Anosognosia is a frequent symptom in Alzheimer disease (AD). The objective of this article is to describe prevalence of this condition at time of diagnosis and analyse any predisposing factors and their influence on disease progression.
MethodsObservational, prospective, and analytical multi-centre study in an outpatient setting. Patients recently diagnosed with AD (NINCDS-ADRDA criteria) were included. Each patient underwent 2 cognitive, functional, and neuropsychiatric assessments separated by an interval of 18 months. The Clinical Insight Rating Scale was employed as a measure of anosognosia (CIR, scored 0-8). Progression was defined as an increase in the Clinical Dementia Rating Scale-sum of boxes of more than 2.5 points. The predictor variables were analysed using binary logistic regression.
ResultsThe study included 127 patients, and 94 completed both assessments. Of the total, 31.5% displayed severe anosognosia (CIR 7-8); 39.4%, altered level of consciousness (CIR 3–6); and 29.1%, normal awareness (CIR 0-2). The median baseline CIR in this cohort was 4 (Q1–Q3: 1-7), and at 18 months, 6 (Q1–Q3: 3-8), P<.001. Advanced age (odds ratio (OR) 2.43; CI 95%: 1.14-5.19), lower educational level (OR 2.15; CI 95%: 1.01-4.58), and more marked neuropsychiatric symptoms (OR 2.66; 95% CI: 1.23-5.74) were predictor variables of anosognosia. Baseline CIR was similar in the groups with and without significant clinical progression.
ConclusionsThe large majority of patients with AD at the time of diagnosis showed significant anosognosia, and this condition was associated with advanced age, lower educational level, and more marked behavioural symptoms. Our results did not show that anosognosia had an effect on the initial clinical progression of AD after diagnosis.
La anosognosia es frecuente en la enfermedad de Alzheimer (EA). El objetivo fue describir su prevalencia en el momento del diagnóstico y analizar los factores predisponentes y su influencia en la evolución posterior de la EA.
MétodosEstudio observacional, multicéntrico, prospectivo, analítico, realizado en consultas de neurología general. Se incluyó a pacientes recién diagnosticados de EA (criterios NINCDS-ADRDA). Se realizaron 2 evaluaciones — cognitivas, funcionales y neuropsiquiátricas—, con un intervalo de 18 meses. Se empleó la Clinical Insight Rating scale como medida de anosognosia (CIR, rango 0-8). El criterio de progresión fue un incremento en la Clinical Dementia Rating-sum of boxes mayor a 2,5 puntos. Las variables predictoras se analizaron mediante regresión logística.
ResultadosSe incluyó a 127 pacientes, 94 completaron las 2 evaluaciones. El 31,5% mostraba anosognosia grave (CIR 7-8), el 39,4% conciencia alterada (CIR 3-6) y el 29,1% conciencia normal (CIR 0-2). La mediana del CIR basal en la cohorte fue 4 (Q1-Q3: 1-7) y a los 18 meses 6 (Q1-Q3: 3-8); p<0,001. La edad avanzada (odds ratio [OR] 2,43; IC del 95%, 1,14-5,19), menor escolaridad (OR 2,15; IC del 95%, 1,01-4,58) y mayor afectación neuropsiquiátrica (OR 2,66; IC del 95%, 1,23-5,74) fueron variables predictoras de anosognosia. El CIR basal fue similar en los grupos con y sin progresión clínica significativa.
ConclusionesLa gran mayoría de los pacientes con EA en el momento del diagnóstico muestran un grado significativo de anosognosia que se asocia a mayor edad, menor escolaridad y mayor afectación conductual. No se demostró influencia de la anosognosia sobre la evolución inicial de la EA tras el diagnóstico.
Anosognosia is defined as the inability to identify the presence of the deficits characteristic of a disease (cognitive, motor, sensory, or affective) as well as their magnitude, progression, and how they limit daily life.1 It is a common manifestation in right hemisphere infarcts, as described by Babinski in 1914. Anosognosia is a very frequent symptom in Alzheimer disease (AD), even at early stages, although it is also frequent in such other entities as frontotemporal dementia and schizophrenia.2 Anosognosia has no anatomical or biochemical correlate since it is a complex and multidimensional phenomenon of varying nature and intensity. Recent studies with perfusion SPECT and functional MRI suggest that anosognosia is associated with involvement of the right dorsolateral frontal lobe,3 right inferior frontal gyrus,4 anterior cingulate cortex, and right parietal-temporal region.5 Patients with this symptom showed poorer performance on the neuropsychological tests that assess these areas as well as those evaluating executive and visuospatial functions.6
The relevance of anosognosia in AD resides in its high frequency and its personal, family, and social health consequences. Lack of awareness of a disease is associated with a greater risk of dangerous behaviours7 and increased caregiver burden.8,9 In real life, it can represent an obstacle to treatment adherence and to such basic tasks as driving, managing personal finances, or making a will.
Previous research on prevalence of anosognosia in AD consists of cross-sectional studies or data from memory clinics. The conceptual disparities and the different measurement tools used explain the differences in prevalence rates, which range between 5% and 70%.1,10,11 A recent study conducted in Spain by Turro-Garriga et al.12 reported a prevalence rate of 46.7%, which increased to 91% in cases of moderate to advanced dementia.
The DEMDIAG prospective study (for the evaluation of Alzheimer-type dementia at the time of diagnosis) was designed to assess different clinical, sociodemographic, and nutritional characteristics of AD at the time of diagnosis and analyse their influence on disease progression at 18 months. The purpose of our study was to evaluate the prevalence of anosognosia, its predisposing factors, and its influence on AD progression.
Patients and methodsStudy designWe conducted a prospective, observational, multicentre, analytic study at the outpatient neurology clinics of the Hospital Universitario Río Hortega de Valladolid and the Complejo Hospitalario de Segovia. Patients underwent 2 evaluations. The patients included in the baseline evaluation were prospectively recruited from October 2009 to March 2011. The second evaluation was conducted 18 months (±1) after the baseline evaluation, from April 2011 to October 2012.
Inclusion criteriaThe inclusion criteria were: (1) patients of any age and sex; (2) living in Castile-Leon; (3) who had visited the neurology clinic for the first time due to symptoms of cognitive impairment of any degree; (4) who were subsequently diagnosed with dementia with AD; and (5) who had a reliable caregiver or informant. We did not include institutionalised patients. The diagnosis of dementia with AD was based on the NINCDS-ADRDA criteria for probable AD.13
Study protocolThe patients underwent 2 neurological and neuropsychological assessments (at baseline and at 18 months). The neurologists in charge, experts in assessing and treating dementia, followed up the patients during the period between the 2 assessments. The baseline assessment included a structured interview aimed at gathering different sociodemographic variables and data on years of schooling. The education level was classified into ‘no studies’ (illiterate patients or those who had not completed primary education), ‘primary education’, or ‘secondary or higher education’. We gathered data from the patients’ medical histories, with a special emphasis on vascular risk factors, lifestyle habits, and a family history of dementia. The differential diagnosis of secondary dementias included a blood test (ions, renal function, thyroid hormones, and vitamin B12). APOE genotype was determined. All patients underwent neuroimaging studies, preferably a brain MRI scan, or when unavailable, a brain CT. Delays in diagnosis were expressed as the number of months elapsed between symptom onset and AD diagnosis.
Measurement instrumentsWe used the Clinical Insight Rating scale (CIR),14 whose results are based on the information provided by patients and their caregivers during the clinical interview, to assess the level of anosognosia. The CIR scale assesses 4 aspects: (1) awareness of the situation (reason for consultation and the surrounding circumstances); (2) awareness of the memory deficit and other cognitive problems; (3) awareness of the functional deficit in activities of daily living; and (4) awareness of the progression of cognitive impairment. In this scale, full awareness is scored 0, impaired awareness 1, and no awareness 2. The total score of this scale (range, 0-8) is the sum of scores on each item. The highest score indicates no awareness of the disease.
To assess the cognitive state we used the Spanish-language version of the Cambridge Cognitive Examination-Revised (CAMCOG-R; range, 0-105),15 which includes the Folstein Mini–Mental State Examination (MMSE). We used the Spanish-language version of the Rapid Disability Rating Scale-2 (RDRS-2; range, 18-72)16 for the functional evaluation. To evaluate behaviour we used the Spanish-language version of the Neuropsychiatric Inventory Questionnaire [NPI-Q])17, which measures the presence and severity of 12 behavioural changes, as well as the distress caused to caregivers. NPI-Q severity score ranges from 0 to 36 and NPI-Q distress score ranges from 0 to 60. Caregiver burden was evaluated using the Zarit Burden Inventory (range, 22-110).18 We used the Clinical Dementia Rating (CDR) as an overall measurement of dementia severity.19 The total score was calculated using the algorithm proposed by Morris.20 Additionally, we calculated the CDR sum of boxes (CDR-SOB) as the sum of the individual scores on each of the 6 domains evaluated in the scale.21 Progression of AD between the 2 evaluations was defined as an increase of more than 2.5 points on the CDR-SOB.22
Statistical analysisThe clinical and sociodemographic characteristics were analysed in terms of absolute and relative frequencies for qualitative variables and measures of central tendency and dispersion for quantitative variables. Normal distribution was assessed using the Kolmogorov–Smirnov test to determine whether a parametric or a non-parametric test was appropriate. We used the chi-square test to compare proportions. For purposes of contrasting hypotheses, we used the t-test for normal variables (independent or paired data) and one-way ANOVA. For ordinal quantitative variables and non-normal quantitative variables, we used the Mann–Whitney U test or Kruskal–Wallis H test for independent variables, and the Wilcoxon rank sum test for paired data.
For the analysis of predictive variables of anosognosia, we divided the CIR score into baseline scores below, equal to, and above the median (dependent variable: CIR score ≥5) and we conducted a forward stepwise multiple logistic regression analysis. We classified all the quantitative variables included in the model according to their median value in the baseline evaluation. We calculated the adjusted odds ratio (OR) with 95% confidence intervals (95% CI).
We had previously conducted an inter-rater reliability study of the CIR scale in 35 patients with AD. Inter-rater agreement showed an intraclass correlation coefficient (ICC) of 0.93 (95% CI: 0.87-0.96; P<.01), with a Kappa coefficient of 0.6. The test–retest reliability (intraobserver agreement) showed an ICC of 0.89 (95% CI: 0.77-0.95; P<.01), with a Kappa coefficient of 0.43. For all the analyses, CIR scores were classified as preserved awareness (score 0-2), impaired awareness (score 3-6), and anosognosia (scores 7 and 8).
Statistical analysis was completed using SPSS software v. 22.0 (IBM Corp, Armonk, NY, USA). The significance level was established at P<.05.
Ethical considerationsThe study was approved by the Clinical Research Ethics Committee at the Hospital Universitario Río Hortega de Valladolid and the Complejo Hospitalario de Segovia. Informed consent forms were signed by all patients or their legal representatives or closest family member in case of disability.
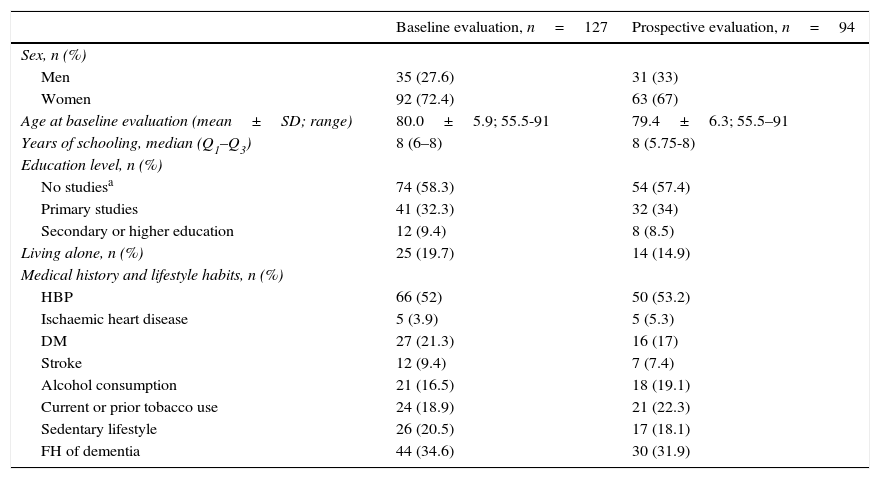
ResultsWe included 127 patients with dementia with AD in the baseline evaluation. Twenty-one patients were lost to follow-up, 3 were excluded due to a severe disease, and 9 died. A total of 94 patients completed the 2 evaluations. Table 1 shows some sociodemographic characteristics and the most relevant medical history of all the subjects included in our study and those who completed the 2 evaluations. The percentage of women was higher (87.8% vs 67%, P<.05) in the group completing one evaluation (n=33) than in the group completing 2 (n=94). The patients in the group completing one evaluation were older (mean age: 81.7 vs 79.4 years; P=.06). No differences were observed in the remaining sociodemographic characteristics between the 2 groups.
Sociodemographic characteristics of all the evaluated subjects.
| Baseline evaluation, n=127 | Prospective evaluation, n=94 | |
|---|---|---|
| Sex, n (%) | ||
| Men | 35 (27.6) | 31 (33) |
| Women | 92 (72.4) | 63 (67) |
| Age at baseline evaluation (mean±SD; range) | 80.0±5.9; 55.5-91 | 79.4±6.3; 55.5–91 |
| Years of schooling, median (Q1–Q3) | 8 (6–8) | 8 (5.75-8) |
| Education level, n (%) | ||
| No studiesa | 74 (58.3) | 54 (57.4) |
| Primary studies | 41 (32.3) | 32 (34) |
| Secondary or higher education | 12 (9.4) | 8 (8.5) |
| Living alone, n (%) | 25 (19.7) | 14 (14.9) |
| Medical history and lifestyle habits, n (%) | ||
| HBP | 66 (52) | 50 (53.2) |
| Ischaemic heart disease | 5 (3.9) | 5 (5.3) |
| DM | 27 (21.3) | 16 (17) |
| Stroke | 12 (9.4) | 7 (7.4) |
| Alcohol consumption | 21 (16.5) | 18 (19.1) |
| Current or prior tobacco use | 24 (18.9) | 21 (22.3) |
| Sedentary lifestyle | 26 (20.5) | 17 (18.1) |
| FH of dementia | 44 (34.6) | 30 (31.9) |
FH: family history; DM: diabetes mellitus; HBP: high blood pressure.
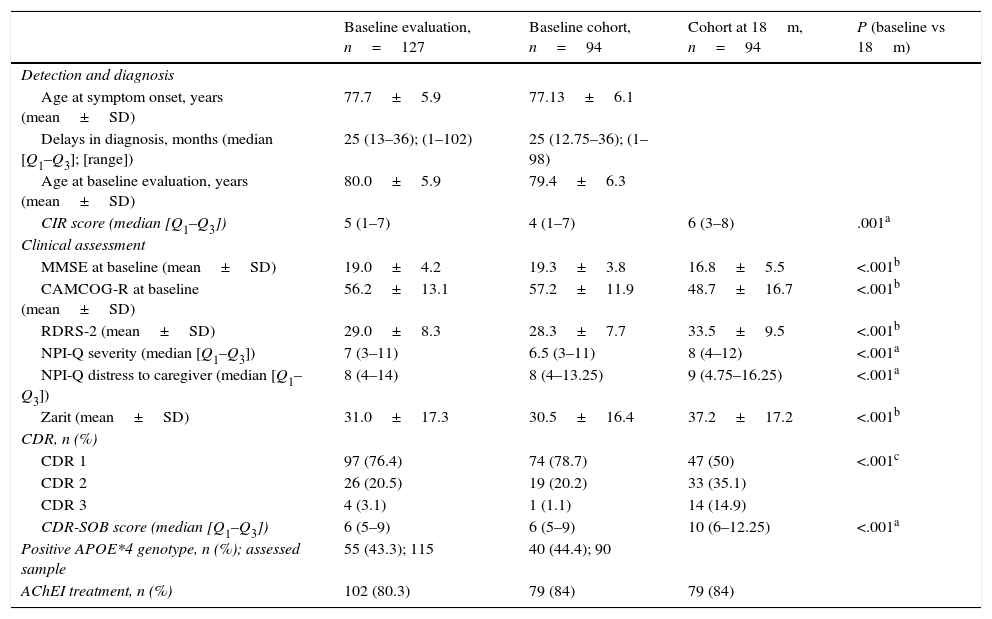
Table 2 shows the clinical characteristics of dementia in all the included patients and in the patients who completed the 2 evaluations, as well as the differences between the baseline evaluation and the evaluation at 18 months. APOE genotype was determined in 115 patients; 55 (43.3%) were carriers of the APOE*4. The percentage of patients with severe dementia (CDR score=3) was higher in the group completing 1 evaluation than in the group completing 2 (9.1% vs 1.1%, P<0.5). Scores for the evaluation at 18 months were significantly worse than baseline scores in all scales. At the second evaluation, 84% of the patients were following treatment with cholinesterase inhibitors. The median CIR score was 5 (Q1–Q3: 1–7) for the 127 patients included in the baseline evaluation. Disease awareness was preserved in 37 patients (29.1%) and impaired in 50 (39.4%), and 40 presented anosognosia (31.5%).
Clinical characteristics of dementia.
| Baseline evaluation, n=127 | Baseline cohort, n=94 | Cohort at 18m, n=94 | P (baseline vs 18m) | |
|---|---|---|---|---|
| Detection and diagnosis | ||||
| Age at symptom onset, years (mean±SD) | 77.7±5.9 | 77.13±6.1 | ||
| Delays in diagnosis, months (median [Q1–Q3]; [range]) | 25 (13–36); (1–102) | 25 (12.75–36); (1–98) | ||
| Age at baseline evaluation, years (mean±SD) | 80.0±5.9 | 79.4±6.3 | ||
| CIR score (median [Q1–Q3]) | 5 (1–7) | 4 (1–7) | 6 (3–8) | .001a |
| Clinical assessment | ||||
| MMSE at baseline (mean±SD) | 19.0±4.2 | 19.3±3.8 | 16.8±5.5 | <.001b |
| CAMCOG-R at baseline (mean±SD) | 56.2±13.1 | 57.2±11.9 | 48.7±16.7 | <.001b |
| RDRS-2 (mean±SD) | 29.0±8.3 | 28.3±7.7 | 33.5±9.5 | <.001b |
| NPI-Q severity (median [Q1–Q3]) | 7 (3–11) | 6.5 (3–11) | 8 (4–12) | <.001a |
| NPI-Q distress to caregiver (median [Q1–Q3]) | 8 (4–14) | 8 (4–13.25) | 9 (4.75–16.25) | <.001a |
| Zarit (mean±SD) | 31.0±17.3 | 30.5±16.4 | 37.2±17.2 | <.001b |
| CDR, n (%) | ||||
| CDR 1 | 97 (76.4) | 74 (78.7) | 47 (50) | <.001c |
| CDR 2 | 26 (20.5) | 19 (20.2) | 33 (35.1) | |
| CDR 3 | 4 (3.1) | 1 (1.1) | 14 (14.9) | |
| CDR-SOB score (median [Q1–Q3]) | 6 (5–9) | 6 (5–9) | 10 (6–12.25) | <.001a |
| Positive APOE*4 genotype, n (%); assessed sample | 55 (43.3); 115 | 40 (44.4); 90 | ||
| AChEI treatment, n (%) | 102 (80.3) | 79 (84) | 79 (84) | |
CAMCOG-R: cognitive section of the Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating scale; CDR-SOB: Clinical Dementia Rating sum of boxes; AChEI: acetylcholinesterase inhibitors; MMSE: Mini–Mental State Examination; NPI-Q: Neuropsychiatric Inventory Questionnaire; RDRS-2: Rapid Disability Rating Scale-2.
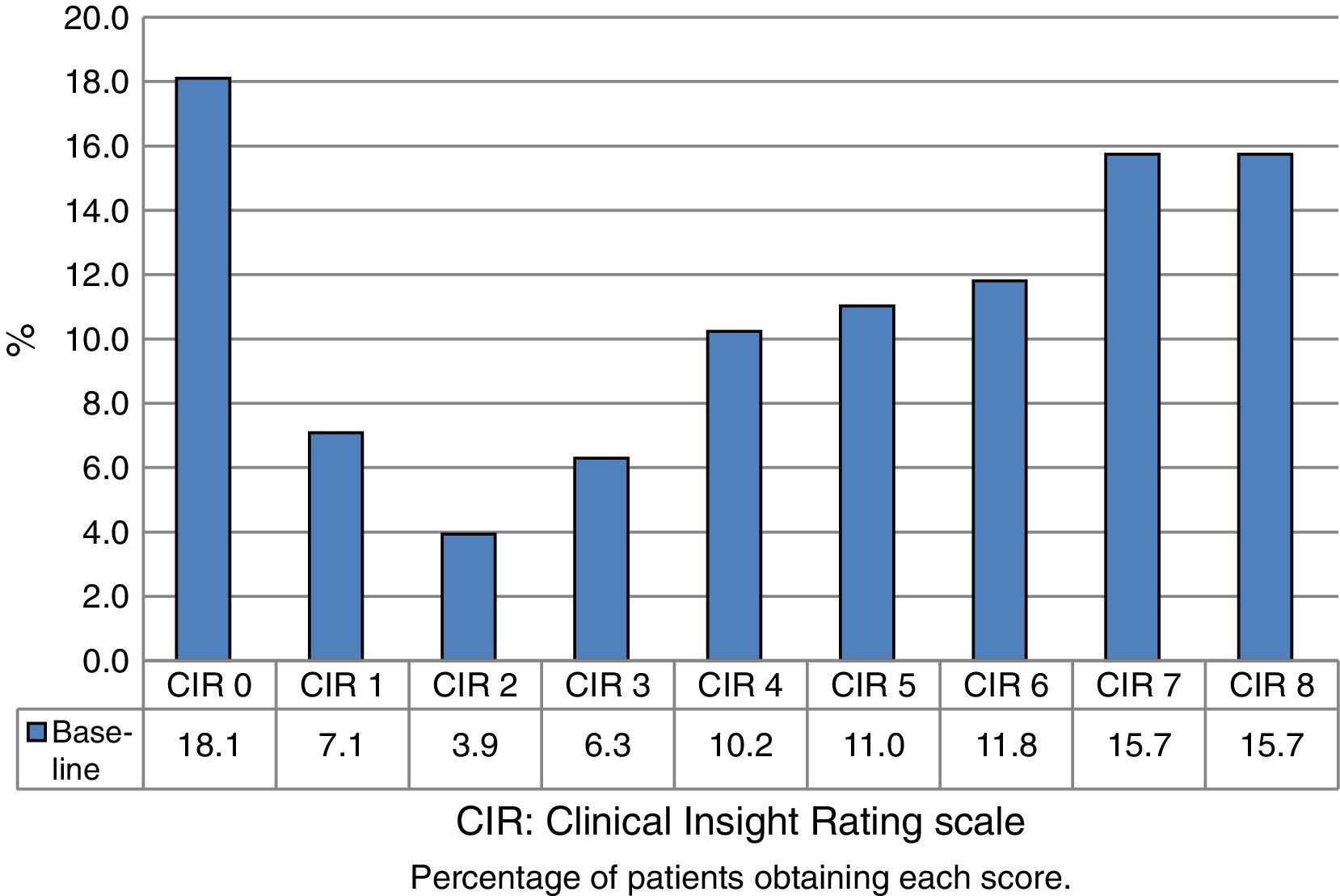
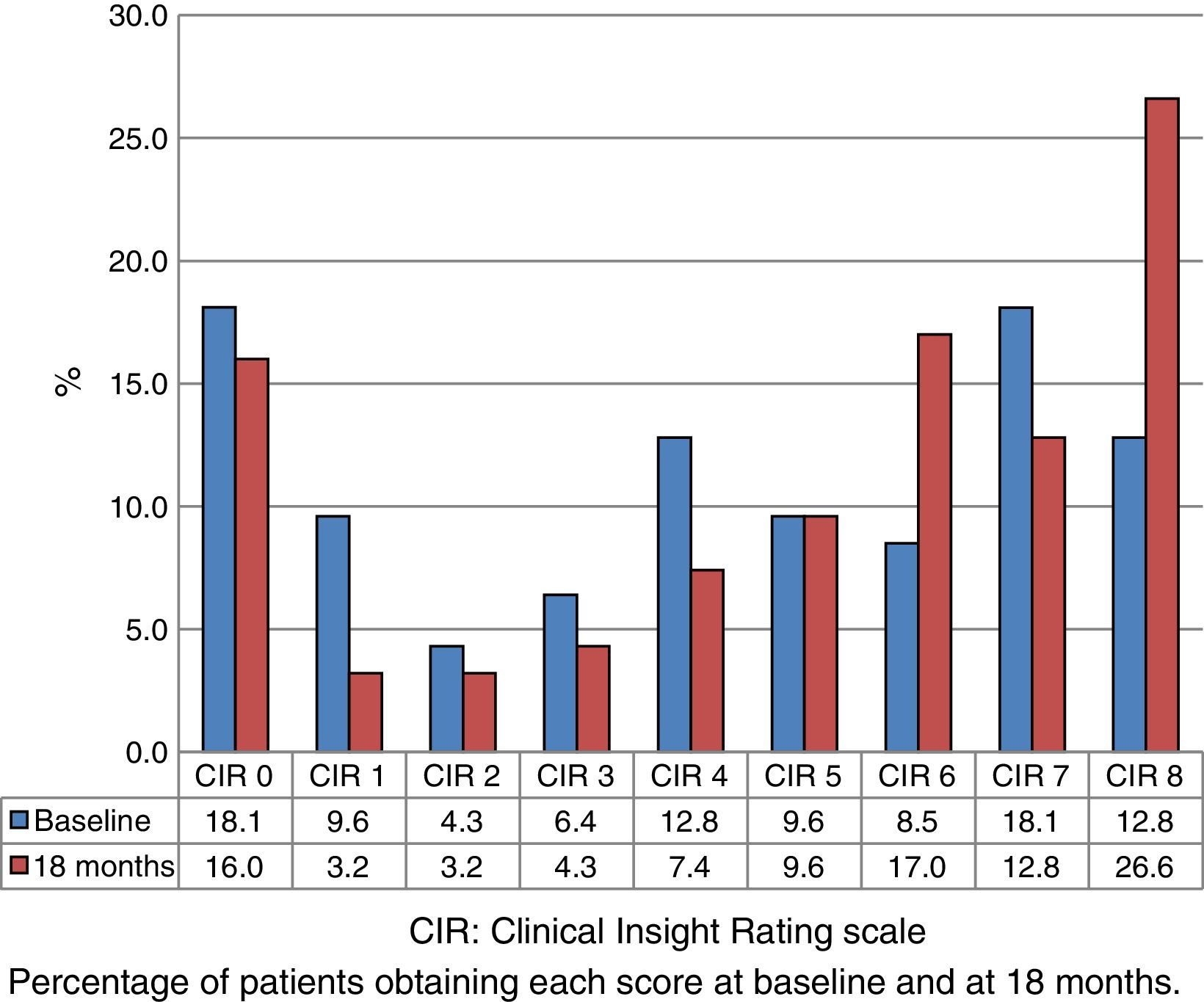
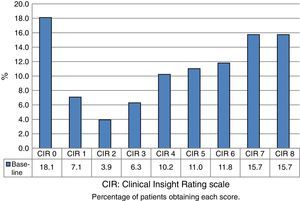
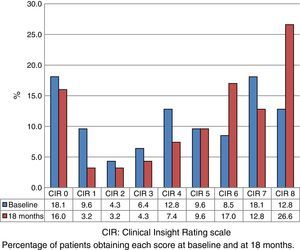
Fig. 1 shows the distribution of CIR scores in the baseline evaluation of all the assessed patients. This distribution is bimodal, with higher representation of the highest and lowest scores. Fig. 2 displays the differences in scores between the 2 evaluations. The percentage of patients with anosognosia increased from 30.9% to 39.4%, and in the case of impaired awareness, from 37.2% to 38.3%. Preserved awareness decreased from 31.9% to 22.3%. The lack of awareness of disease progression was the factor most contributing to the overall score on the CIR scale, although no statistically significant differences were observed between this and the other 3 factors.
Table 3 shows the sociodemographic and clinical characteristics by degree of anosognosia observed in the baseline evaluation of all the included patients. Greater cognitive impairment according to MMSE and CAMCOG-R results was associated with lower disease awareness. The frequency of the analysed vascular risk factors (arterial hypertension, ischaemic heart disease, a history of stroke, diabetes mellitus, smoking, and sedentary lifestyle) was similar between the different degrees of anosognosia and showed no statistically significant differences. The median CIR score was significantly higher in patients with negative APOE*4 than in those with positive APOE*4 (5 [Q1–Q3: 2.25–7] vs 4 [Q1–Q3: 1–6]; P<.05). Baseline scores on cognitive tests were similar between groups with positive and negative APOE *4 genotype (MMSE 19 and 19.1, respectively; P=.93; CAMCOG-R 57.5 and 55.2, respectively; P=.35). No differences were observed in the median CIR score by sex (men 5 [Q1–Q3: 3–7], women 5 [Q1–Q3: 1–7]; P=.33) or education level (no studies 5 [Q1–Q3: 2–7], primary or higher education 4 [Q1–Q3: 1–6]; P=.21]). Changes in CIR scores at 18 months were negatively correlated with the change in scores on the MMSE (r=−0.43, P<.001) and CAMCOG-R (r=−0.34, P<.001), and positively correlated with the change in scores on the CDR (r=0.28, P<.01) and CDR-SOB (r=.025, P<.05).
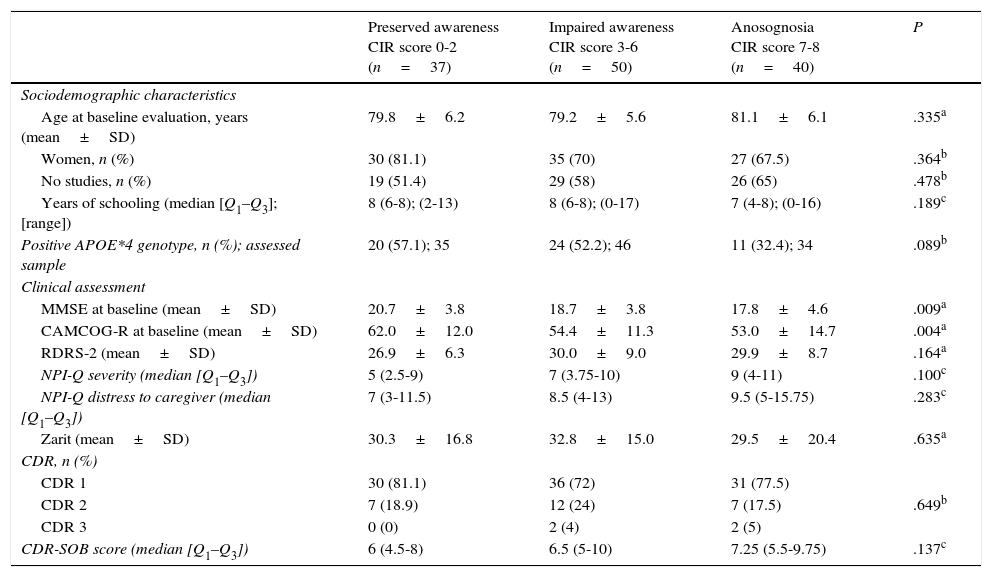
Sociodemographic and clinical characteristics by degree of anosognosia observed at the baseline evaluation (n=127).
| Preserved awareness CIR score 0-2 (n=37) | Impaired awareness CIR score 3-6 (n=50) | Anosognosia CIR score 7-8 (n=40) | P | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age at baseline evaluation, years (mean±SD) | 79.8±6.2 | 79.2±5.6 | 81.1±6.1 | .335a |
| Women, n (%) | 30 (81.1) | 35 (70) | 27 (67.5) | .364b |
| No studies, n (%) | 19 (51.4) | 29 (58) | 26 (65) | .478b |
| Years of schooling (median [Q1–Q3]; [range]) | 8 (6-8); (2-13) | 8 (6-8); (0-17) | 7 (4-8); (0-16) | .189c |
| Positive APOE*4 genotype, n (%); assessed sample | 20 (57.1); 35 | 24 (52.2); 46 | 11 (32.4); 34 | .089b |
| Clinical assessment | ||||
| MMSE at baseline (mean±SD) | 20.7±3.8 | 18.7±3.8 | 17.8±4.6 | .009a |
| CAMCOG-R at baseline (mean±SD) | 62.0±12.0 | 54.4±11.3 | 53.0±14.7 | .004a |
| RDRS-2 (mean±SD) | 26.9±6.3 | 30.0±9.0 | 29.9±8.7 | .164a |
| NPI-Q severity (median [Q1–Q3]) | 5 (2.5-9) | 7 (3.75-10) | 9 (4-11) | .100c |
| NPI-Q distress to caregiver (median [Q1–Q3]) | 7 (3-11.5) | 8.5 (4-13) | 9.5 (5-15.75) | .283c |
| Zarit (mean±SD) | 30.3±16.8 | 32.8±15.0 | 29.5±20.4 | .635a |
| CDR, n (%) | ||||
| CDR 1 | 30 (81.1) | 36 (72) | 31 (77.5) | |
| CDR 2 | 7 (18.9) | 12 (24) | 7 (17.5) | .649b |
| CDR 3 | 0 (0) | 2 (4) | 2 (5) | |
| CDR-SOB score (median [Q1–Q3]) | 6 (4.5-8) | 6.5 (5-10) | 7.25 (5.5-9.75) | .137c |
CAMCOG-R: cognitive section of the Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating; CDR-SOB: Clinical Dementia Rating sum of boxes; MMSE: Mini–Mental State Examination; NPI-Q: Neuropsychiatric Inventory Questionnaire; RDRS-2: Rapid Disability Rating Scale-2.
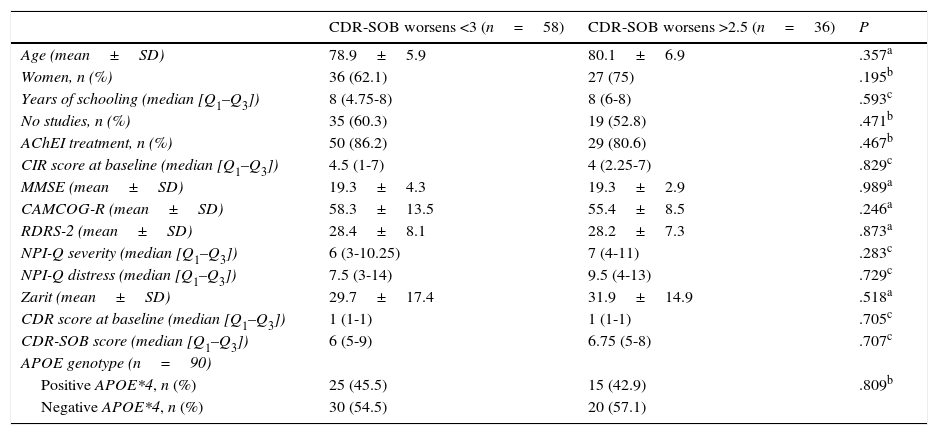
Table 4 shows some clinical and demographic characteristics and baselines scores on the evaluation scales according to disease progression at 18 months. At that time, 58 patients remained stable according to the established criterion and 36 showed greater cognitive impairment. The median CIR score was similar in the baseline evaluation of both groups (4.5 [Q1–Q3: 1–7] vs 4 [Q1–Q3: 2.25–7]; P<.83).
Sociodemographic and clinical characteristics by progression (n=94).
| CDR-SOB worsens <3 (n=58) | CDR-SOB worsens >2.5 (n=36) | P | |
|---|---|---|---|
| Age (mean±SD) | 78.9±5.9 | 80.1±6.9 | .357a |
| Women, n (%) | 36 (62.1) | 27 (75) | .195b |
| Years of schooling (median [Q1–Q3]) | 8 (4.75-8) | 8 (6-8) | .593c |
| No studies, n (%) | 35 (60.3) | 19 (52.8) | .471b |
| AChEI treatment, n (%) | 50 (86.2) | 29 (80.6) | .467b |
| CIR score at baseline (median [Q1–Q3]) | 4.5 (1-7) | 4 (2.25-7) | .829c |
| MMSE (mean±SD) | 19.3±4.3 | 19.3±2.9 | .989a |
| CAMCOG-R (mean±SD) | 58.3±13.5 | 55.4±8.5 | .246a |
| RDRS-2 (mean±SD) | 28.4±8.1 | 28.2±7.3 | .873a |
| NPI-Q severity (median [Q1–Q3]) | 6 (3-10.25) | 7 (4-11) | .283c |
| NPI-Q distress (median [Q1–Q3]) | 7.5 (3-14) | 9.5 (4-13) | .729c |
| Zarit (mean±SD) | 29.7±17.4 | 31.9±14.9 | .518a |
| CDR score at baseline (median [Q1–Q3]) | 1 (1-1) | 1 (1-1) | .705c |
| CDR-SOB score (median [Q1–Q3]) | 6 (5-9) | 6.75 (5-8) | .707c |
| APOE genotype (n=90) | |||
| Positive APOE*4, n (%) | 25 (45.5) | 15 (42.9) | .809b |
| Negative APOE*4, n (%) | 30 (54.5) | 20 (57.1) |
CAMCOG-R: cognitive section of the Cambridge Cognitive Examination-Revised; CDR: Clinical Dementia Rating; CDR-SOB: Clinical Dementia Rating sum of boxes; MMSE: Mini–Mental State Examination; NPI-Q: Neuropsychiatric Inventory Questionnaire; RDRS-2: Rapid Disability Rating Scale-2.
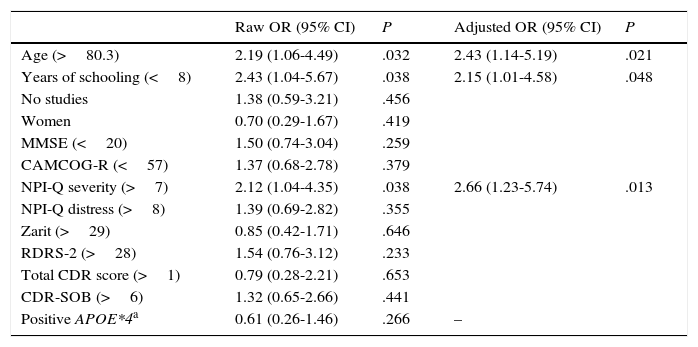
Table 5 shows an analysis of the risk factors for anosognosia in the whole study population (dependent variable: CIR score ≥5). Older ages (OR: 2.43 for patients older than 80.3 years), years of schooling (OR: 2.15 for fewer than 8 years), and NPI-Q severity subscale scores (OR: 2.7 for scores >7) were included in the final model of predictive variables for anosognosia.
Analysis of risk factors for anosognosia (dependent variable: CIR≥5 at baseline evaluation), n=127.
| Raw OR (95% CI) | P | Adjusted OR (95% CI) | P | |
|---|---|---|---|---|
| Age (>80.3) | 2.19 (1.06-4.49) | .032 | 2.43 (1.14-5.19) | .021 |
| Years of schooling (<8) | 2.43 (1.04-5.67) | .038 | 2.15 (1.01-4.58) | .048 |
| No studies | 1.38 (0.59-3.21) | .456 | ||
| Women | 0.70 (0.29-1.67) | .419 | ||
| MMSE (<20) | 1.50 (0.74-3.04) | .259 | ||
| CAMCOG-R (<57) | 1.37 (0.68-2.78) | .379 | ||
| NPI-Q severity (>7) | 2.12 (1.04-4.35) | .038 | 2.66 (1.23-5.74) | .013 |
| NPI-Q distress (>8) | 1.39 (0.69-2.82) | .355 | ||
| Zarit (>29) | 0.85 (0.42-1.71) | .646 | ||
| RDRS-2 (>28) | 1.54 (0.76-3.12) | .233 | ||
| Total CDR score (>1) | 0.79 (0.28-2.21) | .653 | ||
| CDR-SOB (>6) | 1.32 (0.65-2.66) | .441 | ||
| Positive APOE*4a | 0.61 (0.26-1.46) | .266 | – |
Variables included in the logistic regression model: age, years of schooling, NPI-Q severity, and APOE*4 genotype.
The measurement tool is an essential element in the analysis of anosognosia in populations with AD, since no well-established or acknowledged instrument for this aim has been developed.23 Anosognosia in patients with dementia can be assessed using several methods: clinical scales, questionnaires, performance-based methods, phenomenological methods, and multidimensional methods.24 The most widely used techniques to date are clinical judgement in structured interviews (clinical scales) and comparison of patient and caregiver reports on the same questionnaire.23,25 Although CIR analyses few dimensions and is a simple tool, we decided to use it in our study since it is an easy-to-use and fast clinical scale with acceptable reliability parameters. In our sample, prevalence of impaired awareness of the disease or anosognosia (CIR score ≥3) was 71%; this rate is higher than those obtained in other studies using different measurement tools, which makes them difficult to compare. Prevalence values in previous studies ranged between 20% and 68%.1,10–12,25–27 Some of them have focused on describing this symptom in the context of mild AD. In a study of amnesic mild cognitive impairment and mild AD, Orfei et al.28 reported anosognosia prevalence rates of 3% and 42%, respectively. Kalbe et al.29 conducted a study with patients with MMSE scores ≥24 and found that patients with mild AD were significantly more prone to underestimate cognitive dysfunction than patients with mild cognitive impairment.
The main distinguishing feature of the DEMDIAG study is that it is intrinsically linked to a specific situation: the time of AD diagnosis. This situation may have significant practical implications since it represents the first contact of the specialist with patients and their disease. In fact, it can provide a first prognostic approach if factors associated with a more aggressive progression of the disease are detected. This undoubtedly justifies performing these studies at the moment of diagnosis. Logically enough, it is highly influenced by the characteristics of healthcare (model used, territorial differences, ease of access to a specialist), cultural and social factors (identification of symptoms, global impact, family history of AD), and clinical factors (initial symptoms, presence of behavioural symptoms, and comorbidities). Almost all patients in our study were diagnosed with mild to moderate AD (CDR score 1 and 2, mean MMSE of 19), had a mean age of 79 years, and showed a median delay of 25 months between initial symptoms and diagnosis. These figures are very similar to those from the EACE observational study, which consisted of an extensive evaluation of the developmental stage of AD conducted in neurology and geriatric specialist clinics.30 We can therefore state that they reflect the Spanish reality of AD at the moment of diagnosis.
The DEMDIAG study offers a prospective view of disease progression over the first 18 months after diagnosis. Progression was defined as an increase in the CDR-SOB of more than 3 points during that period. The annual rate of change in CDR-SOB scores is 1.9 in patients with a baseline CDR score of 1, as reported by a recent extensive longitudinal study. This rate is higher in older patients with an APOE*4 allele.22 In view of its psychometric properties, the CDR-SOB has recently been proposed as a primary outcome measurement tool for clinical trials of AD.31 In our series, only 38% of the patients showed progression according to the definition, although scores on all the scales were significantly worse in the second evaluation than at baseline. Baseline CIR scores were similar in the group which remained stable and the group showing disease progression; therefore, anosognosia does not seem to be associated with a higher risk of progression, at least in the first few months following diagnosis. Few prospective studies have assessed this aspect of the disease. Starkstein et al.32 described a significant increase in the frequency of anosognosia during follow-up of a series of 62 patients with AD. The patients with severe anosognosia showed longer disease duration.
The bivariate analysis showed a clear association between the lack of disease awareness and greater cognitive impairment. Furthermore, scores on cognitive tests and global scales got worse in parallel with the degree of anosognosia. These findings have already been reported by previous studies.1,6,11,12,33,34 However, we found no differences in such other variables as sex, age, or education level, in contrast with previous studies.7,11,12,35 Likewise, we found no association with poorer functional status or greater caregiver burden, which have been linked to anosognosia in other studies.8,9,12,36 It is striking that the degree of anosognosia was significantly lower in the patients with positive APOE*4 genotype. Any attempt to explain this phenomenon would be merely speculative.
According to the multivariate analysis, predictive values for anosognosia were older ages, lower education level, and greater behavioural impairment. Previous studies report contradictory data on these variables. Clare et al.37 described the opposite effect with regards to age: they found a greater prevalence of anosognosia in younger patients. Aalten et al.38 and Turro-Garriga et al.,12 however, have described an association between older age and higher level of anosognosia, in line with our findings. Regarding education level, a lower cognitive reserve (measured as education, occupation, and reading ability) has been associated with a greater degree of anosognosia.39 Furthermore, the effect of behaviour disorders has recently been assessed by Horning et al.40 in 107 patients with probable AD. As could be expected, good disease awareness was associated with depression and anxiety, even after controlling for the variable of cognitive performance. However, anosognosia was associated with higher levels of apathy.
The DEMDIAG study presents some limitations. The first of these is the small sample size and the number of patients lost to follow-up, especially elderly patients. The results from the previous reliability analysis of the CIR scale were not optimal, particularly regarding intraobserver agreement (test–retest). Likewise, classifying patients by scores on the scale is an artificial approach. In addition, progression on the CDR-SOB scale may have been strict, considering the mean age at the baseline evaluation and the frequency of APOE*4. Lastly, the lower proportion of carriers of this allele in the group with anosognosia does not allow us to rule out the possible inclusion of patients with non-Alzheimer dementia in this group. In any case, we feel that the main limitation stems from the fact that anosognosia is a complex and fluctuating symptom in terms of both dimension and magnitude, and is closely related to the perception of a third person, the caregiver.
Our results confirm that anosognosia is a highly prevalent symptom in AD which is already present at time of diagnosis in most cases. However, the level of awareness of a disease does not seem to have an impact on overall progression of the disease after diagnosis. Achieving a better management of such a frequent symptom in AD could help improve adherence to treatment for dementia or other comorbidities and especially prevent dangerous behaviours in these patients.
FundingThe DEMDIAG study received public funding from the Health Department of the Regional Government of Castile-Leon (dossier GRS 556/A/10).
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the staff of the neurology departments at Complejo Hospitalario de Segovia and Hospital Universitario Río Hortega de Valladolid for their help in the selection of patients.
Please cite this article as: Castrillo Sanz A, Andrés Calvo M, Repiso Gento I, Izquierdo Delgado E, Gutierrez Ríos R, Rodríguez Herrero R, et al. Anosognosia en la enfermedad de Alzheimer: prevalencia, factores asociados e influencia en la evolución de la enfermedad. Neurología. 2016;31:296–304.
This work was partially presented as an oral communication at the 65th Annual Meeting of the Spanish Society of Neurology, Barcelona, November 2013 and the 66th Annual Meeting of the Spanish Society of Neurology, Valencia, November 2014.