To analyse cognitive skills in patients with severe unilateral hearing loss versus those in subjects with normal hearing.
Methods40 adults participated: 20 patients (10 women and 10 men) with severe unilateral hearing loss and 20 healthy subjects matched to the study group. Cognitive abilities were measured with the Spanish version of the Woodcock Johnson Battery-Revised; central auditory processing was assessed with monaural psychoacoustic tests. Box plots were drawn and t tests were performed for samples with a significance of P≤.05.
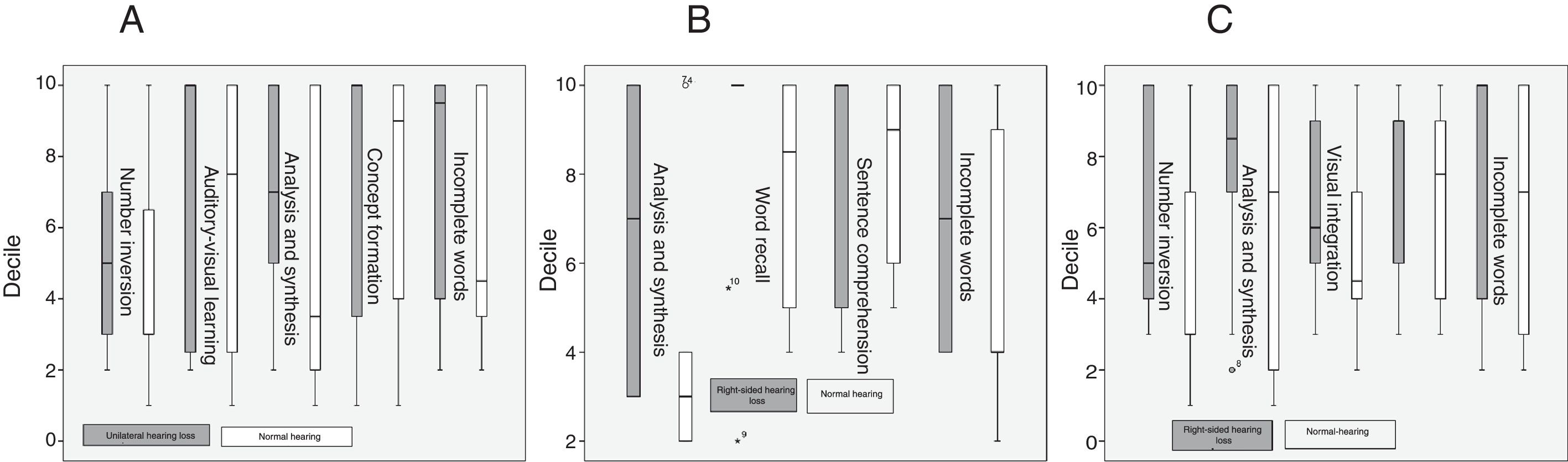
ResultsA comparison of performances on the filtered word testing and time-compressed disyllabic word tests between patients and controls revealed a statistically significant difference (P≤.05) with greater variability among responses by hearing impaired subjects. This same group also showed a better cognitive performance on the numbers reversed, visual auditory learning, analysis synthesis, concept formation, and incomplete words tests.
ConclusionsPatients with hearing loss performed more poorly than controls on the filtered word and time-compressed disyllabic word tests, but more competently on memory, reasoning, and auditory processing tasks. Complementary tests, such as those assessing central auditory processes and cognitive ability tests, are important and helpful for designing habilitation/rehabilitation and therapeutic strategies intended to optimise and stimulate cognitive skills in subjects with unilateral hearing impairment.
Analizar la asociación de competencias cognitivas en sujetos con hipoacusia unilateral severa versus sujetos con audición normal.
MétodosParticiparon 40 adultos; 20 pacientes, 10 de cada género, con hipoacusia unilateral sensorial severa y 20 sujetos sanos pareados al grupo de estudio. Las habilidades cognitivas se midieron con la batería Woodcock Muñoz-revisada y los procesos centrales auditivos con pruebas psicoacústicas monoaurales. Se realizaron gráficas de caja y prueba t de Student para muestras relacionadas con significación p ≤ 0,05.
ResultadosAl comparar el desempeño en las pruebas palabra filtrada y bisílabos comprimidos, se encontró diferencia estadísticamente significativa p ≤ 0,05, con mayor variabilidad de respuesta en los hipoacúsicos, los cuales también tuvieron mejor desempeño cognitivo en las subpruebas inversión de números, aprendizaje visual auditivo, análisis y síntesis, formación de conceptos y palabras incompletas.
ConclusionesLos hipoacúsicos presentaron bajo desempeño en palabra filtrada y bisílabos comprimidos, y mayor habilidad para memoria, razonamiento y procesamiento auditivo. Es importante realizar pruebas complementarias, tales como procesos centrales auditivos y habilidades cognitivas que permitan establecer estrategias de habilitación, rehabilitación y terapéuticas con la finalidad de optimizar y estimular las habilidades de los sujetos con hipoacusia unilateral.
According to World Health Organization data, 275 million people had moderate to profound hearing loss in 20121; this number rose to 360 million in 2013.2
In Mexico, the 2010 population census revealed that 12.1% of disabled Mexican patients had hearing problems.3 According to the Mexican National Household Income and Expenditures Survey, this number reached 16.5% in 2012.4
In the United States, unilateral hearing loss affects around 83 in every 100000 live births; the National Health and Nutrition Examination Survey reports an incidence of 3% among school-age children.5
Hearing impairment is defined as a partial or complete inability to hear, and may affect one or both ears.6
Central auditory processes are the auditory mechanisms responsible for the behavioural phenomena of sound localisation and lateralisation, auditory discrimination, auditory pattern recognition, temporal processing, and auditory performance in the presence of degraded or competing acoustic signals.7
Central auditory processing disorders (CAPD) involve deficiencies in the neural pathways responsible for auditory information processing.6 Patients with CAPDs have difficulty focusing attention, recalling oral information, following complex instructions, perceiving language in noisy settings, and understanding fast or degraded acoustic signals (time-compressed, filtered, interrupted, or competing).8
Published evidence suggests that unilateral hearing loss results in poor academic performance, behavioural issues, and the need for educational assistance.9 Patients with unilateral hearing loss have been found to have lower intelligence quotient scores than their normal-hearing peers, especially the areas of working and phonological memory, attention, and processing speed.10 They also present emotional, social, and language problems,9 particularly affecting comprehension.11,12 Several studies suggest that these patients develop certain compensatory mechanisms which enable them to achieve better comprehension, language, and verbal function scores, despite which their performance is worse than that of their normal-hearing peers.12–14
We aimed to analyse cognitive and central auditory performance in a sample of adult patients with severe and profound unilateral hearing loss and a group of normal-hearing controls.
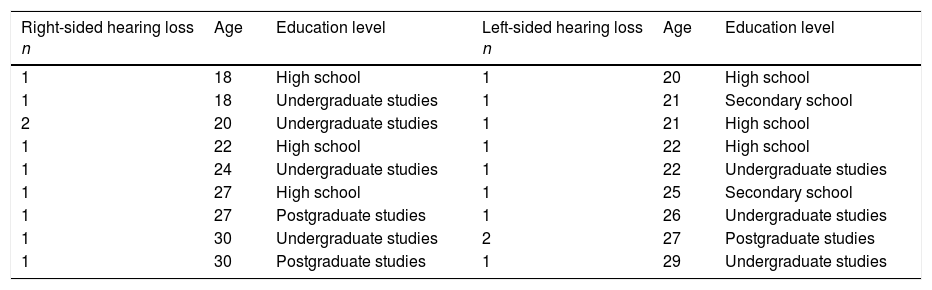
Material and methodsForty adults participated in the study. The study group included 20 patients (10 men and 10 women) with a diagnosis of severe or profound acquired unilateral sensory hearing loss (10 with right-sided hearing loss, 10 with left-sided hearing loss) under follow-up at the audiology department of the National Institute of Rehabilitation Luis Guillermo Ibarra Ibarra. Progression time was below 10 years (range, 1-7) in 10 patients and over 10 years (range, 10-27) in the remaining 10. Mean age was 23.8±3.8 years (range, 18-30). The control group included 20 sex-, age-, and education-matched normal-hearing individuals. Completed undergraduate study was the most frequent education level, and secondary school the least frequent. Age ranged from 18 to 30 years in the group of patients with right-sided hearing loss, and from 20 to 29 among patients with left-sided hearing loss. In both groups, the modal age was 27 years, followed by 22 and 20 years (Table 1).
Age and education level of patients with right-sided and left-sided hearing loss.
| Right-sided hearing loss n | Age | Education level | Left-sided hearing loss n | Age | Education level |
|---|---|---|---|---|---|
| 1 | 18 | High school | 1 | 20 | High school |
| 1 | 18 | Undergraduate studies | 1 | 21 | Secondary school |
| 2 | 20 | Undergraduate studies | 1 | 21 | High school |
| 1 | 22 | High school | 1 | 22 | High school |
| 1 | 24 | Undergraduate studies | 1 | 22 | Undergraduate studies |
| 1 | 27 | High school | 1 | 25 | Secondary school |
| 1 | 27 | Postgraduate studies | 1 | 26 | Undergraduate studies |
| 1 | 30 | Undergraduate studies | 2 | 27 | Postgraduate studies |
| 1 | 30 | Postgraduate studies | 1 | 29 | Undergraduate studies |
Undergraduate studies constitute the most frequent level of education. Modal age: 27 years. Mean age: 23.8333 years (95% CI, 21.8791-25.7875).
The study protocol was approved by our centre's research ethics committee. All participants were informed about the study and signed informed consent forms, as stipulated by the Declaration of Helsinki. A questionnaire was administered to gather information on participants’ medical histories and current health status. We also examined the participants with an otoscope to evaluate the permeability of the external auditory canal and the integrity and characteristics of the tympanic membrane. A tympanometry was performed to measure middle ear compliance (normal range, 0.5-1.5) and pressure (+50 to −100daPa); tympanograms were classified according to the system developed by Jergen. Participants also underwent pure tone audiometry (increasing frequencies from 125 to 8000Hz). To evaluate the hearing-impaired ear we masked the air-conduction pathway with white noise (random acoustic signal with the same power spectral density across the frequency band) at 30dB above the hearing threshold for each frequency in the normal-hearing ear. We subsequently evaluated the bone-conduction pathway by placing a bone vibrator on the mastoid bone of the hearing-impaired ear and masking the pathway with white noise at 40dB above the hearing threshold of the normal-hearing ear. Following our centre's protocol, the hearing threshold was established for each frequency between 250 and 4000Hz. No response or an air-bone gap of below 10dB was regarded as sensory hearing loss.
In the evaluation of central auditory processes, both groups completed 3 monaural psychoacoustic tests (filtered words test, compressed two-syllable word test, and speech-in-noise test) in a soundproof booth, using the same equipment as in the audiometry plus a calibrated compact disc. The control group underwent these tests in the same ear as did their hearing-impaired peers. The filtered words test consists of a series of 25 words, and the compressed two-syllable word test includes 25 two-syllable words. Words were reproduced at 50dB above the 1-kHz threshold in the ear being tested; the contralateral ear was masked with white noise at 30dB lower than the intensity of the stimulus used for the tested ear. For the speech-in-noise test, we used an acoustic stimulus 50dB above the 1-kHz threshold for the tested ear, which was at the same time masked with white noise 10dB below the intensity used.
All participants were assessed with the revised version of the Woodcock-Muñoz battery (WMB-R). The battery includes tests for the assessment of cognitive function and acquired abilities associated with formal instruction, and evaluates deficiencies and preserved functions, providing data on cognitive skills, verbal language, and academic performance in individuals aged 2-90 years. WMB-R was used to assess the following skills: name recall, sentence recall, visual pairing, incomplete words, visual integration, drawing vocabulary, analysis and synthesis, auditory-visual learning, word recall, crossing out, sound integration, picture recognition, verbal vocabulary, concept formation, number inversion, spatial relationships, sentence comprehension, verbal analogies, mathematical calculation, and dictation. The battery was administered in a controlled setting in a session lasting approximately 2hours; information was presented from in front of the participant, which forced hearing-impaired individuals to orient the unaffected ear towards the source of sound. For the purposes of this study, crude scores on each test were calculated in deciles: performance was therefore scored 0-10.
For data analysis, we created multivariate contingency tables with the results from the WMB-R and the tests for CAPDs. Boxplots were used to analyse the position of the median with regard to the second and third quartiles, since WMB-R data were expressed as medians.
The t test for paired samples was used to analyse quantitative data from the filtered words and compressed two-syllable word tests. Statistical significance was set at P≤.05.
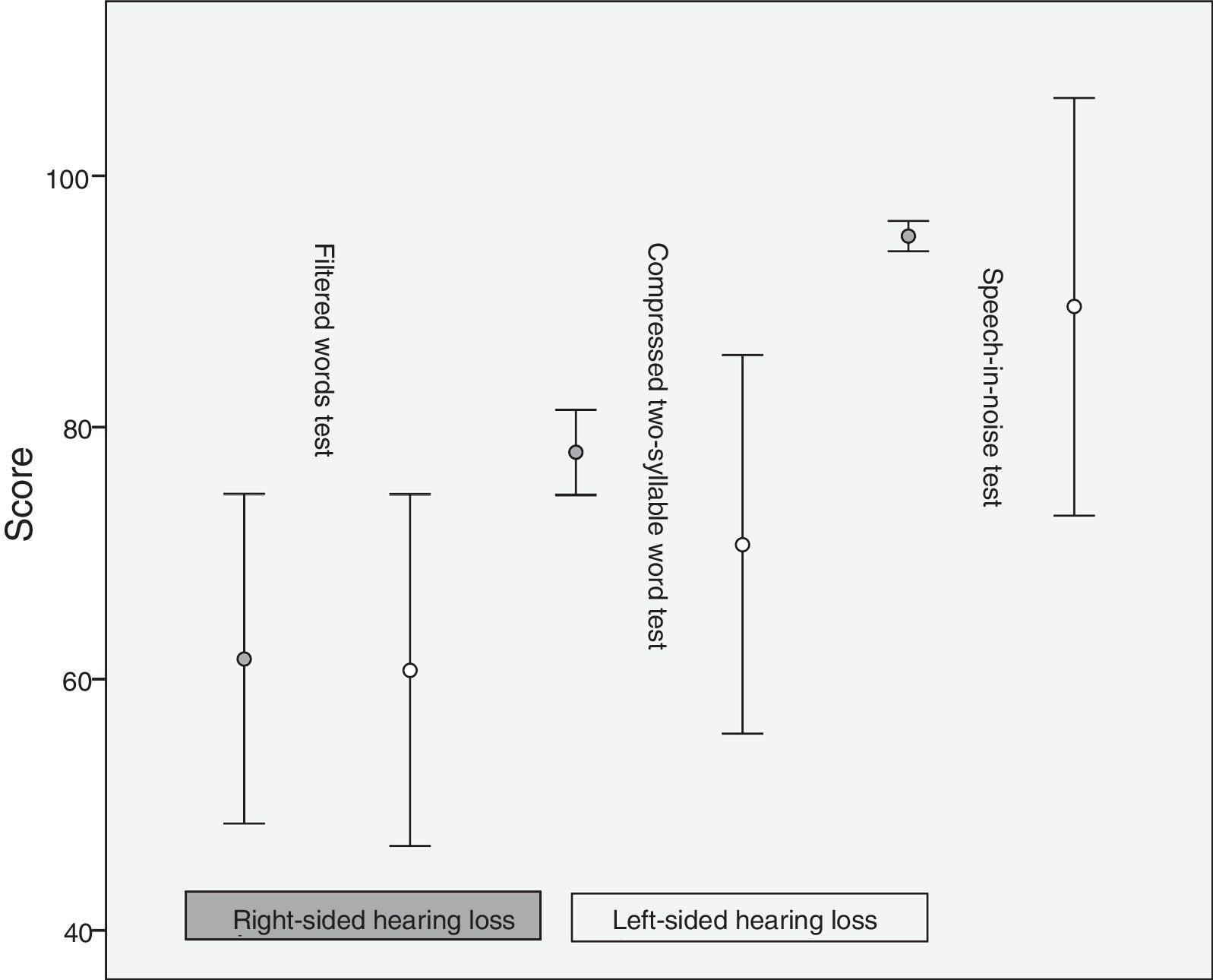
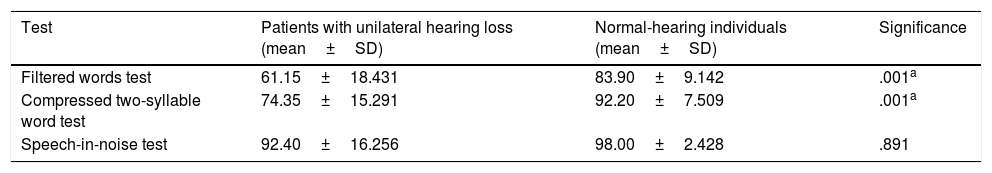
ResultsIn the monaural tests for CAPDs, we found significant intergroup differences in the results of the filtered words test and the compressed two-syllable word test, but not the speech-in-noise test (Table 2).
Scores on the tests for central auditory processing disorders for patients with unilateral hearing loss and normal-hearing individuals.
| Test | Patients with unilateral hearing loss (mean±SD) | Normal-hearing individuals (mean±SD) | Significance |
|---|---|---|---|
| Filtered words test | 61.15±18.431 | 83.90±9.142 | .001a |
| Compressed two-syllable word test | 74.35±15.291 | 92.20±7.509 | .001a |
| Speech-in-noise test | 92.40±16.256 | 98.00±2.428 | .891 |
SD: standard deviation.
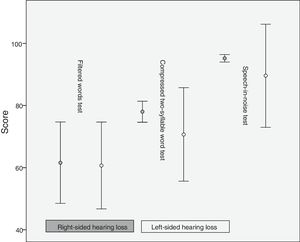
The percentage of error was 61.6±18.3 for the right ear and 60.7±19.53 for the left in the filtered words test, 78±4.71 (right) and 70.7±21.01 (left) in the compressed two-syllable word test, and 95.2±1.68 (right) and 89.6±23.18 (left) in the speech-in-noise test. Differences in mean scores between patients and controls were more marked in the compressed two-syllable word and the speech-in-noise tests than in the filtered words test (Fig. 1).
Regarding the number of repetition errors, all right-sided hearing-impaired patients made mistakes in the words “tres” and “dot”, whereas 9/10 left-sided hearing-impaired patients made mistakes in the word “dot”. In the compressed two-syllable word test, 8/10 right-sided hearing-impaired patients made mistakes in the word “reto”, whereas 7/10 left-sided hearing-impaired patients made mistakes with “quepa”. In the speech-in-noise test, 8/10 of patients in each group made mistakes with the word “tiñoso”.
In the WMB-R, patients scored higher than controls in number inversion, auditory-visual learning, analysis and synthesis, concept formation, and incomplete words, and scored lower in mathematical calculation, verbal vocabulary, and drawing vocabulary.
Right-sided hearing-impaired patients scored higher than normal-hearing individuals in analysis and synthesis, word recall, sentence comprehension, and incomplete words, and scored lower in concept formation, verbal vocabulary, and drawing vocabulary.
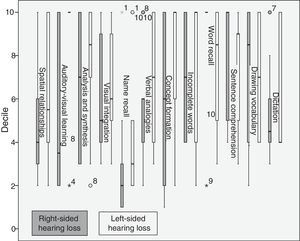
Compared to normal-hearing individuals, left-sided hearing-impaired patients scored higher in number inversion, analysis and synthesis, visual integration, sentence comprehension, and incomplete words, and scored lower in mathematical calculation, picture recognition, word recall, verbal vocabulary, and drawing vocabulary (Fig. 2).
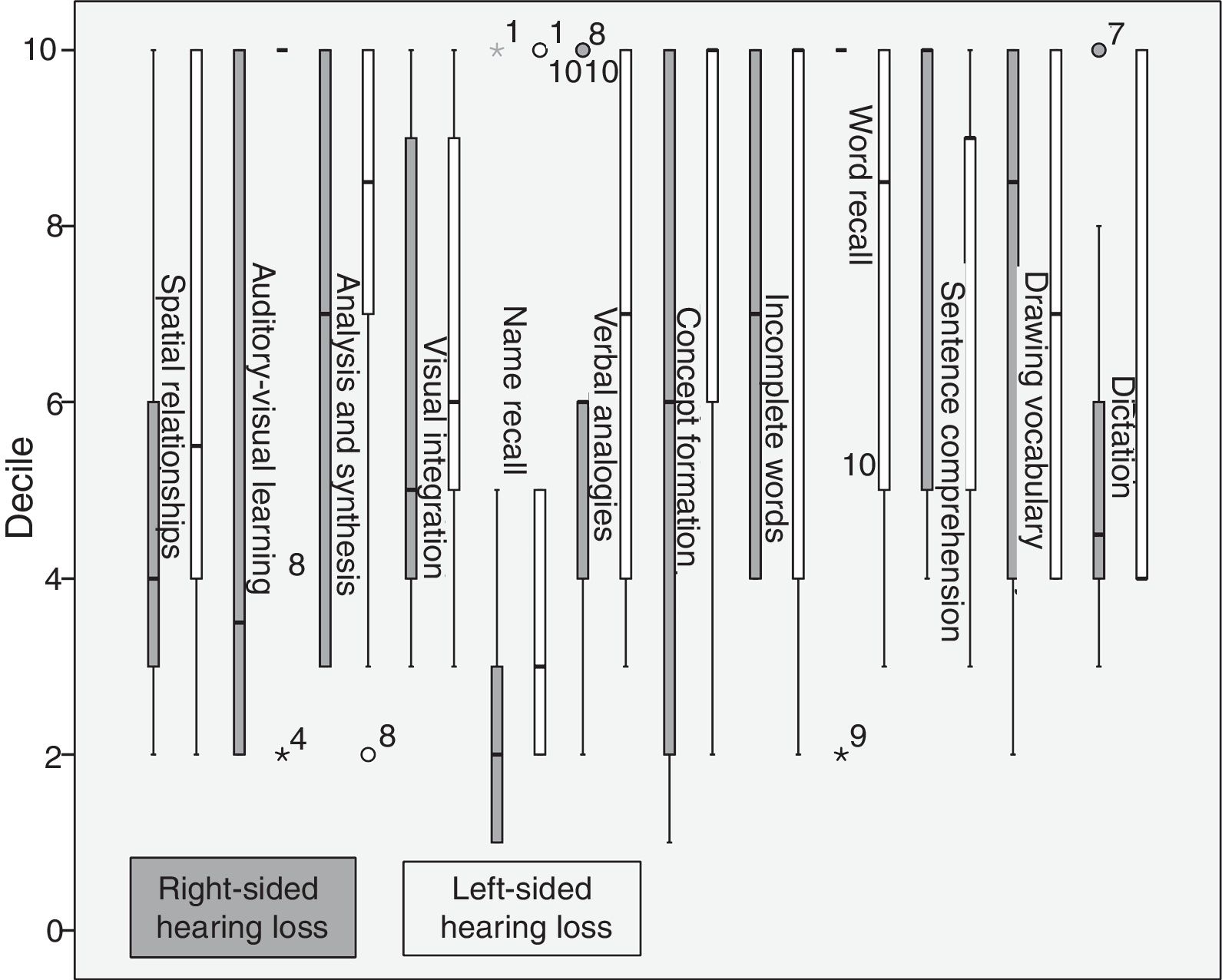
Compared to right-sided hearing-impaired patients, left-sided hearing-impaired patients scored higher on spatial relationships, auditory-visual learning, analysis and synthesis, visual integration, name recall, dictation, verbal analogies, concept formation, and incomplete words, and scored lower on word recall, sentence comprehension, and verbal vocabulary. Right-sided hearing-impaired patients scored higher on word recall, sentence comprehension, drawing vocabulary, and dictation (Fig. 3).
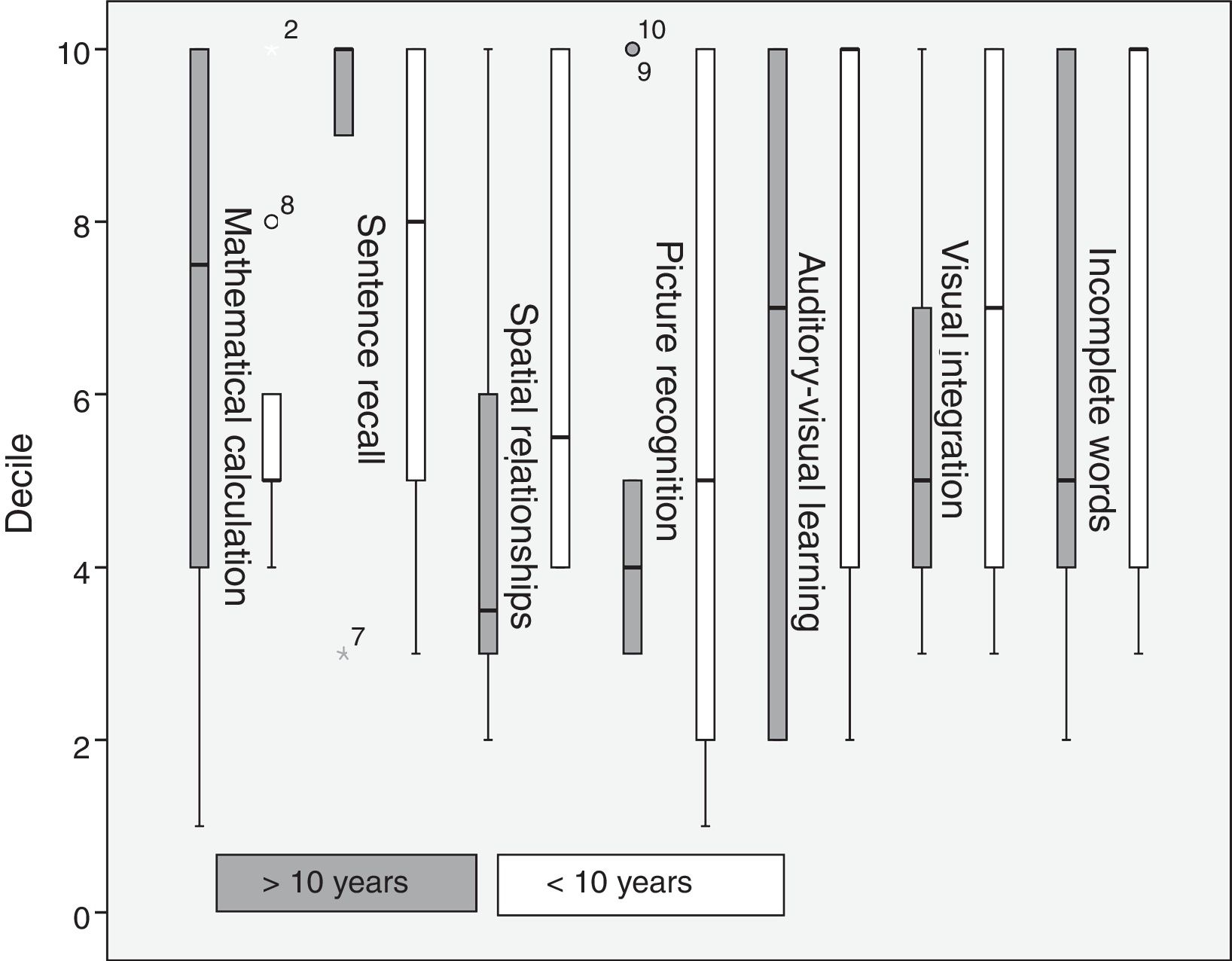
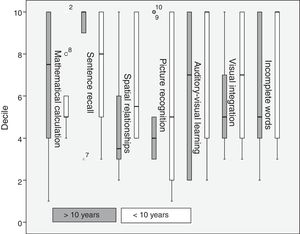
Regarding disease progression time, patients with a progression time of over 10 years scored higher in mathematical calculation and sentence recall, whereas those with a progression time below 10 years scored higher in spatial relationships, picture recognition, auditory-visual learning, visual integration, and incomplete words (Fig. 4).
The sign test was used for those assessment tools showing greater score variability, yielding α=0.125 in analysis and synthesis between right-sided hearing-impaired patients and controls, and α=0.2187 in auditory-visual learning between right-sided and left-sided hearing-impaired patients. These differences were not significant.
DiscussionPatients with unilateral hearing loss showed poor auditory performance in the filtered words and compressed two-syllable word tests compared to normal-hearing individuals. In the speech-in-noise test, the t test for paired samples showed no statistically significant differences between patients and controls.
The filtered words test is monaural and measures the ability to draw meaning from acoustic signals.15 Poor performance in this test may explain the language difficulties reported in patients with unilateral hearing loss10,14,16: in our sample, word comprehension was deficient despite our patients having normal hearing in one ear. The compressed two-syllable word test is moderately sensitive for identifying CNS dysfunctions but not for determining their exact location.15 Poor performance in patients with unilateral hearing loss may be explained by the fact that cortical reorganisation in these patients is different from that of normal-hearing individuals.17,18 We used the WMB-R in both groups to confirm this hypothesis. The speech-in-noise test, in which the stimulus and the mask are applied to the same ear, revealed no significant differences between patients with unilateral hearing loss and normal-hearing individuals. According to other studies performed in a controlled setting, discrimination is adequate in spite of the mask when the stimulus is applied to the normal-hearing ear19; this task required a greater level of attention in patients with unilateral hearing loss.
In the tests for CAPDs, the patients with right-sided hearing loss showed better discrimination ability and lower variability in the results of the compressed two-syllable word test and the speech-in-noise test than the patients with left-sided hearing loss. This contrasts with the structural model proposed by Kimura, according to which the left hemisphere is dominant for language processing in most people: when the contralateral auditory pathway is stimulated and the ipsilateral auditory pathway is suppressed, the right ear has an advantage over the left ear since the information reaches the left hemisphere directly, whereas information delivered to the left ear has to travel from the right to the left hemisphere via the corpus callosum.20 Based on the above, we would expect right-sided hearing-impaired patients to perform worse than left-sided hearing-impaired patients. We should bear in mind, however, that the Kimura model is based on dichotic listening and demonstrates right-ear advantage when a stimulus is presented simultaneously to both ears. This is not true for patients with unilateral hearing loss, since they only have one pathway by which to receive information.
In 2012, Burton et al.17 conducted a study using functional MRI, finding that patients with unilateral hearing loss receiving monaural stimulation showed greater activation of the ipsilateral auditory cortex than did normal-hearing individuals, who displayed activation of the contralateral hemisphere.
According to their results, right-sided hearing-impaired patients show greater activation of the left hemisphere (ipsilateral to the preserved auditory afferences) than left-sided hearing-impaired patients in relation to the right hemisphere (preserved right-hemisphere auditory afferences). This suggests greater resilience of the speech functions in the left hemisphere against reduced crossed inputs from a deafferented right ear.17 These findings better explain the fact that right-sided hearing-impaired patients perform better in monaural tests for CAPDs: in these cases, left-sided afferences are preserved, resulting in ipsilateral stimulation. As the left hemisphere is dominant for language, response to left hemisphere stimulation is better than direct stimulation to the right hemisphere, as would occur with left-sided hearing-impaired patients.
Unlike in other studies conducting tests similar to the WMB-R,14,18 in our study, hearing-impaired patients performed better than normal-hearing individuals in the following tests: incomplete words, analysis and synthesis, auditory-visual learning, and number inversion. The incomplete word test measures auditory analysis and closure, phonological awareness, and phonetic coding.21 Our results stand in contrast with those of other studies assessing language performance in patients with unilateral hearing loss.9,10 The patients with a progression time of over 10 years performed significantly worse than those patients with shorter progression times; the same was true for auditory-visual learning. This suggests that, although patients performed better than controls in these tests, these skills deteriorate as the condition progresses, or simply do not develop fully, as reported in some studies on the development of language skills in children with unilateral hearing loss.9,14,20 Progression time was found to have no impact on performance in tests for analysis and synthesis or number inversion, which evaluate working memory and fluid reasoning. Functional MRI has shown that patients with unilateral hearing loss display greater activity in the area associated with working memory; this area may become stronger due to the need for deeper concentration in completing certain tasks.11
Patients with left-sided hearing loss perform better in analysis and synthesis and in visual integration than those with right-sided hearing loss; according to some studies using functional MRI, this may be explained by changes in functional cytoarchitecture. This increase in default mode network activity suggests a mechanism of functional compensation and/or recovery preventing cognitive impairment, given that hearing loss contributes to loss of grey matter volume in the auditory cortex,22 decreasing the ability of cortical areas to react to auditory stimulation. These changes may affect cognitive processing in the brain's functional networks. The brain undergoes plastic reorganisation to compensate for the sensory deficits caused by hearing loss, in order to limit the consequences of neurological damage and help maintain cognitive function.23 Plastic reorganisation results in increased functional connectivity in some brain regions involving the default mode network.18
Regarding the poor performance of right-sided hearing-impaired patients for visual integration and most cognitive tasks compared to left-sided hearing-impaired patients, we should point out that patients with early-onset right-sided hearing loss show recruitment of visual pathways. This reorganisation may result in disintegration of auditory areas of the cortex, which may negatively affect some cognitive functions.24 We also observed increased connectivity in the right primary auditory cortex and left primary visual cortex, which suggests that long-term right-sided hearing loss promotes multimodal functional reorganisation, probably also affecting the patient's cognitive skills.18 Furthermore, patients with right-sided hearing loss performed better on word recall (short-term memory)21 than left-sided hearing-impaired patients; this may be explained by these patients’ increased attention in everyday activities. These findings are compatible with the results of monaural tests for CAPDs, in which right-sided hearing-impaired patients scored higher. This may be due to increased attention as compensation for right-sided deafferentation. This mechanism benefits auditory discrimination but is insufficient to compensate for cognitive deficiencies.
Patients with left-sided hearing loss perform better on cognitive tasks. These patients have left-sided auditory deafferentation, and show greater brain plasticity and better adaptation to their surroundings than patients with right-sided hearing loss.
Further studies should include larger samples and compare the performance of children with congenital left-sided hearing loss who are not eligible for sound amplifying devices to that of normal-hearing children. We also need further longitudinal studies with paired samples, a controlled setting, and follow-up times of at least 12 years to assess cognitive performance.
This study shows the importance of complementary tests (tests for CAPDs and cognitive skills) for establishing habilitation, rehabilitation, and treatment techniques, with a view to optimising and stimulating skills in patients with unilateral hearing loss.
Auditory deficiencies in patients with unilateral hearing loss result in poor performance in the filtered words and the compressed two-syllable word tests due to difficulties understanding words and the fact that cortical activity in these patients is different from that of normal-hearing individuals. Right-sided hearing-impaired individuals show greater auditory discrimination than left-sided hearing-impaired patients, which is explained by the ipsilateral stimulation of the left hemisphere and left-hemisphere dominance for language processing, which increases the plasticity of language function in the left hemisphere. Regarding cognitive skills, patients with unilateral hearing loss performed better than controls in incomplete words, analysis and synthesis, auditory-visual learning, and number inversion. However, patients with a progression time of less than 10 years showed better cognitive skills. This suggests that some skills may deteriorate over time or may not develop fully in patients with unilateral hearing loss. Patients with unilateral hearing loss showed greater activity in areas associated with working memory. Those patients with left-sided auditory deafferentation seem to be at an advantage over right-sided hearing-impaired patients as they display greater brain plasticity and are better adapted to their surroundings.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Calderón-Leyva I, Díaz-Leines S, Arch-Tirado E, Lino-González AL. Análisis de la relación entre habilidades cognitivas e hipoacusia sensorial severa unilateral. Neurología. 2018;33:283–289.
This study has not been presented at any congress or meeting.