42
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Acknowledgement
Our acknowledgement and appreciation to the Neurology Services of "Dr. José Eleuterio González" University Hospital, particularly to Dr. Hector Jorge Villarreal, for all the facilities provided.
Received: May 2013;
Accepted: December 2013
* Corresponding author:
Madero y Gonzalitos Avenue, Z.P.
64700, Monterrey, N. L., Mexico.
Telephone/Fax: (81) 8333 7798.
E-mail address: drjavierisordia@gmail.com (J. Isordia-Martínez).