The choroid plexuses, blood vessels, and brain barriers are closely related both in terms of morphology and function. Hypertension causes changes in cerebral blood flow and in small vessels and capillaries of the brain. This review studies the effects of high blood pressure (HBP) on the choroid plexuses and brain barriers.
DevelopmentThe choroid plexuses (ChP) are structures located in the cerebral ventricles, and are highly conserved both phylogenetically and ontogenetically. The ChPs develop during embryogenesis, forming a functional barrier during the first weeks of gestation. They are composed of highly vascularised epithelial tissue covered by microvilli, and their main function is cerebrospinal fluid (CSF) production. The central nervous system (CNS) is protected by the blood–brain barrier (BBB) and the blood–CSF barrier (BCSFB). While the BBB is formed by endothelial cells of the microvasculature of the CNS, the BCSFB is formed by epithelial cells of the choroid plexuses. Chronic hypertension induces vascular remodelling. This prevents hyperperfusion at HBPs, but increases the risk of ischaemia at low blood pressures. In normotensive individuals, in contrast, cerebral circulation is self-regulated, blood flow remains constant, and the integrity of the BBB is preserved.
ConclusionsHBP induces changes in the choroid plexuses that affect the stroma, blood vessels, and CSF production. HBP also exacerbates age-related ChP dysfunction and causes alterations in the brain barriers, which are more marked in the BCSFB than in the BBB. Brain barrier damage may be determined by quantifying blood S-100β and TTRm levels.
Los plexos coroideos, los vasos sanguíneos y las barreras cerebrales están íntimamente relacionados tanto morfológica como funcionalmente. Por otro lado, la hipertensión produce cambios en el flujo sanguíneo y en los pequeños vasos y capilares cerebrales. El propósito de la presente revisión es estudiar los efectos de la hipertensión arterial sobre los plexos coroideos y las barreras cerebrales.
DesarrolloLos plexos coroideos (PC) son una estructura del cerebro situada en los ventrículos cerebrales, altamente conservada filogenética y ontogénicamente. Los PC se desarrollan temprano durante la embriogénesis y constituyen una barrera funcional en las primeras semanas de gestación. Están compuestos por tejido epitelial altamente vascularizado, cubiertos por microvellosidades y su función principal es la producción del líquido cefalorraquídeo (LCR). El sistema nervioso central se encuentra aislado y protegido por la barrera hematoencefálica (BHE) y por la barrera sangre-LCR (BSLCR). Mientras que la BHE se localiza al nivel de las células endoteliales en la microvasculatura del encéfalo, la BSLCR está formada por las células epiteliales de los plexos coroideos. La hipertensión arterial crónica induce una remodelación vascular para adaptarse a los valores elevados de presión arterial, con lo que se evita el riesgo de hiperperfusión ante presiones elevadas, pero se incrementa el riesgo de isquemia a presiones bajas; en cambio, en las personas normotensas la circulación cerebral se autorregula y el flujo sanguíneo permanece constante y se mantiene la integridad de la BHE.
ConclusionesLa hipertensión arterial induce variaciones en los plexos coroideos que afecta al estroma, los vasos sanguíneos, la producción de LCR y agrava el deterioro de los PC producidos por envejecimiento. Además, la hipertensión también produce alteraciones en las barreras cerebrales que son más ostensibles en la BSLCR que en BHE; estos daños de las barreras cerebrales se podrían determinar detectando los niveles en sangre de S100β y TTRm.
The choroid plexuses (ChP) are made up of a monostratified epithelium (Fig. 1) and a central vascular fibrous axis, the stroma. Epithelial cells are cuboidal, with a rounded nucleus located in the centre or base of the cell. They possess numerous mitochondria, which are more densely distributed in the base and apical poles of the cell, occupying 15% of the cytoplasm in primates.1–4 The total number of epithelial cells is estimated at 100 million, with the cells measuring approximately 15μm long.2,5
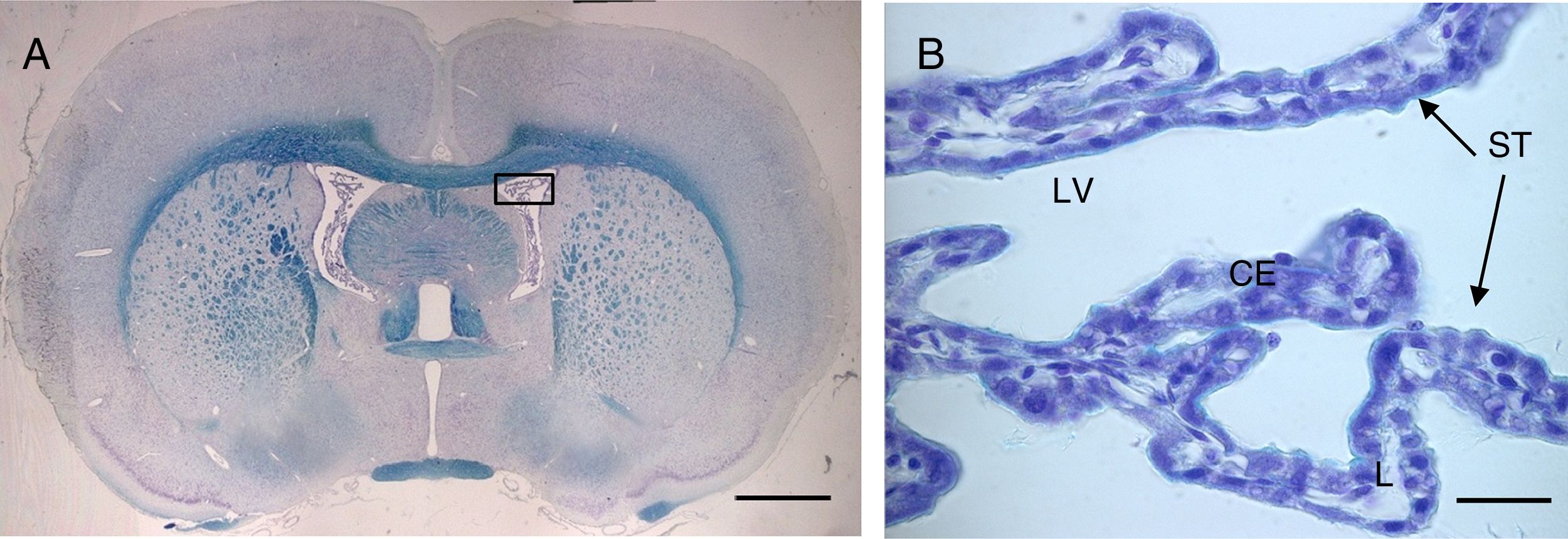
(A) Optical microscopy image (Klüver–Barrera staining) of a coronal slice from the brain of a rat, showing the choroid plexus within the lateral ventricles. B) Closer image of the choroid plexus (64×), with the same staining technique.
Scale bar: (A) 1600μm; (B) 40μm.
CE: choroid plexus epithelium; L: lumen; LV: lateral ventricle; ST: stroma.
The apical poles of these cells present numerous interlaced microvilli of uniform diameter, with few cilia. In rats, the total surface area of the ChP microvilli is approximately 75cm2, comparable to the surface area of the capillaries of the blood–brain barrier (BBB) (155cm2).6 At the basal poles, epithelial cells are separated from the stroma by the basolateral membrane. The stroma contains numerous interdigitations with multiple type IV collagen fibres and few dendritic cells, with macrophages, fibroblasts, and large capillaries with fenestrated endothelium.2 ChPs are richly irrigated, presenting blood flow almost 10 times greater than that of the cerebral cortex in rats.7 In humans, the ChP of the lateral ventricle is supplied by the anterior and posterior choroidal arteries, with drainage into the choroidal vein. The anterior choroidal artery is usually a branch of the internal carotid artery, whereas the posterior choroidal artery originates from the posterior cerebral artery. The posterior choroidal artery also supplies the ChP of the third ventricle.8
Functions of the choroid plexusThe ChP's functions include the production of cerebrospinal fluid (CSF), the secretion of numerous molecules,9 and the reabsorption of substances from the CSF.10 It constitutes a selective barrier between the blood and the CSF (the blood–CSF barrier [BCSFB]), which is probably involved in the immune surveillance of the brain.11,12 ChP epithelial cells play an important role in many of these tasks and in the synthesis of numerous CSF proteins, including prostaglandins and growth factors.1,2 The efficiency of the BCSFB is dependent on the efficacy of the epithelial tight junctions; studies conducted in the last decade support the hypothesis that functional dysregulation of ChPs (and therefore of the BCSFB) may be a common pathophysiological mechanism in a wide variety of neurological disorders.13
Numerous transport proteins are expressed in the apical and basal poles of epithelial cells; this enables the entry and exit of molecules and the clearance of organic anions and cations from the CSF, preventing the accumulation of potentially harmful substances in the brain.12,14 The ChPs participate in brain repair processes by secreting neuroprotective substances, and act as a site of neurogenesis, suggesting an important role in cell repair and replacement in the central nervous system (CNS).15,16
Secretion of proteins and peptides by the choroid plexusesThe ChPs synthesise numerous neuropeptides, growth factors, and cytokines (Table 1).17,18 One of the main proteins synthesised by the ChPs is transthyretin (TTR), which is mainly found in the plasma and the CSF.19–21 TTR is mainly synthesised in the liver, the ChP, and the retinal pigment epithelium. TTR synthesised in the liver is secreted into the blood, where it acts as a transport protein for thyroid hormone.22 TTR synthesised by the ChPs is secreted into the CSF, and is involved in transporting thyroid hormone from the blood to the brain.22–25
Polypeptides identified in the choroid plexus and molecules for which receptors have been identified in the choroid plexus.
| Polypeptides identified in the ChP | Molecules for which receptors have been identified in the ChP |
|---|---|
| Adrenomedullin | Angiotensin II |
| Apolipoprotein J | Apolipoprotein E |
| Arginine | Apolipoprotein J/clusterin |
| Basic fibroblast growth factor | Atrial natriuretic peptide |
| β-Amyloid precursor protein | Basic fibroblast growth factor |
| Brain-derived neurotrophic factor | Bradykinin |
| Cystatin C | Brain-derived neurotrophic factor |
| Endothelin-1 | Corticotropin-releasing factor |
| Hepatocyte growth factor | Endothelin |
| Insulin-like growth factor | Growth hormone |
| Interleukin-1β | Insulin |
| Interleukin-6 | Insulin-like growth factor-1 |
| Nerve growth factor | Interleukin-1 |
| Neurotrophin 3 and 4 | Leptin |
| Prostaglandin D synthase | Nerve growth factor |
| Transferrin | Neurotrophin 4 |
| Transthyretin | Prolactin |
| Tumour necrosis factor | Vascular endothelial growth factor |
| Vascular endothelial growth factor | Vasoactive intestinal polypeptide |
| Vasopressin | Vasopressin |
Adapted from Chodobski and Szmydynger-Chodobska.15
The first discoveries about the BBB, a vascular barrier separating the blood and the CNS, were made over 100 years ago. In the 1880s, Paul Ehrlich26 found that when he injected certain dyes into the vascular system, they reached all organs but the brain and spinal cord. Erhlich26 interpreted these results as the nervous system's lack of affinity for these dyes. Soon after, Goldmann27 demonstrated that when the same dyes were injected into the CSF, they easily reached the nervous tissue but no other tissue, suggesting that once within the CNS, dyes did not cross into the blood circulation. Additional studies have demonstrated that neurotoxic agents affect brain function only when they are injected directly into the brain, but not when injected into the vascular system.28,29 Only with advances in electron microscopy was it possible to correlate the ultrastructural location of the BBB with the endothelial cells of cerebral vessels.30
The CSF is separated from the vascular system by the BCSFB, while the BBB, which maintains cerebral homeostasis, is located between the brain parenchyma and the vascular system. While both barriers serve similar purposes, they differ in terms of morphological and functional properties. Both barrier systems are permeable not only to small molecules, but also to macromolecules and circulating cells. The transportation of molecules across the BBB and the BCSFB is regulated by passive diffusion (e.g., albumin, immunoglobulins) and facilitated or active transport (e.g., glucose). The BCSFB may also serve as a route of drug administration to the CNS.31,32 The volume of the extracellular space, potassium buffering, CSF circulation, and interstitial fluid absorption are mainly regulated by aquaporin-4 channels, which are abundant in blood–brain and brain-CSF interfaces.32–36
These barriers protect the CNS, preventing or restricting the passage of undesired molecules. However, this description does not fully capture the dynamic function of the cerebral barriers, which includes the regulation of transporters, signalling between endothelial cells and neurovascular structures, and, importantly, the regulation of CSF composition via the ChP during development and ageing.37
The composition of the CSF shows a high dynamic range, with the levels of different proteins depending on several factors, such as the place of production (brain or derived from blood), the sampling site (ventricular or lumbar), the flow of CSF (BCSFB function), daily fluctuations in the rate of CSF production, and the molecular size of blood-derived proteins (IgM vs albumin) and circadian rhythm (glucose, prostaglandin-D synthase).33–36
The majority of studies into the brain barriers have addressed the BBB; however, some researchers stress the importance of the BCSFB, made up of ChP epithelial cells and their junctions, which constitutes a truly selective barrier between the blood and the CSF.33–36 Its permeability increases during embryogenesis, enabling compounds of low molecular weight to enter the brain more easily than in adults. During embryonic development, the ChPs occupy a proportionally larger volume than the brain, and are essential for the correct formation of the nervous system, secreting morphogens, mitogens, and specific growth factors into the CSF.10,34–36
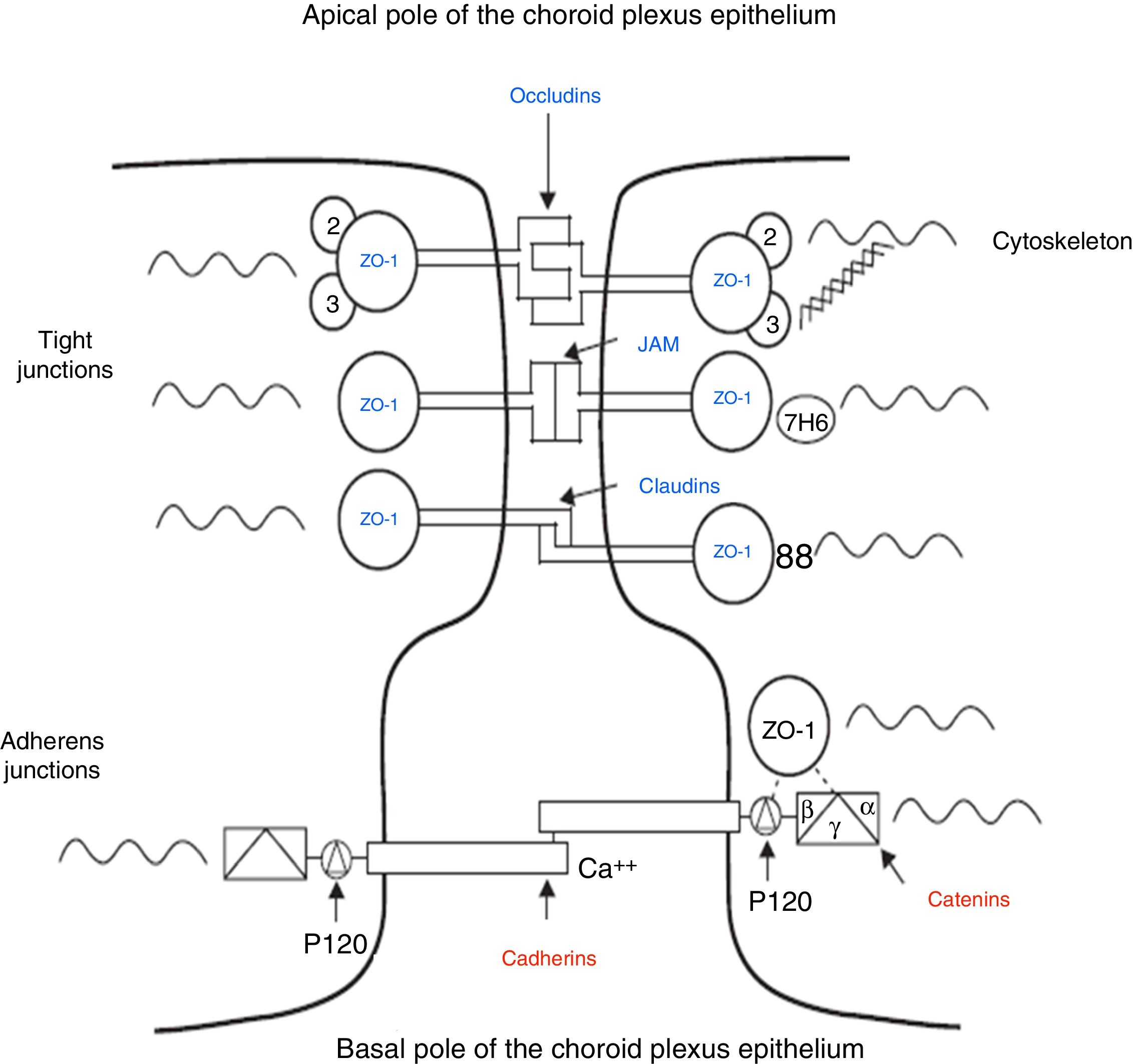
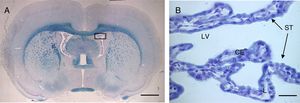
The intercellular junctions between ChP epithelial cells include both tight and adherens junctions (Fig. 2).38 Tight junctions are made up of 3 proteins (claudins, occludins, and cell adhesion molecules), whereas adherens junctions are formed by cadherins. The associated cytoplasmic accessory proteins (zonula occludens proteins in tight junctions and catenins in adherens junctions) connect transmembrane proteins to the cell cytoskeleton (Fig. 2).38,39 These junctions are responsible for maintaining the structural integrity of the epithelium, forming the BCSFB (Fig. 2).
Schematic diagram of the tight and adherens junctions present between the cells of the choroid plexus epithelium. Adapted from Vorbrodt and Dobrogowska.39
The BCSFB prevents passive diffusion of TTR from the blood to the CSF; therefore, the TTR found in CSF is produced almost exclusively in the ChP.40 In rats, serum TTR concentrations are 10 times higher than CSF concentrations, despite the proportion of TTR protein in the CSF (estimated at 25% of intraventricular proteins) being much higher than in the serum (0.5% of total proteins).40 In vitro, TTR accounts for 20% of recently synthesised proteins and 50% of proteins secreted by the ChP.36 TTR levels are lower in CSF than in plasma (15mg/mL vs 200mg/mL); these data support the hypothesis that CSF is not an ultrafiltrate of plasma, but rather is actively secreted by the ChP.41
However, researchers including Hladky and Barrand42 argue that although the BBB is crucial for correct brain function, it is not evident that the ChPs are essential in adults. Indeed, while secretion from the ChPs explains most of the net fluid entry to the brain, an appropriate disposition of transporter proteins in the BBB could easily produce a similar secretion. Subtler reasons are needed to explain the role of the ChPs as the main source of CSF. Firstly, it may allow the BBB to serve local needs, without the restrictions of having to maintain fluid secretion, for example in response to changes in nervous activity. Secondly, it prevents the need to provide pathways and sufficient pressure gradients for all the fluid entering the brain to flow across the parenchyma. Thirdly, secretion of fluids directly into the ventricles may contribute to maintaining their permeability. The presence of these spaces, which are of variable volume and present little flow resistance, enables compensation for changes in blood volume during the cardiac and respiratory cycles and in response to postural changes. It should also be noted that the ChPs are necessary in fluid secretion during the development of the CNS, and allowing this process to continue in adults may be an economical solution.42,43
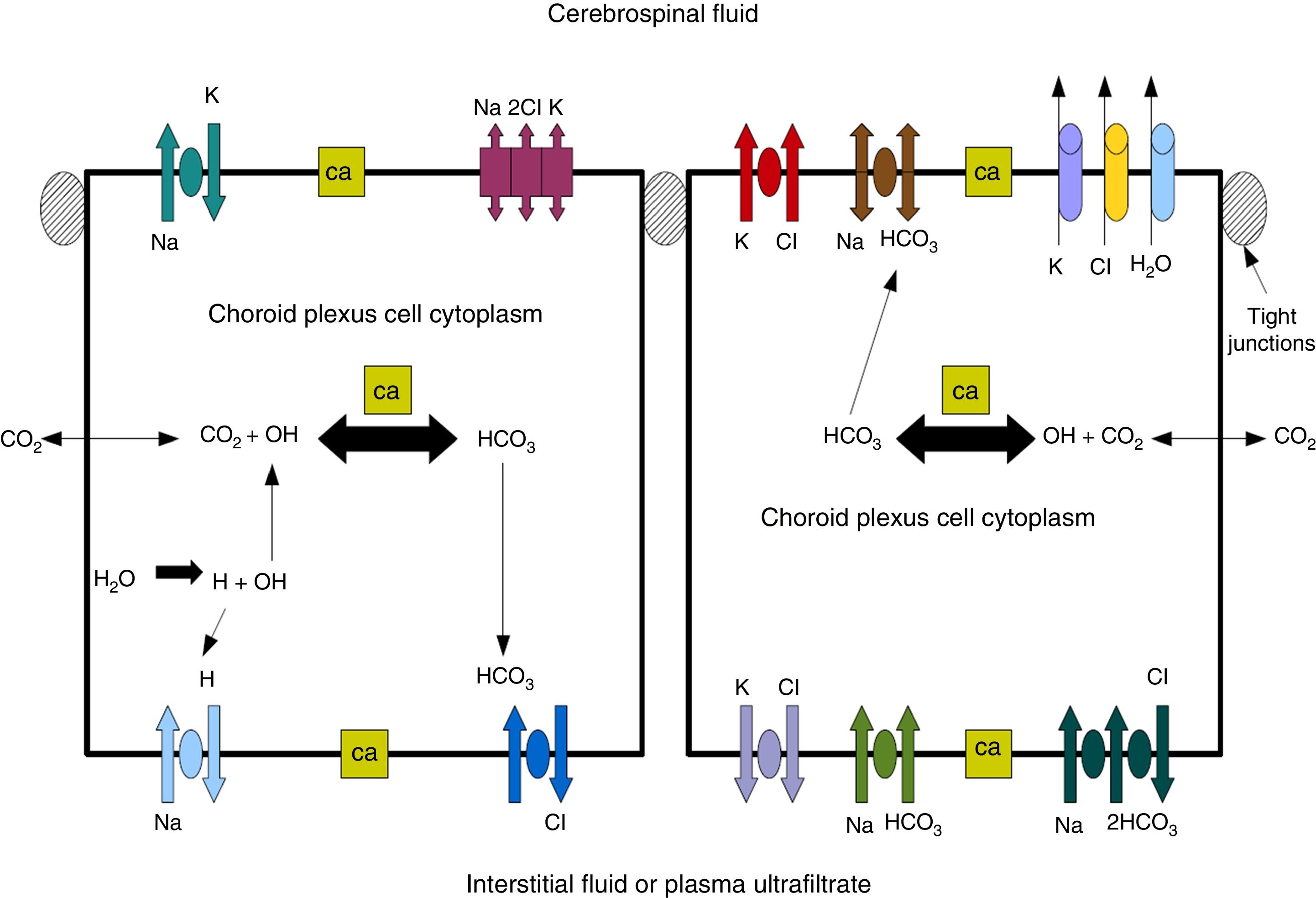
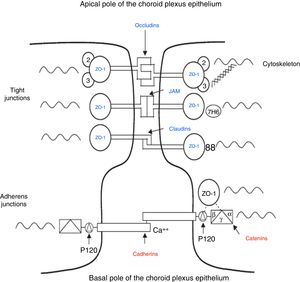
Cerebrospinal fluidCSF production in the ChP of the cerebral ventricles occurs in 2 phases: first, fluid is passively filtered through capillaries of the ChP, facilitated by hydrostatic pressure44; and second, proteins are actively secreted from the ChP epithelium into the ventricle.5,44–50 CSF is produced at a rate of 3 and 350μL/minute in rats and in humans, respectively (Fig. 3).45,50–54
Diagram demonstrating ion transport in the choroid plexus for cerebrospinal fluid production. Adapted from Johanson et al.52
One of the most important proteins in the passive filtration of fluid through the ChP is the water channel aquaporin-1 (AQP1). This protein has been identified in the apical pole of the membranes of ChP epithelial cells, although its location may vary during embryonic development and in the context of hydrocephalus or ventricular dilation.50–56 The aquaporins are a family of integral membrane proteins that function as water channels. The production of CSF in the ChPs involves an osmotic gradient, which is mainly caused by the action of carbonic anhydrase and Na+/K+-ATPase.48–52 AQP1 enables water to follow the osmotic gradient in the apical membrane, making this protein highly important for the transfer of water across the ChPs.45–51 AQP1 is also involved in maintaining this osmotic permeability: permeability is 4.8 times lower in mutant mice that do not express the protein.57,58 These mutant mice also produced approximately 20% less CSF than wild-type mice (0.38±0.02 vs 0.30±0.01μLminute–1). Intracranial pressure was also 56% lower in mutant mice lacking AQP1.42,52,53 CSF is not simply an ultrafiltrate of blood, as its composition is different to that of plasma.56 It is composed of 99% water, with a much lower concentration of proteins (≈350mg/L) than in serum (70000mg/L); Na+, K+, Ca2+, and HCO3− levels are much lower than in plasma, and approximately 80% of CSF proteins are present in blood plasma. Seventy percent of the CSF originates in the ChP, with the rest being secreted by minor pathways in the brain parenchyma.43,50–60
Maintenance of the interstitial fluid and the purity of CSF composition depend on efficient clearance mechanisms. As the CNS lacks lymphatic capillaries, CSF-mediated elimination of metabolites and toxins is essential to neuronal function. The ChP plays an important role in clearing these harmful substances. Clearance takes 3 forms: firstly, reabsorption by transporters in the apical membrane of the ChP epithelium actively removes organic anions and peptides from the ventricular CSF.61–63 This clearance by the ChP is complementary to active reabsorption of unnecessary metabolites from the interstitial fluid through brain capillaries.64 Secondly, the continuous production of CSF flowing from inside the brain acts as a drainage mechanism, reducing the concentration of the metabolites passing into the ventricles.13,65,66 A third means of toxin clearance is the passage of circulating metabolites from the CSF through arachnoidal-lymphatic-venous interfaces.52,67
Effects of arterial hypertension on the central nervous systemThe cerebral circulation has control mechanisms guaranteeing adequate blood supply at all times; therefore, if the brain's energy demand increases due to elevated neuronal activity, cerebral blood flow (CBF) also increases, in a phenomenon known as hyperaemia.68 In normal conditions, CBF rate is approximately 50-60mL/100g/minute.69 Increased CBF increases the supply of nutrients, removes metabolic waste, and dissipates heat produced by brain activity; however, it may also affect neuronal activity through its effects on astrocytes.68,70
CBF is defined as the sufficient capacity of blood for adequate perfusion of the brain tissue, according to the formula CBF=cerebral perfusion pressure (CPP)/cerebrovascular resistance.
CPP is defined as the pressure needed to perfuse the nervous tissue for good metabolic function, and represents the difference between arterial pressure driving blood into the cerebral circulation and venous back-pressure. Under normal conditions, venous back-pressure is minimal, meaning that CPP is similar to systemic arterial pressure. Therefore, if CPP is normal, then changes in CBF are explained by changes in cerebrovascular resistance.71
In normotensive individuals, the cerebral circulation is self-regulating: within a broad range (50-150mmHg mean arterial pressure), blood flow remains constant in order to maintain the integrity of the BBB.72 This process is regulated by the calibre of the small arteries and arterioles, which contract when arterial blood pressure increases and dilate when it decreases. Due to the vascular remodelling that accompanies chronic high blood pressure (HBP), self-regulation adapts to the high blood pressure values, displacing the self-regulation curve to the right and thereby reducing the risk of hyperperfusion when blood pressure increases, but also increasing the likelihood of ischaemia when it decreases.73 According to some studies, certain antihypertensive drugs may reverse this alteration.74
CBF is reduced in spontaneously hypertensive rats, and cerebral circulation may be compromised from the age of 4 months.75 One possible explanation for the reduced CBF observed in the context of HBP is that atherosclerosis of the carotid arteries plays a role in the insufficient blood supply to the brain. Whatever the underlying mechanism, reduced CBF seems always to accompany chronic HBP.76–79 Animal models offer good insight into the possible mechanisms underlying the association between reduced CBF and the state of capillaries in the brain. For example, permanent bilateral common carotid artery ligation in rats has been suggested as a model of chronic cerebral hypoperfusion, reducing blood supply to approximately 70%.80,81 Similarly, hypertensive rats present structural anomalies in cerebral vessels after 14 months of cerebral hypoperfusion, manifesting as thickening of the basement membrane due to accumulation of collagen in hippocampal blood vessels.82 As a result, it has been suggested that chronic hypoperfusion is functionally related to cerebral small vessel damage, with cerebral hypoperfusion triggering the ultrastructural alterations and the vascular deformities observed.75 These findings further support the hypothesis of a causal interaction between chronic reduction of CBF and microvascular degeneration in patients with HBP.
Despite the physiological phenomenon of the brain's self-regulation, sustained high blood pressure results in constant vasoconstriction of cerebral arterioles and small arteries, leading to structural changes to vessels and the appearance of several types of brain lesions. These changes are fundamentally characterised by “vascular remodelling,” the reordering of the layers of smooth muscle cells of the vessel walls, as well as adaptive and degenerative structural changes, such as atherosclerosis, arteriosclerosis, arterial wall thickening, reduced arterial lumen, and smooth muscle hypertrophy.71,82–85As the vascular lumen continues to decrease in diameter, cerebral perfusion is reduced, potentially causing lacunar infarcts and/or periventricular or deep white matter ischaemic lesions; this process is known as leukoaraiosis.86,87 Hypertension also leads to vascular stiffness, with increased collagen content in the tissue.88 Finally, hypertensive rats also present variations in CSF proteins and in certain circumventricular organs in addition to the ChPs (e.g., the subfornical organ, which lacks BBB, and the subcommissural organ, which does have an effective BBB).89–91
This article reviews the effects of HBP on the structure, morphology, and function of the ChP. We also aim to interpret whether these effects are present in the brain barriers, and whether they are of greater relevance in the BCSFB or the BBB.
DevelopmentEffects of arterial hypertension on the structure and morphology of the choroid plexusSpontaneously hypertensive rats present increased stromal thickness, flattening of the epithelium, and increased vascular lumen diameter in the ChP as compared to controls; these changes become more marked with age, with the combination of the changes associated with HBP and age-related changes.86,92–94 However, it should also be stressed that the increased vascular lumen diameter in the ChP stands in contrast to the effects observed in other vessels of the brain, where HBP causes lumen diameter to decrease.95–97 Previous studies have classified vessels according to lumen diameter, with a first group of diameter 5.0-15μm, a second group of diameter 15-30μm, and a third group of diameter greater than 30μm. The mean vessel lumen diameter was greater in hypertensive rats than in controls in the 3 ranges selected; in fact, control rats did not present vessels with diameter greater than 30μm.94–97 These results suggest that HBP and cerebral hypoperfusion reduce the lumen diameter of vessels in the brain parenchyma, with the opposite being the case in choroidal vessels. Probably because of their highly vascularised structure, the ChPs receive 10 times more blood than cerebral vessels, and are therefore more sensitive to changes in blood perfusion.7,97 Regarding this contradictory effect, it is possible that the increase in lumen diameter may be a compensatory mechanism in response to a lack of oxygen and nutrients due to poor cerebral perfusion; in this case, maintaining blood pressure would not require a decrease in diameter, as occurs in cerebral vessels. For this reason, the vessels of the ChP are thought to be much more sensitive to reduced perfusion and to present greater changes than cerebral vessels.
Effects of arterial hypertension on choroid plexus functionMorphological changes secondary to HBP may lead to changes in protein secretion, function, and expression in the ChPs.
TTR accounts for 25% of the total protein synthesised by the ChP and 5% of protein present in the CSF47; therefore, TTR expression is indicative of the state of the ChP's secretory function. Decreased ChP TTR levels are reported in middle-aged hypertensive rats as compared against controls, potentially indicating reduced secretory function, but the most striking observation is the small increase in TTR levels in older hypertensive animals with respect to controls. This may be a compensatory mechanism in response to reduced CSF TTR levels in hypertensive rats secondary to BCSFB disruption93; this matter is addressed later in the article.
CSF production is one of the most important functions of the ChPs. As mentioned above, AQP1 protein in the apical poles of epithelial cells is responsible for allowing water to pass from the blood to the CSF via a transcellular mechanism; therefore, AQP1 is a good indicator for evaluating CSF production.57 Previous studies report elevated AQP1 expression in hypertensive rats as compared to controls. This may be the cause of the ventricular dilation described by Ritter et al.98 in spontaneously hypertensive rats, and may be explained by the fact that HBP increases the amount of water in the ChPs, which are responsible for eliminating this excess, hence the increased AQP1 levels.93,94,99,100 However, AQP1 levels also decrease in line with age, perhaps in association with the reduction in water flow across the ChPs when CSF production by the ChPs decreases in the contexts of older age or HBP; this process results in a decrease in CSF circulation, which affects the clearance of toxins from the brain and their reabsorption.92,94 We may deduce that the structural and morphological changes caused in the ChP due to HBP (and ageing) will accelerate the deterioration of such processes as CSF production.
Studies with aged rats have found that the microvilli of ChP epithelial cells decrease considerably in number and size in comparison to younger animals; these changes are only observed in the ChP of the lateral and third ventricles (not in the ChP of the fourth ventricle).92 In a recent study, AQP1 was detected in the CSF, suggesting that the appearance of this protein may be caused by a loss of choroidal microvilli, whose content (including AQP1) would pass into the CSF.96 Interestingly, some studies report a relationship between the amount of AQP1 in the ChPs and in the CSF, with greater labelling of the protein in the ChPs being associated with lower concentration in the CSF, and vice versa.94,101 In addition, some of the few studies evaluating AQP1 in the CSF relate this finding to the loss of microvilli with ageing, with the consequent release of AQP1 into the ChP.101 Therefore, HBP may accelerate ageing and increase the loss of epithelial microvilli and consequently of AQP1, which has a negative impact on CSF circulation.
Effects of high blood pressure on brain barriersSome studies suggest that HBP causes alterations in the brain barriers; specifically, radioactively labelled sucrose has been used to evaluate the permeability of these barriers, with the BCSFB reported to show greater disruption than the BBB.93,100,102 We may deduce from this that HBP causes greater damage to the cerebral structures constituting the BCSFB (i.e., the ChP) than to BBB structures. Marchi et al.103 describe a method of evaluating BCSFB permeability by determining levels of TTR monomer (which has a molecular weight of 14 kilodaltons [kDa]) in the blood. TTR is a homotetramer made up of different subunits, with tetrameric (55kDa), dimeric (28kDa), and monomeric (14kDa) forms also existing. In the blood, TTR is mainly present in the tetrameric form, whereas the monomeric form is more common in the CSF.103 Marchi et al.103 analysed serum TTR monomer levels to measure BBB openings induced with intra-arterial mannitol. Based on the hypothesis that HBP itself may cause BCSFB opening, some studies report markedly lower levels of TTR monomer in the CSF of hypertensive rats than in controls, and considerably higher levels in the blood of hypertensive rats than in controls.93,94 According to several studies, TTR monomer is transferred from the CSF to the blood in hypertensive individuals due to a possible disruption of the BCSFB caused by the effects of HBP.93,94,103
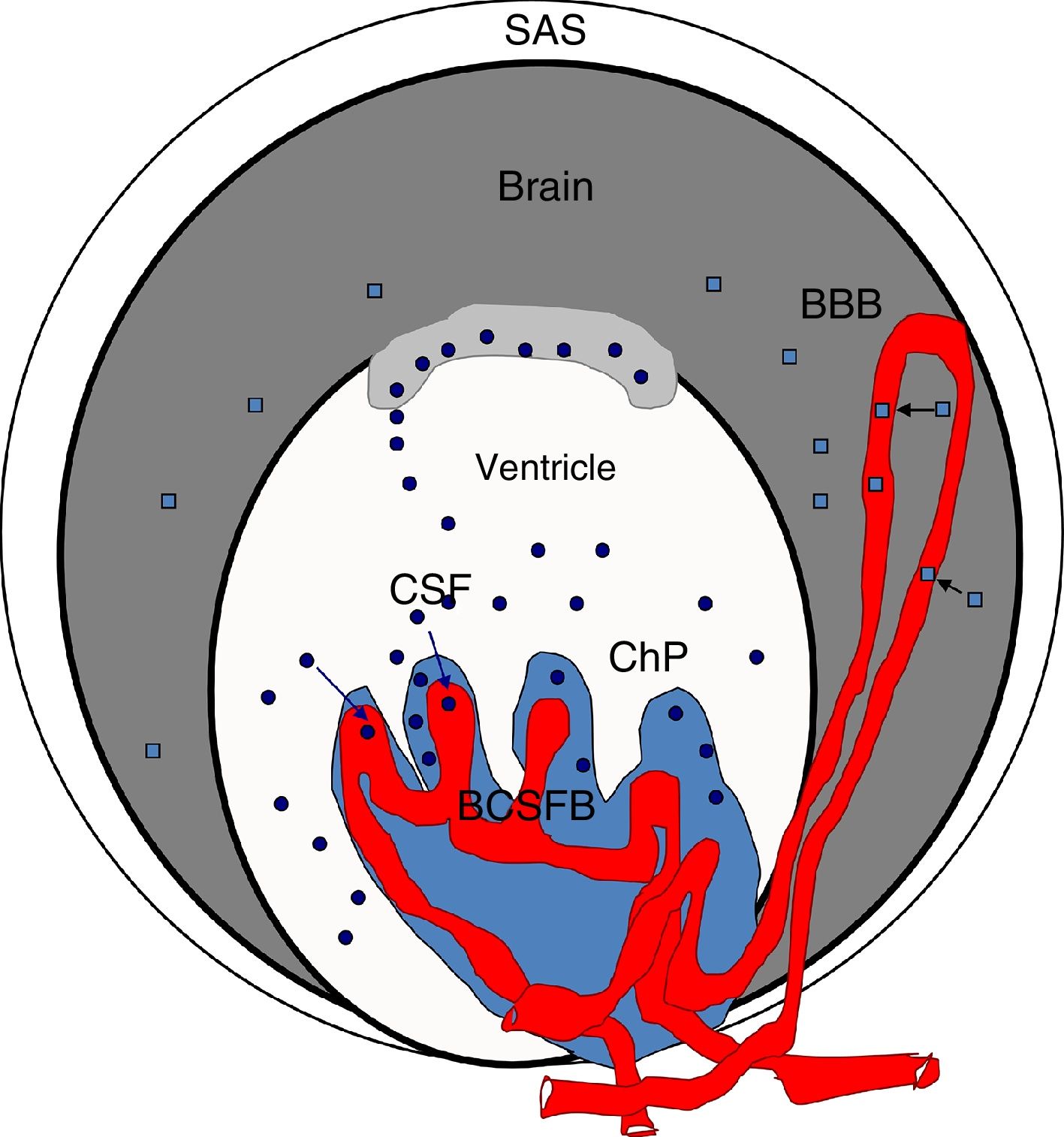
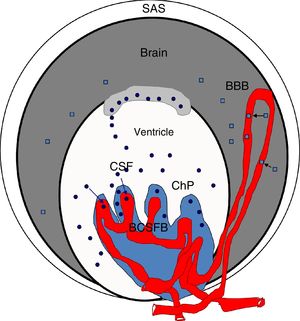
Blood S100β protein levels are measured to ascertain the effects of hypertension on the BBB. According to some authors, blood S100β concentrations are normally low but increase in certain pathological situations; the protein is mainly expressed in astrocyte endfeet and released into the blood if the BBB is disrupted, although it is also detected in the CSF.98,100,103–107 Therefore, blood S100β levels are higher in hypertensive animals than in controls, suggesting BBB damage in these animals. According to Al-Sarraf and Philip,100 the BCSFB is damaged to a greater extent than the BBB, as the quantity of TTR monomer transferred from the CSF to the blood is almost double the level of S100β in the blood of hypertensive animals, and the differences between hypertensive animals and controls are greater for TTR than for S100β (Fig. 4).
Diagram of the brain barriers and potential biomarkers of their disruption. BBB: blood–brain barrier; BCSFB: blood–CSF barrier; ChP: choroid plexus; CSF: cerebrospinal fluid; SAS: subarachnoid space; S100β: S100 calcium-binding protein B (low serum concentration).
Squares: disruption of the blood–brain barrier by S100β; circles: disruption of the blood–cerebrospinal fluid barrier by monomeric transthyretin.
Source: González-Marrero et al.,94 Al-Sarraf and Philip,100 Marchi et al.103.
Several other studies report that increases in the levels of certain proteins, including albumin, immunoglobulin G (IgG), and haptoglobin, may be caused by damage to the BCSFB, which allows them to cross into the CSF.59,60,94,100,104 A proteomics study of the CSF of hypertensive rats found differences in these proteins, with higher CSF levels of albumin, IgG, and haptoglobin in hypertensive rats.88 These data show that HBP causes an increase in the levels of some plasma proteins in the CSF, which also supports the hypothesis of BCSFB disruption. These studies also describe an accumulation of IgG in the ChP of hypertensive animals, which would also suggest that chronic hypertension damages the BCSFB, increasing its permeability.93,94
ConclusionsMorphological changes to the ChP due to hypertension accentuate the changes caused by ageing due to the decrease in cerebral perfusion. Data from analysis of TTR and AQP1 levels suggest that HBP decreases the secretory function of the ChP and its production of CSF. Furthermore, determining levels of S100β and TTR monomer in the serum and CSF has made it possible to verify that the brain barriers are disrupted in the context of HBP, that the changes in the BCSFB become more marked as untreated hypertension progresses, and that the BCSFB is more severely affected than the BBB. Future studies should address the clinical use of these biomarkers to assess the state of the brain barriers, which may clarify whether these changes can be prevented or reduced if hypertension is treated.
FundingThis study received funding from the Canarian Institute of Research and Science (INIPRO); project no. 03/14.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gonzalez-Marrero I, Hernández-Abad LG, Castañeyra-Ruiz L, Carmona-Calero EM, Castañeyra-Perdomo A. Variaciones de los plexos coroideos y las barreras cerebrales en la hipertensión arterial y el envejecimiento. Neurología. 2022;37:371–382.