When CNS infiltration is present in lymphoplasmacytic lymphoma or Waldenström macroglobulinaemia (WM), the entity is called Bing-Neel syndrome (BNS). CNS involvement as an initial sign of WM has only very rarely been described in the literature, and it is even less frequent than co-present orbital and CNS involvement in WM.1
We present the case of a 76-year-old man with CNS involvement of lymphoplasmacytic lymphoma; this condition manifested as chronic meningitis several years before WM was diagnosed and orbital infiltration also developed.
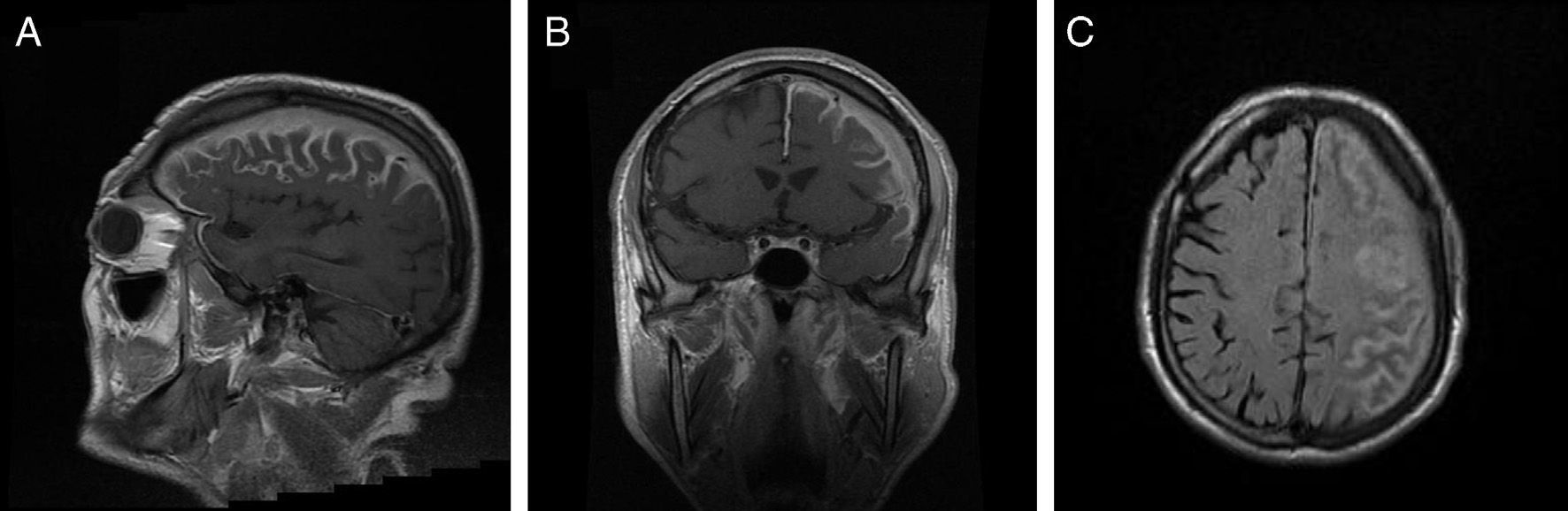
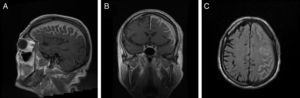
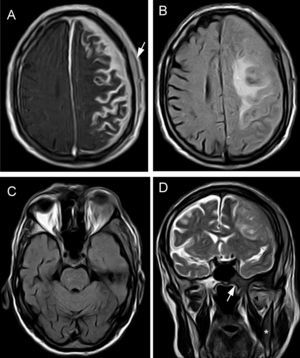
Our patient had a history of chronic atrial fibrillation, depression, and lumbar disc herniation. He had been attending follow-up appointments in another centre for 3 years due to chronic meningitis of unknown origin. Clinical symptoms appeared progressively, including impaired speech production, right upper limb functional impairment in manipulation tasks, and gait impairment due to left lower limb weakness, as well as motor partial seizures with secondary generalisation. Although the patient displayed intact language comprehension, the examination revealed dysarthria, resulting in non-fluent and sometimes unintelligible speech, right-limb paresis (4/5 power) with exaggerated deep tendon reflexes, and right-sided Babinski reflex. A brain MRI study with gadolinium (Fig. 1) revealed diffuse leptomeningeal and dura mater enhancement in the left frontoparietal convexity and signal alterations in the spinal cord of the left parietal bone, greater wing of the sphenoid bone, and base of the pterygoid processes. We conducted an immunology study (antinuclear antibodies, anticytoplasmic antibodies, anti-DNA, VSG, rheumatoid factor, anti-SS-A, anti-SS-B, anti-Scl, anti-Jo, anticentromere), as well as VSG, syphilis serological test, Borrelia test, ACE, Mantoux test, and chest radiography, to rule out infectious and granulomatous diseases (Wegener syndrome, tuberculosis, neurosarcoidosis) that might explain meningeal involvement. All tests yielded negative results. The total protein test showed an IgM concentration of 2429mg/dL and an IgM kappa monoclonal spike. Bone marrow biopsy revealed hypercellularity in the space between trabeculae, with cells pertaining to all 3 cell types; we found poorly-defined groups of small cells with little or no cytoplasm that were also present in the interstitium. The cytology evidenced plasmacytosis and lymphocytosis of mature lymphocytes with no blasts. The immunohistochemical study yielded positive results for CD20, BCL2, and kappa, and negative results for CD10, CD43, CD23, and lambda. CD5 study revealed isolated positive cells, while CD38 revealed mild plasmacytosis. These findings were compatible with low-grade lymphoproliferative syndrome, and the immunophenotype was suggestive of WM. Computed tomography of the chest, abdomen, and pelvis showed no involvement in those areas. Lumbar puncture values were as follows: cell count, 2 leukocytes/mm3; glucose, 72mg/dL; and total protein, 176mg/dL. Analysis of Ig in CSF revealed elevated IgM levels: 22.9mg/dL (reference value<0.2). The cytology study yielded negative results. Findings from fungal and mycobacterial cultures were also negative. Immunophenotype of CSF cells was nonspecific. Electromyography results were compatible with sensorimotor demyelinating polyneuropathy affecting all 4 limbs.
Brain MRI. (A) and (B) Coronal and sagittal T1-weighted sequences with gadolinium contrast showing enhancement of the left frontoparietal cortical sulci, subarachnoid space, and left subdural space. (C) Axial FLAIR sequence showing enhancement of the left frontoparietal cortical sulci, subarachnoid space, and left subdural space.
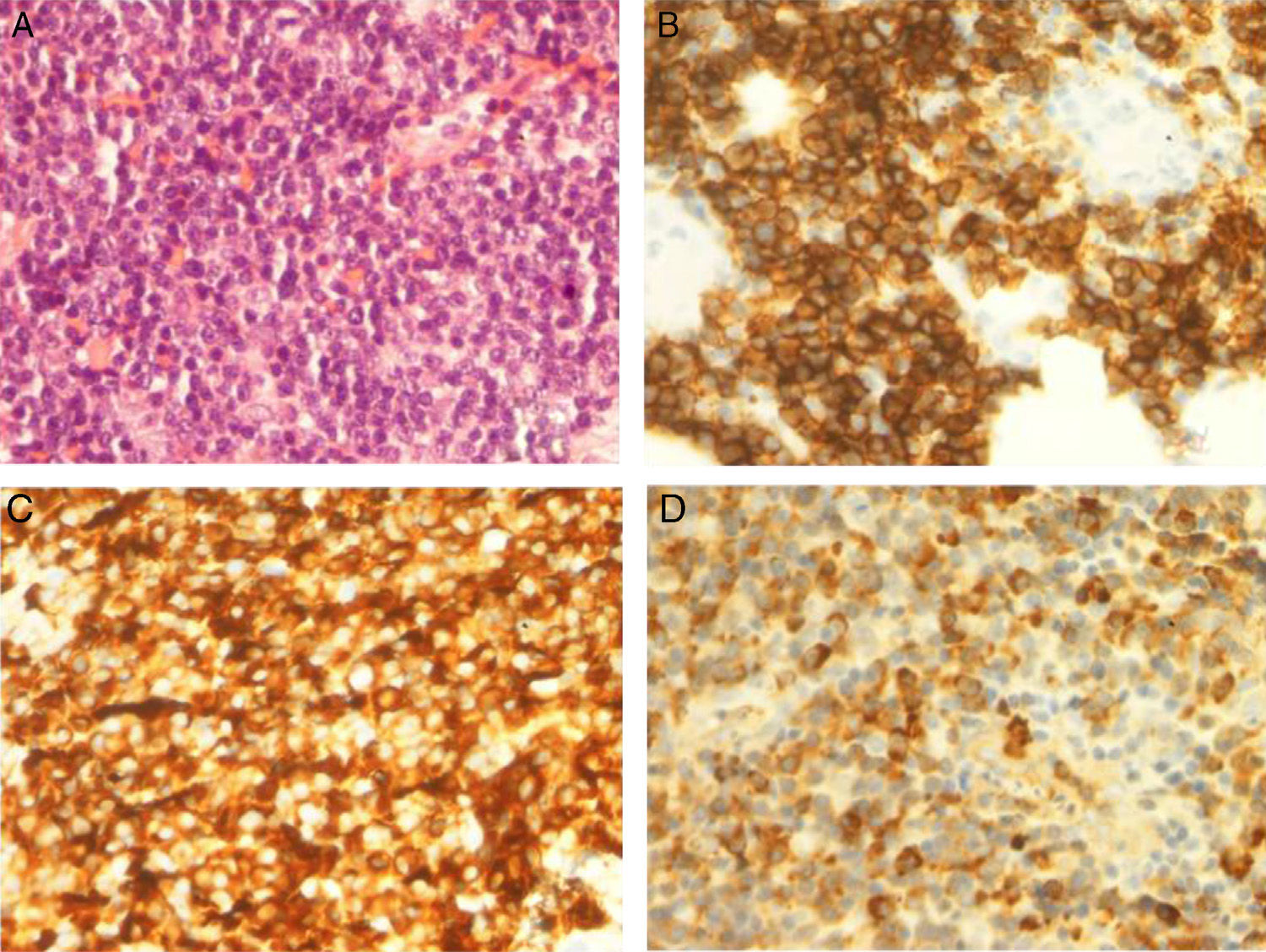
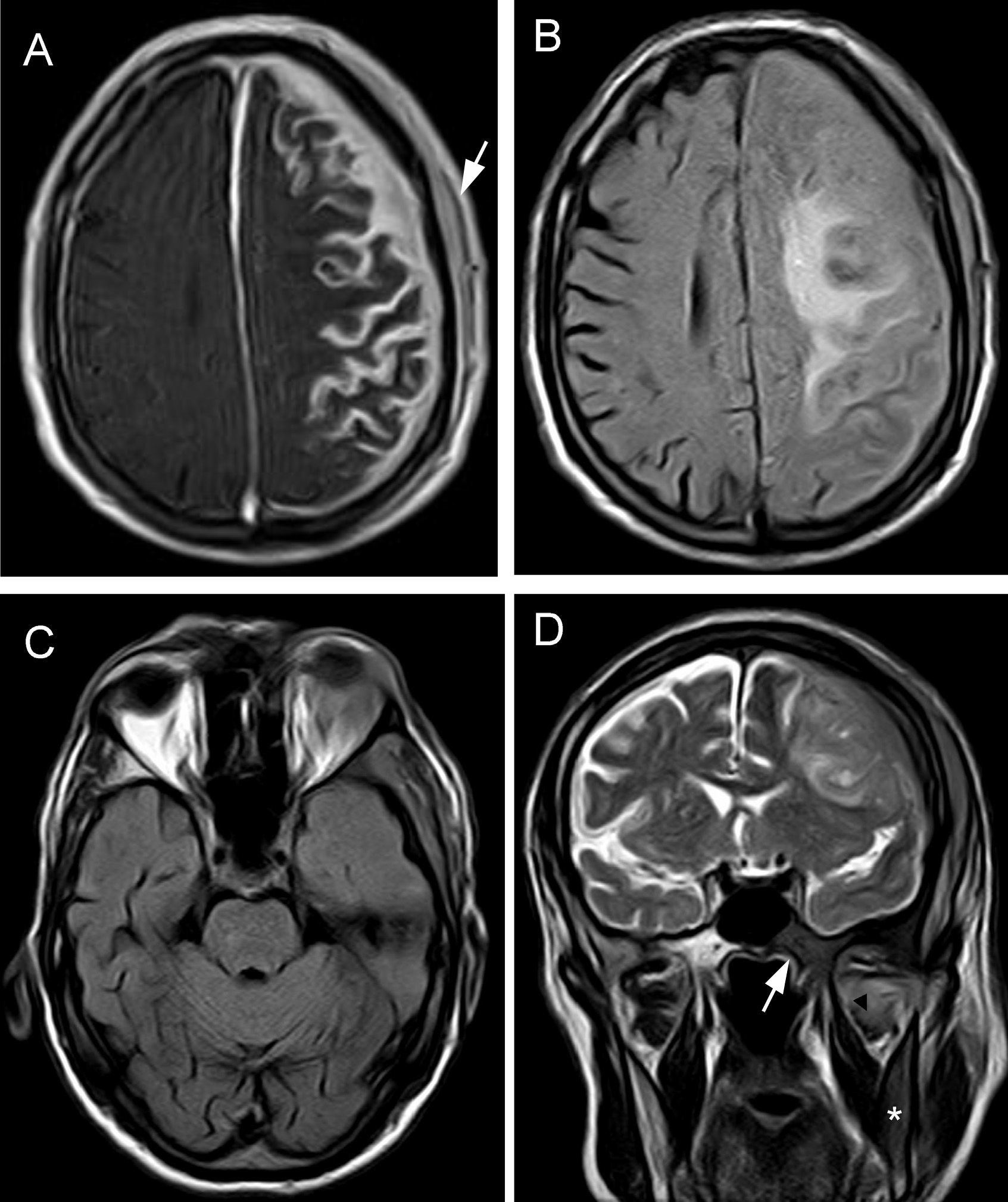
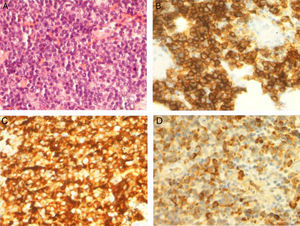
The patient was treated with rituximab and Leukeran®. Six months later a tumour developed in his left eye and neurological focal signs worsened. We performed another MRI study (Fig. 2) and found lesions in the left hemimandible, sphenoid bone, and cranial vault, as well as epicranial soft tissue mass infiltrating the temporal muscle, dura, and leptomeninges, with a mass effect on the parenchyma. MRI scan also showed intraorbital and lacrimal gland invasion with lesions extending to the septum and the upper lid. Results from the palpebral biopsy indicated infiltration of IgM kappa lymphoplasmacytic lymphoma (Fig. 3). The patient underwent chemotherapy with R-CHOP and later with methotrexate and fludarabine. After several cycles, he experienced clinical and radiological improvements, especially resolution of proptosis; improvements related to brain infiltration were less marked.
Brain MRI. (A) Axial T1-weighted sequence with magnetisation transfer contrast and gadolinum. Extensive leptomeningeal enhancement is seen in the left frontoparietal convexity, with dura mater thickening due to intradiploic involvement and epicranial soft tissue lesions (arrow). (B) FLAIR sequence: intraparenchymal vasogenic component of oedema in the left centrum semiovale. (C) Axial FLAIR sequence showing orbital mass affecting the left lacrimal gland and unilateral exophthalmos. D) Coronal T2-weighted TSE sequence: pathological hypointensity on the right side of the body of the sphenoid bone (arrow), base of the pterygoid process, and horizontal ramus of the mandible (asterisk). Signal changes were also seen in the lateral pterygoid muscle (arrowhead). The left side of the skull displays meningeal and epicranial infiltration.
In 1937, Bing and Neel described a syndrome characterised by anaemia and neurological signs associated with increased levels of serum and CSF immunoglobulins.2 Eight years later, Waldenström described the first case of macroglobulinaemia associated with constitutional symptoms, anaemia, thrombocytopenia, and bone marrow infiltration of lymphocytes and lymphoplasmacytic cells.3 CNS manifestations of WM are called Bing-Neel syndrome.4,5 Fintelmann et al.6 conducted a review of the literature including 50 cases of BNS and suggested establishing 2 categories: (a) CNS involvement caused by lymphoplasmacytoid cells infiltrating the brain parenchyma, dura, meninges, or CSF, and (b) a non-cellular form in which IgM deposition might be the cause of the neurological symptoms. In both categories, neurological symptoms will develop after the patient has been diagnosed with macroglobulinaemia. There are only 2 reported cases in which diagnosis was made at time of symptom onset. Arias et al.7 describe a case of rapidly progressing dementia which also coincided with a diagnosis of WM. We found only one study8 in the literature describing a 54-year-old patient whose BNS manifested as a cauda equina syndrome that was present for 3 years before he was diagnosed with WM. In our patient, neurological symptoms presented insidiously and they were the only manifestations occurring in a 3-year period. He was diagnosed with WM after undergoing a study to determine the aetiology of his chronic meningitis. At this point, his symptoms may have been identified as group B in the Fintelmann classification since no lymphoplasmacytic cells were found in CSF and no biopsy had been performed.
Orbital involvement in WM is also rare. The most frequent ocular complications are those caused by hyperviscosity syndrome secondary to increased serum IgM levels, which result in such vaso-occlusive syndromes as retinal haemorrhages and optic nerve oedema.1 Isolated cases of tumours in the orbit, eyelid, bulbar conjunctiva, and lacrimal gland have been described.9 Simultaneous brain and orbital involvement in WM is even more infrequent. Stacy et al.1 describe a case opposite to our own: orbital involvement preceding meningeal, cauda equina, and CSF involvement. These authors broaden the spectrum of BNS group A by including orbital involvement in that category. In our case, a histopathological study confirming the presence of WM cells in the orbit in addition to intracranial infiltration places our patient in category A. This supports Fintelmann's theory6 that group B represents an earlier stage of the disease in which perivascular and parenchymal deposition of IgM causes the blood-brain barrier to break, leading to invasion by lymphoplasmacytic cells. Currently, there is no consensus regarding treatment of BNS. A variety of therapeutic approaches have delivered different results. They include intravenous and intrathecal chemotherapy and brain radiotherapy.10,11 In our case, orbital and brain lesions went into partial remission following several cycles of chemotherapy.
In conclusion, we would like to highlight the importance of understanding CNS and orbital infiltration in this blood dyscrasia. These alterations may occur not only during the course of the disease but also before diagnosis. Furthermore, infiltration can even be present in both locations simultaneously during disease progression.
This study was presented in poster format at the 64th Annual Meeting of the Spanish Society of Neurology, 2012.
Please cite this article as: Cuenca Hernández R, Guzman de Villoria Lebiedziejewski JA, Roa Martínez E, Menarguez Diaz J. Síndrome de Bing-Neel como debut de una macroglobulinemia de Waldenström asociada a infiltración orbitaria. Neurología. 2015;30:252–255.