Cerebral amyloid angiopathy–related inflammation (CAA-ri) is an entity characterised by an inflammatory response to β-amyloid deposition in the walls of cerebral microvessels.
MethodsWe conducted a retrospective review of a series of patients with a diagnosis of CAA-ri according to histopathological study findings or clinical-radiological diagnostic criteria.
ResultsThe study included 7 patients (5 men) with a mean age of 79 years. Disease onset was acute or subacute in 6 patients. The most frequent symptoms were cognitive impairment (n = 6), behavioural alterations (n = 5), epileptic seizures (n = 5), focal neurological signs (n = 4), and headache (n = 2). Cerebrospinal fluid was abnormal in 3 patients (lymphocytic pleocytosis and high protein levels). The most frequent MRI findings were microbleeds (n = 7), subcortical white matter hyperintensities on T2-FLAIR sequences (n = 7), and leptomeningeal enhancement (n = 6). Lesions were bilateral in 3 patients and most frequently involved the parieto-occipital region (n = 5). Amyloid PET studies were performed in 2 patients, one of whom showed pathological findings. Two patients underwent brain biopsy, which confirmed diagnosis. All patients received immunosuppressive therapy. An initially favourable clinical-radiological response was observed in all cases, with 2 patients presenting radiological recurrence after treatment withdrawal, with a subsequent improvement after treatment was resumed.
ConclusionsEarly diagnosis of CAA-ri is essential: early treatment has been shown to improve prognosis and reduce the risk of recurrence. Although a histopathological study is needed to confirm diagnosis, clinical-radiological criteria enable diagnosis without biopsy.
La inflamación relacionada con angiopatía amiloide es una entidad caracterizada por una respuesta inflamatoria alrededor de los depósitos de beta amiloide de la microcirculación cerebral.
MétodosRevisión retrospectiva de una serie de pacientes con inflamación relacionada con angiopatía amiloide, que cumplieran criterios clínico-radiológicos o con diagnóstico histopatológico confirmado.
ResultadosSe incluyeron 7 pacientes, 5 varones, con edad media de 79 años. El inicio fue agudo o subagudo en 6 de los casos. La clínica más frecuente fue deterioro cognitivo (n = 6), alteraciones de conducta (n = 5), crisis epilépticas (n = 5), focalidad neurológica (n = 4) y cefalea (n = 2). El líquido cefalorraquídeo fue anormal en 3 de 5 casos (pleocitosis linfocitaria e hiperproteinorraquia). Las imágenes de resonancia magnética cerebral más frecuentes consistieron en microhemorragias (n = 7), hiperintensidades subcorticales en secuencia T2-FLAIR (n = 7) y realce leptomeníngeo (n = 6). La afectación fue bilateral en 3 de los casos, con predominio en regiones parieto-occipitales (n = 5). Se realizó PET de amiloide en 2 pacientes, resultando positivo en uno. Se obtuvo confirmación histopatológica mediante biopsia en dos de los casos. Todos los pacientes recibieron tratamiento inmunosupresor, objetivándose una respuesta clínica y radiológica inicial favorable, con recaída radiológica en dos de los casos tras la retirada del tratamiento, y mejorando tras la reinstauración.
ConclusionesEl diagnóstico resulta imprescindible de cara a iniciar un tratamiento precoz, ya que ha demostrado mejorar el pronóstico y disminuir las recurrencias. Si bien el diagnóstico definitivo es histopatológico, los criterios clínico-radiológicos permiten el diagnóstico de esta entidad sin necesidad de biopsia.
Cerebral amyloid angiopathy (CAA) is caused by beta-amyloid (Aβ) deposition in the tunica media and tunica adventitia of the cortical and leptomeningeal microcirculation, particularly in arterioles and capillaries, and less frequently in venules.1,2 One possible complication of CAA is CAA-related inflammation (CAA-ri). CAA-ri is a rare but increasingly well recognised entity characterised by a vascular or perivascular inflammatory response surrounding amyloid deposits.3 Two subtypes of CAA-ri have been described: inflammatory CAA, defined as perivascular inflammation without destruction of vessels, and Aβ-related angiitis (ABRA), when involvement is transmural or intramural, often associated with granuloma formation, an angiodestructive process. Recent studies propose the inclusion of ABRA as a subgroup of primary CNS vasculitis, whereas other authors suggest that it belongs on the CAA-ri spectrum,4 as many patients simultaneously present characteristics of both entities.5,6
This study describes the most relevant clinical, neuroimaging, and histopathological features of this rare entity, as well as its treatment and prognosis, based on information obtained from a series of 7 cases of CAA-ri from our centre and a narrative review of the literature.
Patients and methodsStudy design, location, and participantsThis single-centre retrospective study includes all patients diagnosed with CAA-ri evaluated at a university hospital in the period 2005 to 2020.
Data collectionWe gathered data on patients meeting the clinical/radiological diagnostic criteria for probable or possible CAA-ri proposed by Auriel et al.7 in 2016 (Table 1), or with histopathologically-confirmed diagnosis. The main demographic and clinical data were gathered from the hospital registry, which since 2011 has exclusively used electronic records, including both inpatient and outpatient data. Data were gathered on the following variables:
- 1)
Demographic variables: age at diagnosis, sex, and year of diagnosis.
- 2)
Relevant personal history.
- 3)
Most frequent symptoms: cognitive impairment, behavioural alterations, seizures, focal neurological signs, headache, and such other symptoms as visual/auditory hallucinations or insomnia.
- 4)
Form of onset, characterised as acute (< 48 hours), subacute (48 hours–4 weeks), or chronic (> 4 weeks).
- 5)
Analytical parameters: erythrocyte sedimentation rate (ESR) as an acute-phase reactant and inflammatory indicator, studied in 4 patients.
- 6)
Cerebrospinal fluid (CSF) parameters, which were studied in 6 patients, following standardised methods for sample collection, centrifuging, and storage.
- 7)
Imaging studies, carried out in all patients: head CT scan at baseline and brain MRI study with T2-FLAIR and diffusion-weighted (DWI) sequences, among others, as well as a post-contrast study. The presence and number of microbleeds was evaluated with susceptibility-weighted angiography (SWAN) and/or gradient echo sequences.
- 8)
Electroencephalography (EEG) study, in 5 patients.
- 9)
Amyloid positron emission tomography (PET) scan with florbetapir, in 2 patients.
- 10)
Histopathological data from brain biopsy studies, in 2 patients.
- 11)
Treatment: review of the symptomatic and immunomodulatory treatments received.
- 12)
Follow-up data: main outcomes, disability status (modified Rankin Scale [mRS]), and/or clinical or radiological recurrence.
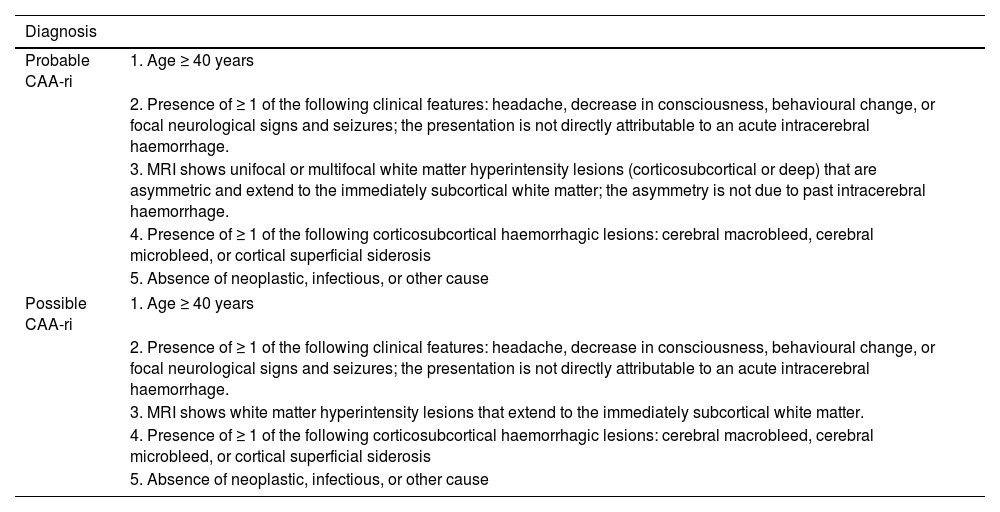
Diagnostic criteria for cerebral amyloid angiopathy–related inflammation.
| Diagnosis | |
|---|---|
| Probable CAA-ri | 1. Age ≥ 40 years |
| 2. Presence of ≥ 1 of the following clinical features: headache, decrease in consciousness, behavioural change, or focal neurological signs and seizures; the presentation is not directly attributable to an acute intracerebral haemorrhage. | |
| 3. MRI shows unifocal or multifocal white matter hyperintensity lesions (corticosubcortical or deep) that are asymmetric and extend to the immediately subcortical white matter; the asymmetry is not due to past intracerebral haemorrhage. | |
| 4. Presence of ≥ 1 of the following corticosubcortical haemorrhagic lesions: cerebral macrobleed, cerebral microbleed, or cortical superficial siderosis | |
| 5. Absence of neoplastic, infectious, or other cause | |
| Possible CAA-ri | 1. Age ≥ 40 years |
| 2. Presence of ≥ 1 of the following clinical features: headache, decrease in consciousness, behavioural change, or focal neurological signs and seizures; the presentation is not directly attributable to an acute intracerebral haemorrhage. | |
| 3. MRI shows white matter hyperintensity lesions that extend to the immediately subcortical white matter. | |
| 4. Presence of ≥ 1 of the following corticosubcortical haemorrhagic lesions: cerebral macrobleed, cerebral microbleed, or cortical superficial siderosis | |
| 5. Absence of neoplastic, infectious, or other cause | |
CAA-ri: cerebral amyloid angiopathy–related inflammation; MRI: magnetic resonance imaging.
Source: Auriel et al.7
A search was conducted on PubMed and MEDLINE on 30 September 2020 to identify articles describing the main clinical, radiological, histopathological, and therapeutic characteristics of CAA-ri. We used the following search terms: “inflammatory cerebral amyloid angiopathy,” “amyloid angiopathy-related inflammation,” and “amyloid-beta-related angiitis.” In addition to the electronic search, we manually searched the references sections of the articles identified.
ResultsOur series included 7 patients (5 men and 2 women). The main data on demographic variables and personal history are summarised in Table 2. Mean age at diagnosis was 79 years (range, 65–94). According to the criteria proposed by Auriel et al.,7 diagnosis was definitive (histopathological confirmation) in 2 cases and probable in 5 (Table 1). The most relevant findings in patients’ personal history included such vascular risk factors as arterial hypertension (in 3 patients) and diabetes mellitus (2). No patient had history of cognitive impairment, epilepsy, or other relevant history of neurological disorders; none had previously been diagnosed with CAA.
Demographic and clinical variables of the patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Age (years) | 81 | 80 | 77 | 94 | 73 | 83 | 65 |
| Sex | M | M | M | M | M | W | W |
| VRF | AHT | DL, DM | AHT, DL | None | AHT, DL, DM | DL | None |
| History of neurological disorders | None | Essential tremor | None | None | None | None | None |
| Onset | Subacute | Subacute | Acute | Chronic | Subacute | Subacute | Acute |
| Symptoms: | |||||||
| Cognitive alterations | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Behavioural disorders | Yes | Yes | Yes | No | Yes | No | Yes |
| Epileptic seizures | No | Yes | Yes | Yes | Yes | Yes | No |
| Focal neurological signs | No | No | Yes | Yes | No | Yes | Yes |
| Headache | No | No | Yes | No | No | Yes | No |
| Hallucinations | Auditory | None | Visual | None | None | None | None |
AHT: arterial hypertension; DL: dyslipidaemia; DM: diabetes mellitus; M: man; VRF: vascular risk factors; W: woman.
The most frequent symptoms of CAA-ri were cognitive impairment (in 6 patients), behavioural alterations (5), seizures (5), focal neurological signs (4), and headache (2). Less frequent symptoms were visual and auditory hallucinations (in one patient each) and insomnia (in one patient).
Table 3 summarises the results of complementary tests. ESR was slightly elevated in 2 of the 4 samples analysed. CSF presented alterations in 3 of the 5 patients who underwent lumbar puncture.
Main characteristics of the complementary tests performed.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| ESR | 37 | 13 | 11 | – | 16 | – | – |
| CSF | |||||||
| Pleocytosis | No | 30 cells/mm3 | No | – | No | No | – |
| Elevated protein level | Yes (1.02) | Yes (0.87) | No | – | No | Yes (0.81) | – |
| OCB | Yes | Yes | Yes | – | No | No | – |
| EEG | Normal | Persistent slowing in posterior region of right hemisphere | PLEDs-plus in posterior region of right hemisphere | – | Diffuse slowing | Diffuse slowing | – |
| CT | Subcortical hypodensities | Subcortical hypodensities | Subcortical hypodensities | Subcortical hypodensities | Normal | Subcortical hypodensities | Subcortical hypodensities |
| MRI | |||||||
| Subcortical FLAIR hyperintensity | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lesion localisation | Parieto-occipital/temporal | Parieto-occipital/temporal | Parieto-occipital/temporal | Parieto-occipital | Temporal | Parieto-occipital | Parieto-occipital |
| Bilateral involvement | Yes | Yes | No | No | Yes | No | No |
| Mass effect | Yes | No | No | No | No | No | No |
| Leptomeningeal contrast uptake | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Cortical superficial siderosis | Yes | No | Yes | No | Yes | No | Yes |
| Lobar microbleeds | > 10 | > 10 | < 10 | > 10 | > 10 | > 10 | > 10 |
| Deep microbleeds | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Microbleeds predominantly in hyperintense area | Yes | Yes | Yes | No | No | Yes | No |
| Amyloid PET | Positive | Negative | |||||
| Biopsy | Positive | Positive | |||||
CSF: cerebrospinal fluid; CT: computed tomography; EEG: electroencephalography; ESR: erythrocyte sedimentation rate; FLAIR: fluid-attenuated inversion recovery; MRI: magnetic resonance imaging; OCB: oligoclonal bands; PET: positron emission tomography; PLEDs: periodic lateralised epileptiform discharges.
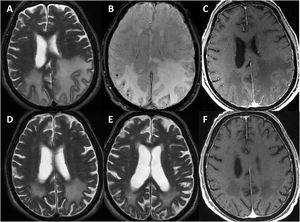
All patients underwent head CT scans at baseline, with 6 presenting subcortical hypodensities. Brain MRI scans were also performed in all patients during the first days after admission; results are shown in Table 3. All patients presented extensive unilateral (4) or bilateral (3) subcortical hyperintensities in the FLAIR sequence, with a clear predominance in posterior regions. Other relevant findings were lobar microbleeds (7), cortical superficial siderosis (4), and leptomeningeal contrast uptake in the region adjacent to the subcortical hyperintensity (6). No patient presented deep microbleeds. Fig. 1 presents the main neuroimaging findings from one of the patients.
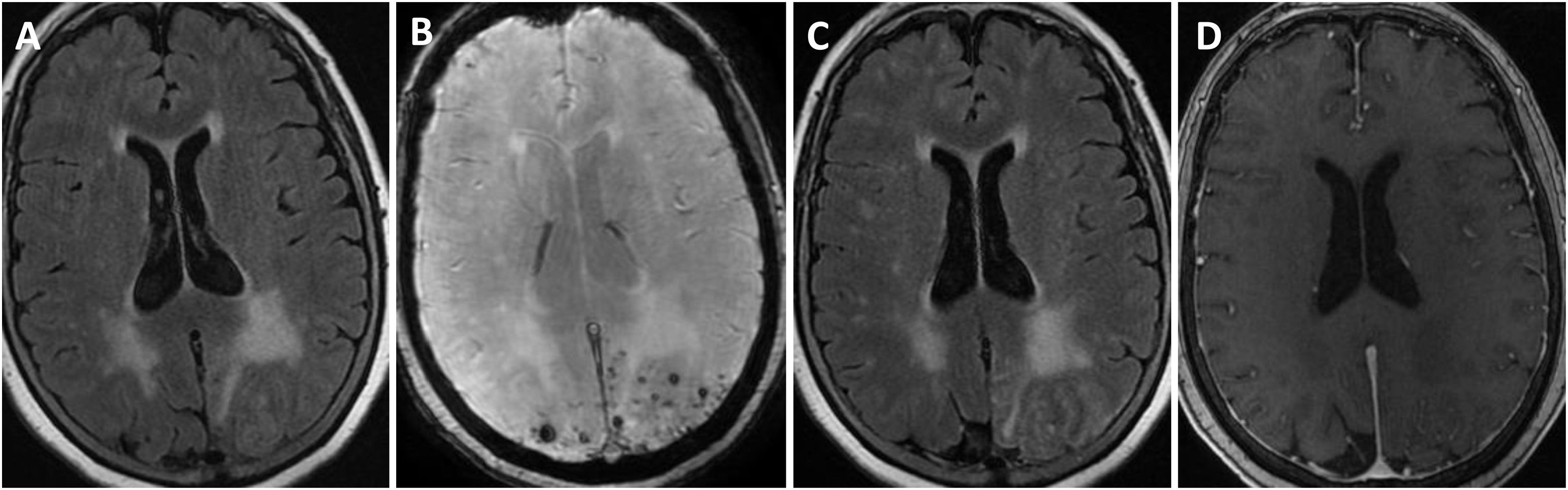
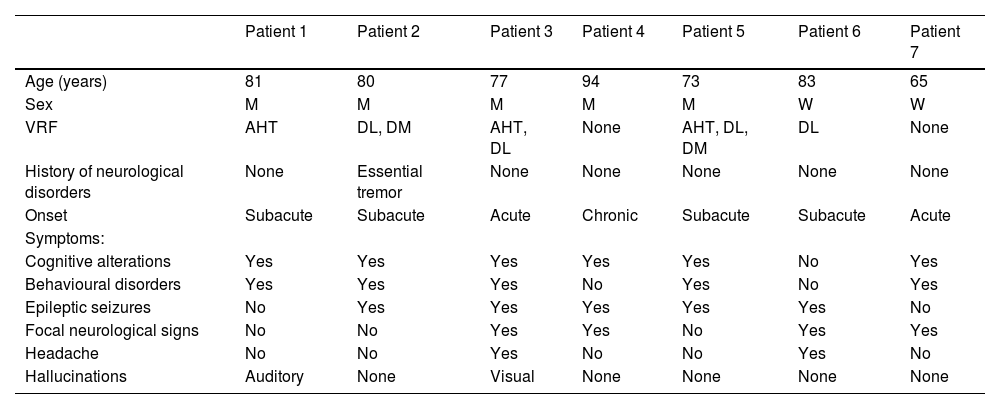
Brain MRI findings from patient 6.
The patient was an 83-year-old woman with acute symptoms of disorientation, confusion, and seizures. Brain MRI shows bilateral involvement of parietal white matter, predominantly affecting the left side (A). Susceptibility-weighted sequences (B) revealed multiple cortical microbleeds at the posterior edge of both parietal lobes; leptomeningeal contrast uptake within the sulci was more clearly identified on FLAIR sequences (C) than on post-contrast T1-weighted sequences (D).
EEG findings were abnormal in 4 of the 5 patients undergoing the study.
Amyloid PET scans were performed in 2 patients, with positive results in one.
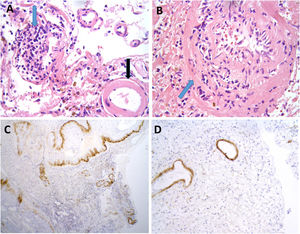
Brain biopsy studies were performed in 2 patients, and in both cases detected amyloid deposition in the walls of cortical and leptomeningeal vessels, as well as transmural inflammatory infiltrates. These findings are suggestive of ABRA (Fig. 2).
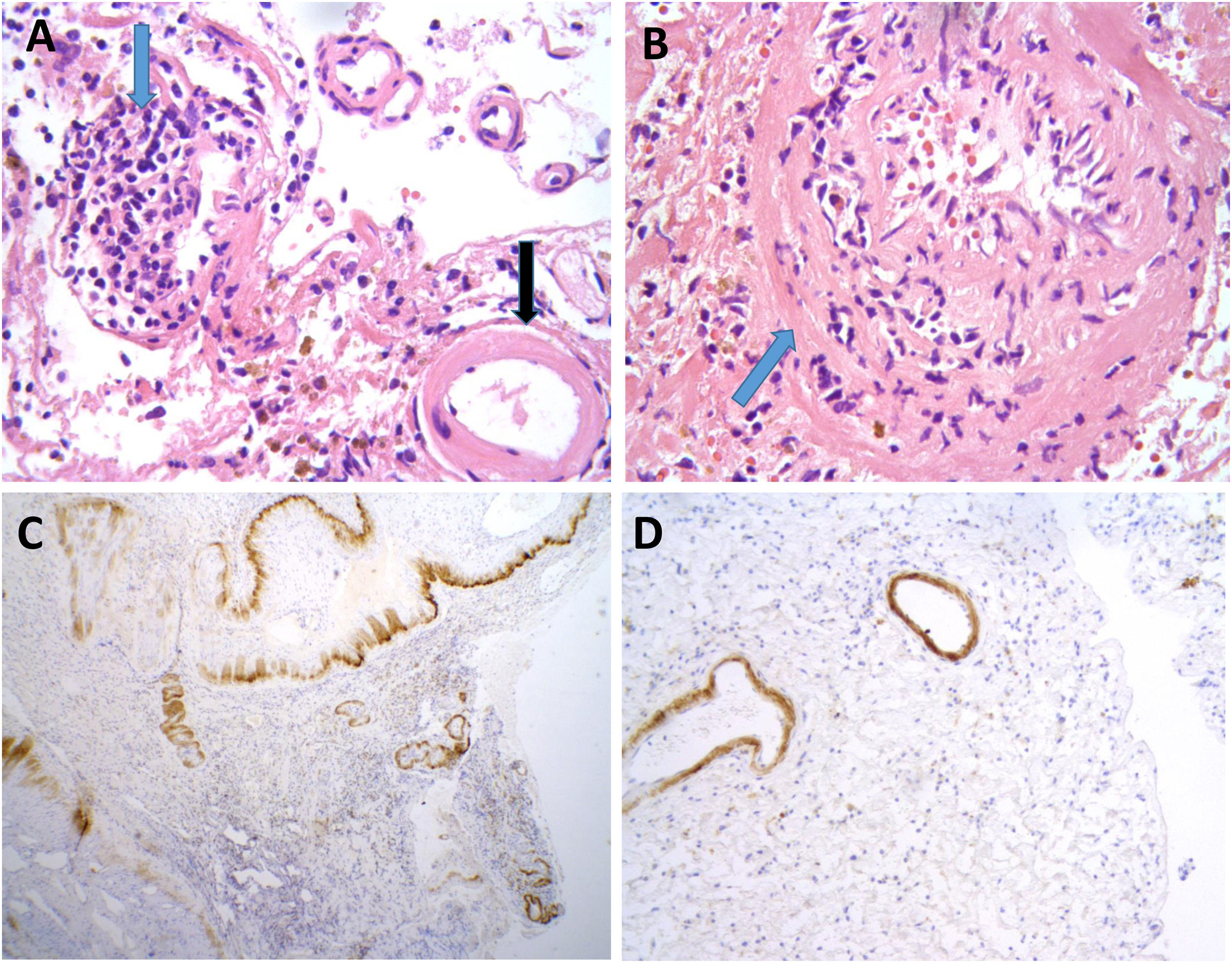
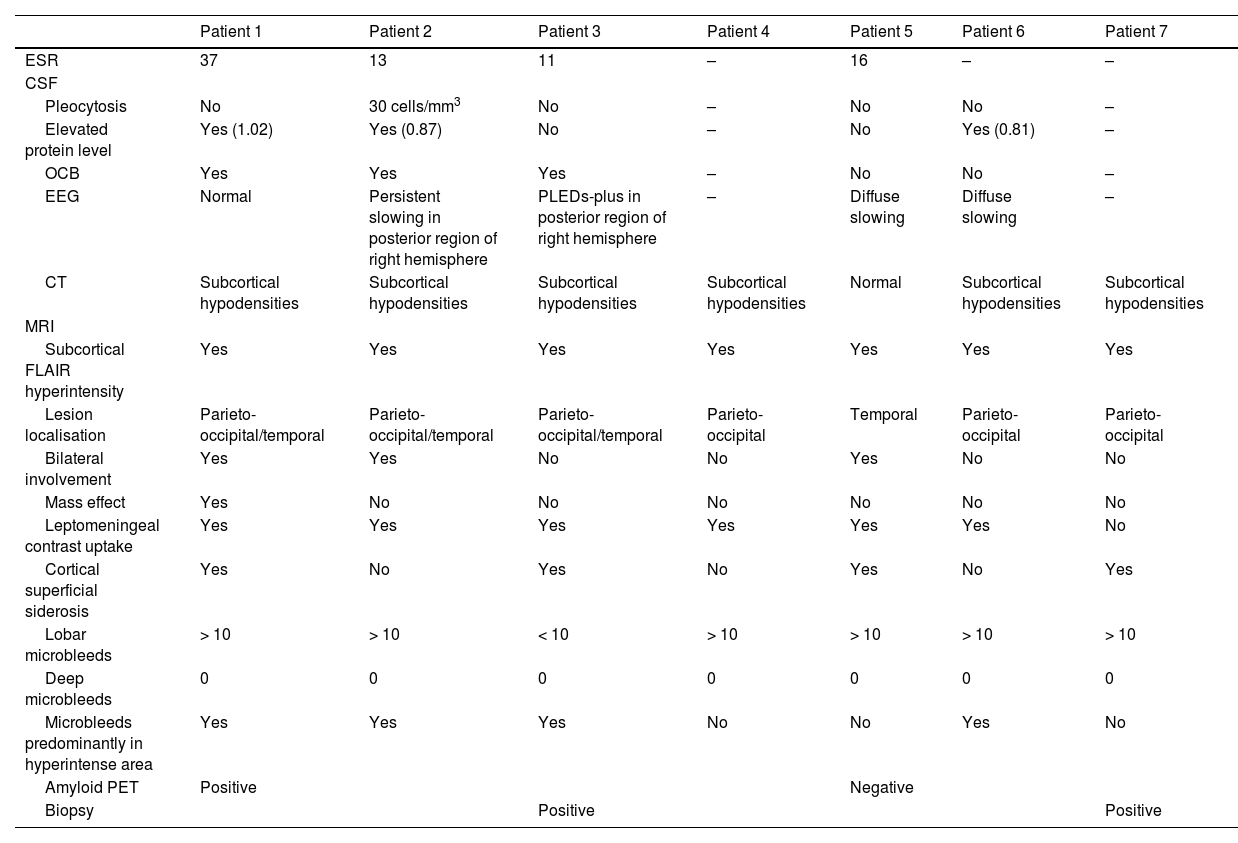
Neuropathological findings from the brain biopsy of patient 3.
Leptomeningeal vessel displaying hyaline thickening of the walls (A, black arrow). Blood vessels show focal invasion of lymphocytic inflammatory infiltrates, with focal evidence of karyorrhexis (A and B, blue arrows). No fibrinoid necrosis, giant cells, or granuloma are observed. Immunohistochemical testing revealed positivity for beta-amyloid in the thickened vessel walls (C and D).
With respect to treatment, all patients received glucocorticoids. Patients presenting seizures were also prescribed antiepileptic drugs, according to the judgement of the treating physician. Therapeutic and prognostic data are presented in Table 4.
Treatment and follow-up of the 7 patients analysed.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Initial treatment | IV methylprednisolone for 3 days | IV methylprednisolone for 5 days | IV methylprednisolone for 5 days | Prednisone 30 mg | Prednisone 30 mg | Prednisone 60 mg | IV methylprednisolone for 5 days |
| Tapering of oral corticosteroids | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Initial clinical response (at one year of follow-up) | Improvement | Improvement | Improvement | Lost to follow-up | Improvement | Improvement | Lack of improvement |
| Initial radiological response (6 months-one year after diagnosis) | Relapse after withdrawal of corticosteroids; improvement after reintroduction | Relapse after withdrawal of corticosteroids; improvement after reintroduction | Improvement | Improvement | Improvement | Improvement | |
| mRS at last assessment by neurology department | 3 | 4 | 3 | 2 | 2 | 5 | |
| Death/cause | Yes/COVID-19 | No | Yes/COVID-19 | No | No | No |
IV: intravenous; mRS: modified Rankin Scale.
One patient was lost to follow-up. The remaining patients were followed up for a mean of 3.25 years (range, 0.5–6). All patients underwent at least one follow-up MRI study, 6 to 12 months after diagnosis (Fig. 3); 2 patients displayed radiological worsening after withdrawal of corticosteroids with a subsequent improvement after they were reintroduced. At the end of the follow-up period, 2 patients had died during the COVID-19 pandemic. According to the most recent reports from before they died, both patients presented moderate disability (mRS score of 3). Of the remaining 4 patients, 2 progressed favourably, with an acceptable level of independence (mRS < 3), and the other 2 presented torpid progression, with progressive cognitive decline and dependence in basic and instrumental activities of daily living (mRS > 3).
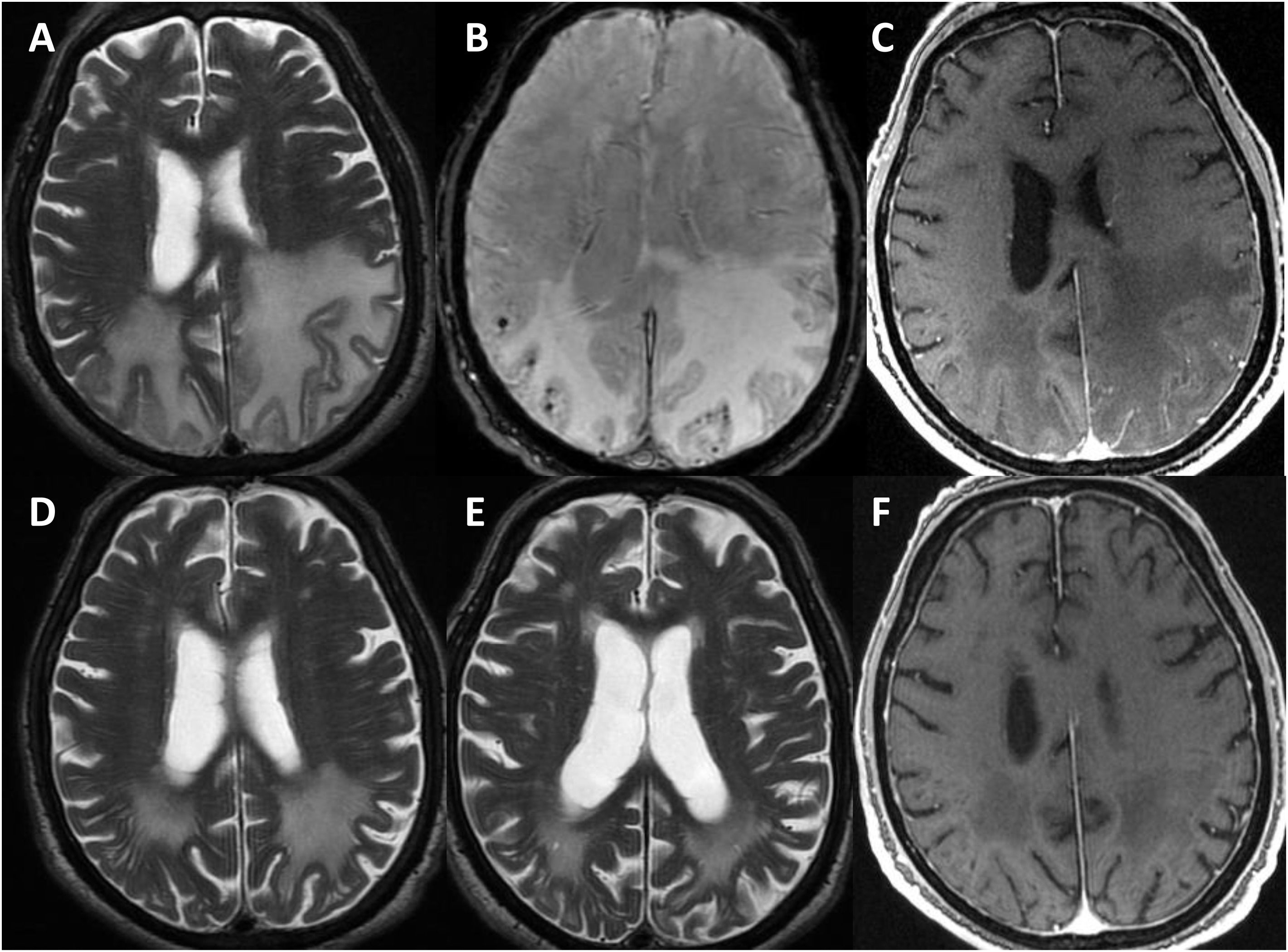
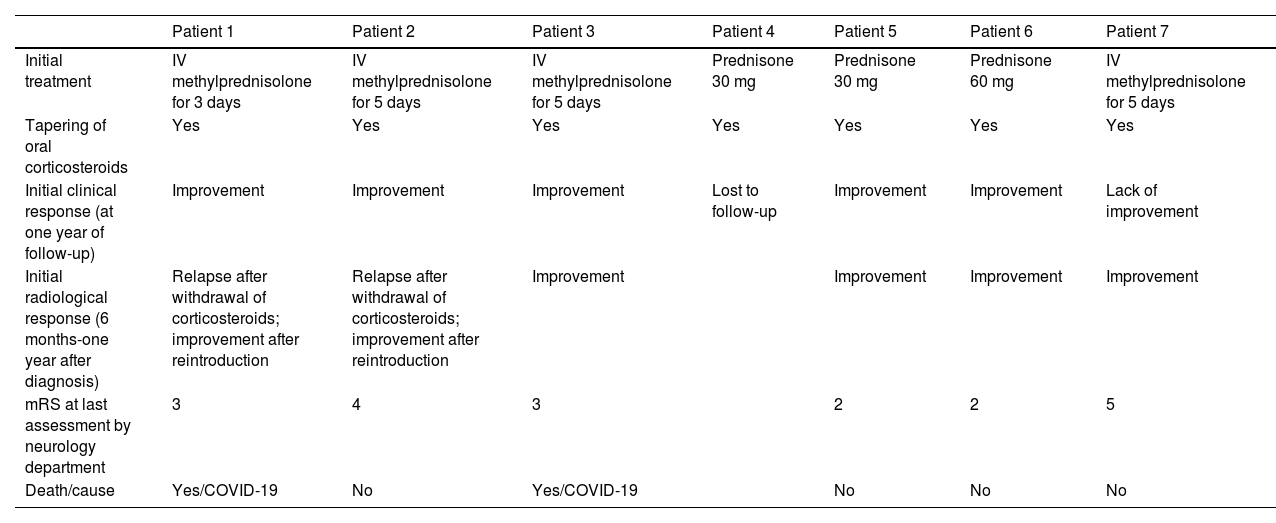
Radiological progression of patient 1 (an 81-year-old man).
Brain MRI revealed extensive bilateral parietal white matter involvement on T2-weighted sequences; involvement was asymmetrical, with a significant mass effect in the left hemisphere (A). White matter involvement coincided with multiple cortical microbleeds on susceptibility-weighted sequences (B) and predominantly left-sided leptomeningeal contrast uptake (C). Follow-up MRI studies performed at one month (D) and 3 months after corticosteroid treatment revealed a marked improvement of white matter involvement (E), with resolution of the mass effect and meningeal uptake (F).
CAA-ri is an increasingly significant clinical problem that is likely to continue growing in the future due to population ageing. We present a series of 7 patients diagnosed with the disease at our centre, and describe their demographic, clinical, laboratory, and neuroimaging characteristics, treatment, and subsequent progression, comparing our findings against those reported by other authors.
Mean age at diagnosis in our series (79 years) was older than in previous reviews (around 67 years); this is consistent with the close relationship between advanced age and amyloid-related brain pathology. Our series shows a greater predominance of male patients (71%) than previous studies, which usually report that around 55% of patients are men.3,8
CAA-ri can present with a wide range of symptoms, with cognitive impairment, seizures, and headache being the most common in the literature and in our own series.9 A striking finding in our series is the fact that the majority of patients presented behavioural alterations. Unlike in classical CAA, intracerebral haemorrhage at onset is rare in CAA-ri. Symptom onset was acute or subacute in the majority of our patients, whereas previous studies report chronic or insidious onset.3
Diagnosis of CAA-ri can be truly challenging. Definitive diagnosis requires histopathological confirmation. Given the invasiveness and the inherent risks of brain biopsy,10,11 new diagnostic criteria have been proposed in recent years.12 Chung et al.13 proposed a set of clinical/radiological criteria for the diagnosis of CAA-ri, which were subsequently validated by Auriel et al.7 in a cohort of 17 patients with histopathological confirmation of the diagnosis. The authors reported sensitivity of 82% and specificity of 97% for these criteria. Although their conclusions are limited by the small sample size, the criteria proposed are consistent with the findings of the present study and of some systematic reviews.3 In fact, application of the criteria would have led to a diagnosis of possible or probable CAA-ri in the 2 patients in whom biopsy studies were performed (these patients presented the disease prior to publication of the criteria).
Imaging studies are of great importance in the diagnosis of this disease. The most common lesions are subcortical hypodensities in CT scans and hyperintensities in T2-FLAIR MRI scans; the latter finding was observed in all our patients. Leptomeningeal contrast uptake is more typical in CAA-ri than in classical CAA, occurring in 70% of patients with perivascular inflammation (vs 86% in our study) and only 7% of those with non-inflammatory CAA. It is not uncommon for images to show a mass effect, mimicking central nervous system neoplasia,5 as observed in patient 1 in our series. SWAN and gradient echo sequences are highly useful in CAA-ri due to their ability to detect microbleeds, which are reported in over 80% of patients in previous reviews; 100% of patients in the present series presented microbleeds. It remains unclear whether microbleeds in CAA-ri constitute a manifestation of the underlying amyloid angiopathy, or whether they are due to inflammatory mechanisms.14 Striking findings in our series were the fact that all the microbleeds detected were superficial, with no deep microbleeds; most patients presented at least 10 such lesions, mainly in the regions displaying hyperintensity on MRI. Lesions predominantly affected parieto-occipital regions; this is consistent with the findings reported by other authors.3,15 On neuroimaging studies, CAA-ri often appears as bilateral multifocal lesions.
Patients with CAA-ri frequently present CSF abnormalities, with elevated protein levels and pleocytosis being the most frequent findings; the majority of patients undergoing lumbar puncture in our series presented these alterations. The disease is associated with a similar CSF biomarker profile to that observed in CAA, including low levels of Aβ 1-40 (Aβ40) and 1-42 (Aβ42). Low CSF Aβ40 levels have been linked to higher numbers of microbleeds and presence of cortical superficial siderosis; this suggests that Aβ40 may play a role as a biomarker of haemorrhagic risk in disorders related to CAA.14,16,17 Some researchers have detected specific anti-Aβ antibodies in the CSF of patients with CAA-ri.18,19 This finding, and the fact that the neuroimaging alterations and some of the clinical characteristics of CAA-ri have previously been detected in patients with Alzheimer disease under treatment with anti-amyloid monoclonal antibodies, suggest that these antibodies may play a pathogenic role. Furthermore, this marker may be useful not only in diagnosis, but also as a marker of disease activity, as high levels of anti-Aβ autoantibodies have been observed during active phases of the disease, with a subsequent return to baseline levels during remission.20
While anti-Aβ autoantibodies are not currently studied in routine practice, the validation of experimental cut-off points is an area of active research,21 and this biomarker may be considered in future as a tool for the diagnosis of CAA-ri and for studying treatment response.22,23
Amyloid PET studies, as performed in 2 of our patients, may also be useful if CAA-ri is suspected, although they do not discriminate well between vascular and parenchymal deposition5,15,22 and the articles published to date acknowledge that their results should be interpreted with caution.14 As amyloid PET images are subject to qualitative study, based on the analysis of 2 or more positive cortical areas by the nuclear medicine physician, the sensitivity of the study is not 100%. Therefore, negative amyloid PET results do not rule out diagnosis of CAA in some contexts. In fact, cases have been reported of patients with amyloid angiopathies confirmed by detection of APP mutations who presented negative amyloid PET results.24,25
Several immunosuppressants have been proposed as treatments for CAA-ri,26–30 although we are yet to identify the optimal treatment schedule; some cases have even been described of spontaneous improvements in the absence of immunosuppression.28 A recent study by Regenhardt et al.23 concluded that individuals receiving immunosuppressive treatment presented a clinical and radiological improvement, with a lower rate of recurrence over the follow-up period.
The treatment was beneficial even for patients receiving a single cycle of corticosteroids, and not only in those receiving such other immunosuppressants as cyclophosphamide. In fact, all patients in our series only received corticosteroids, with nearly all of them presenting a clinical and/or radiological improvement.
The main limitations of this study are its retrospective design (as a result of which data may be missing for some variables, although we carefully reviewed all clinical notes from the patient records) and the small sample size (n = 7), despite this being one of the largest series studied in our setting.
ConclusionsCAA-ri is a rare disease that has been described more frequently in recent years. Early diagnosis is essential, as this is a treatable disease in which early treatment has been shown to improve prognosis in terms of survival, functional status, and recurrences. Although histopathological data are needed to confirm diagnosis, the proposed clinical and radiological criteria present good sensitivity and excellent specificity, enabling us to reach a diagnosis without the need for a brain biopsy.
FundingSara Llamas Velasco is the recipient of a “Juan Rodés” research grant from Instituto de Salud Carlos III.
Víctor Blanco Palmero is the recipient of a “Río Hortega” research grant from Instituto de Salud Carlos III.
Conflicts of interestThe authors have no conflicts of interest to declare.