The prescription pattern of antiepileptic drugs (AEDs) during pregnancy is changing but to what extent this is occurring in Spain remains unknown. The efficacy of newer drugs for controlling seizures is a key issue and may have changed over the years as doctors gained familiarity with these drugs during pregnancy. To assess these 2 topics, we report the results from the Spanish EURAP register gathered over a 12-year period.
Material and methodsAfter signing informed consent forms, patients were included in the register and evaluated at onset of pregnancy, at the end of the second and third trimesters, after delivery, and one year after delivery. For the purposes of this study, we analysed AEDs, type of epilepsy, seizure frequency per trimester and throughout pregnancy, percentage of seizure-free pregnancies, and frequency of congenital malformations. We then compared data from 2 periods (June 2001 to October 2007 and January 2008 to May 2015).
ResultsWe compared 304 monotherapies from the older period to 127 from the more recent one. There was a clear increase in the use of levetiracetam (LEV) with declining use of carbamazepine (CBZ), phenytoin, and phenobarbital; a slight decline in use of valproate (VPA), and a slight increase in the use of lamotrigine (LTG) and oxcarbazepine (OXC). Epilepsy types treated with CBZ and VPA remained unchanged, whereas fewer cases of generalised epilepsy were treated with LTG in the new period. This trend was not associated with significant changes in seizure frequency, but rather linked to better control over de novo seizures in the third trimester. LEV was similar to CBZ and VPA with regard to levels of seizure control, and more effective than LTG. Generalised epilepsy accounted for 64% of the cases treated with LEV.
ConclusionsThe prescription pattern of AEDs during pregnancy has changed in Spain, with diminishing use of CBZ, phenytoin, and phenobarbital. Changes also reflect the type of epilepsy, since there is less use of LTG for generalised epilepsy. LEV provides similar seizure control to that of the older AEDs, and it is more effective and better than LTG.
El patrón de uso de fármacos antiepilépticos (FAE) durante el embarazo difiere entre países y está cambiando. Se desconoce en qué medida ello afecta a la población española. La eficacia de los nuevos fármacos en el control de las crisis es motivo de preocupación y puede haber cambiado a lo largo de los años debido a un mejor conocimiento de su uso durante el embarazo. Con el objetivo de analizar estos 2 aspectos reportamos los resultados del registro EURAP España durante un periodo de 12 años.
Material y métodosTras el consentimiento informado, las pacientes son incluidas en el registro y evaluadas al inicio del embarazo, al final del segundo y tercer trimestres, después del parto y al año del nacimiento. Para los objetivos de este estudio hemos analizado: FAE, tipo de epilepsia, frecuencia de crisis por trimestres y a lo largo del embarazo, porcentaje de pacientes libres de crisis, y frecuencia de malformaciones congénitas mayores. Hemos comparado estas variables en 2periodos (junio de 2001-octubre de 2007 y enero de 2008-mayo de 2015).
ResultadosUn total de 304 monoterapias del periodo antiguo se comparan con 127 del periodo nuevo. Observamos un ascenso del uso de levetiracetam (LEV) y un descenso del uso de carbamacepina (CBZ), fenitoína y fenobarbital; un leve descenso del uso de valproato (VPA), y un leve aumento de lamotrigina (LTG) y oxacarbamacepina (OXC). El tipo de epilepsia se mantiene estable para CBZ y VPA, pero cambia para LTG, con menos epilepsias generalizadas tratadas con este fármaco en el periodo nuevo. Ello no se asocia con un cambio significativo de la frecuencia de crisis, pero sí con un mejor control de las crisis de novo en el tercer trimestre. LEV se asocia a niveles de control de crisis similares a los de CBZ y VPA y mejor que con LTG. De las pacientes tratadas con LEV, un 64% tenían una epilepsia generalizada.
ConclusionesEl patrón de uso de los diferentes FAE durante el embarazo está cambiando en España, con menos uso de CBZ, fenitoína y fenobarbital y un aumento del uso de LEV. El tipo de epilepsia también cambia, con un porcentaje inferior de pacientes tratadas con LTG para epilepsias generalizadas. LEV controla las crisis de manera similar a los fármacos clásicos y mejor que la LTG.
Epilepsy usually has no negative effect on pregnancy, delivery, or the child's health. However, epileptic women receiving antiepileptic drugs (AED) are known to be at a greater risk of experiencing seizure exacerbation during pregnancy, as well as other obstetric complications (miscarriage, preterm labour, low birth weight, and maternal or fetal death); their offspring are more likely to have congenital malformations or developmental delays. This risk increases with higher doses of AEDs.1–3
Management of epilepsy during pregnancy requires us to weigh the possible adverse effects of AEDs on the fetus against the effects of seizures on the mother and the fetus. Maintaining the balance between adequate seizure control during pregnancy and the teratogenic risk of AEDs is a major challenge for neurologists.
Treatment with AEDs varies between countries4 and has changed over the years for a number of reasons.5 The new AEDs are usually better tolerated, have fewer severe adverse effects and drug–drug interactions, and have been found to be similar in efficacy to traditional AEDs in pivotal trials.
Treatment with AEDs during pregnancy is also changing, although we do not know to what extent this is true in our population.
Furthermore, the efficacy of AEDs may have changed over the years through better understanding of their pharmacokinetic properties during pregnancy and their efficacy in the treatment of different types of seizures.
This study analyses changes in the prescription pattern of AEDs during pregnancy in Spain and whether seizure control during pregnancy has changed over the years.
Material and methodsThe European Registry of Antiepileptic Drugs and Pregnancy (EURAP) is a prospective, observational study aiming to gather data on the risk of fetal malformations associated with AED use during pregnancy. This collaborative study between multiple centres from different countries includes women taking AEDs for any reason at the time of conception, although in most cases AED use is associated with epilepsy.
After informed consent forms are signed, patient data are prospectively recorded using 5 forms, completed at pregnancy onset, after the first trimester, after the second trimester, after delivery, and one year after delivery; the latter form can be completed via a telephone interview. Each form includes the following data: type of AED; dose; demographic data (age, parity, parents’ education level); type of epilepsy or epileptic syndrome; family history of malformations or epilepsy; other factors that may increase the risk of malformations (drug use, including tobacco and alcohol, and radiation exposure); intercurrent diseases; number of convulsive and non-convulsive seizures per trimester; obstetric complications; folic acid use and dosage; and information about the foetus and newborn (results from the third trimester ultrasound scan, amniocentesis or corial biopsy [when applicable], Apgar score, weight, head circumference, and length).
Spain joined the EURAP in June 2001. In 2009, our research group published the results of the first 6 years of collaboration in the registry (June 2001 to October 2007). In that study, we concluded that valproate (VPA), whether as monotherapy or as part of polytherapy, was the drug with the greatest teratogenic risk, although we were unable to demonstrate a correlation between dose and risk.2 We also observed that patients treated with VPA achieved greater seizure control than those receiving lamotrigine (LTG).
The present study analyses the patients included in the EURAP-Spain registry between June 2001 and October 2007, and compares these to those included between January 2008 and May 2015.
We subsequently analysed the total number of registered patients receiving the new AEDs LTG, oxcarbazepine (OXC), and levetiracetam (LEV), and made comparisons between these patients and against those receiving the classic AEDs carbamazepine (CBZ) and VPA.
The Fisher exact test was used for statistical analysis.
Results of the comparative studyFrom January 2008 to May 2015, a total of 240 patients were included in the registry; data on all variables analysed (including the one-year follow-up period after delivery) were available for 154 mother–child pairs. Of these, 127 mothers (82%) were receiving monotherapy.
In our previous study, a total of 304 mother–child pairs were included for an equal period of time (6 years, from June 2001 to November 2007); a similar percentage of patients were receiving monotherapy (83%).2
Regarding obstetric outcomes in the present study, there were 141 live births, 8 spontaneous abortions (5%), 2 induced abortions (one due to a chromosomal disorder, none due to congenital malformations), and 3 stillbirths.
By type of epilepsy, 57 patients (37%) had primary generalised epilepsy, 90 (58%) had focal epilepsy, and 7 (4.5%) had undetermined epilepsy. These results are similar to those of the first series.2
The drugs most frequently used in monotherapy in the more recent series were LTG (37 patients, 29%), LEV (25, 20%), CBZ (24, 19%), VPA (26, 20.5%), OXC (6, 5%), topiramate (TPM; 5, 4%), and primidone (1 patient).
Comparing these results to those of the previous series (June 2001 to November 2007),2 CBZ use has decreased from 38% to 19% and VPA use from 25% to 20%, whereas LTG use has increased slightly (21%-29%). LEV use increased considerably, from 1.3% (4 patients) to 20%, whereas use of phenobarbital and phenytoin decreased from 12 (4%) and 10 patients (3%), respectively, to no cases in the more recent series.
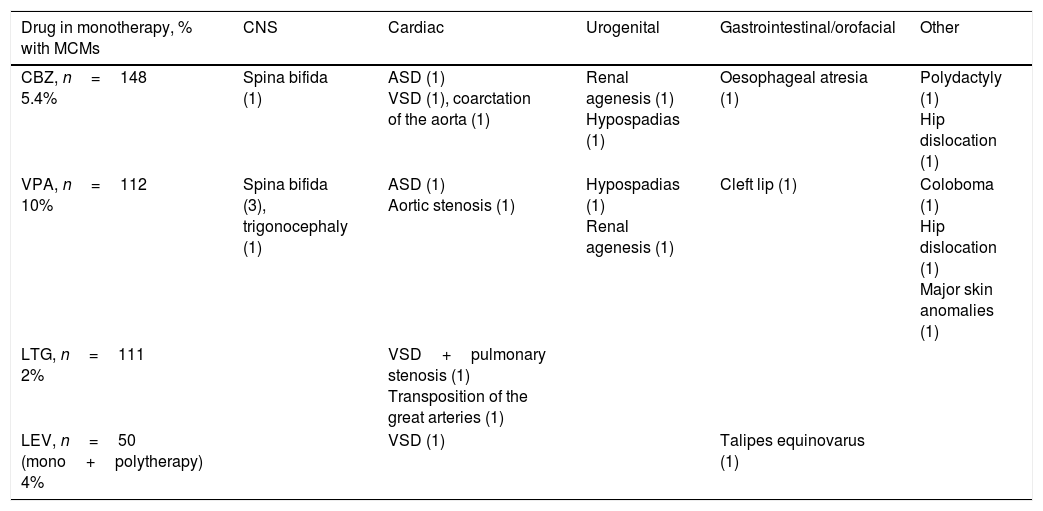
Eight children (5.5% of 144 children, excluding spontaneous abortions and induced abortions not secondary to major congenital malformations [MCM]) had one or more than one MCM; this percentage is similar to that reported in the 2001 to 2007 series.2 We did observe differences in the percentage of children of polymedicated mothers who developed MCMs (7.6% [2/26] vs 12% in the older series); this may be explained by the lower number of patients receiving polytherapy with VPA (6/26 [23%] in the more recent series vs 15/51 [29%] in the 2001-2007 series). The following MCMs were found in children of monotherapy patients: cleft lip and palate (1, mother receiving VPA), aortic stenosis (1, mother receiving VPA), ventricular septal defect (2, 1 mother receiving CBZ and 1 receiving LEV), transposition of the great arteries (1, mother receiving LTG), and talipes equinovarus (1, mother receiving LEV). Children from polymedicated mothers developed hypospadias (1, mother receiving VPA and TPM) and renal agenesis (1, mother receiving ethosuximide and TPM). We found no cases of spina bifida in the present series, compared to 7 cases in the 2001 to 2007 series (3 mothers receiving monotherapy with VPA, 2 receiving polytherapy with VPA, 1 receiving monotherapy with CBZ, and 1 case of a chromosomal disorder associated with VPA [Patau syndrome]).
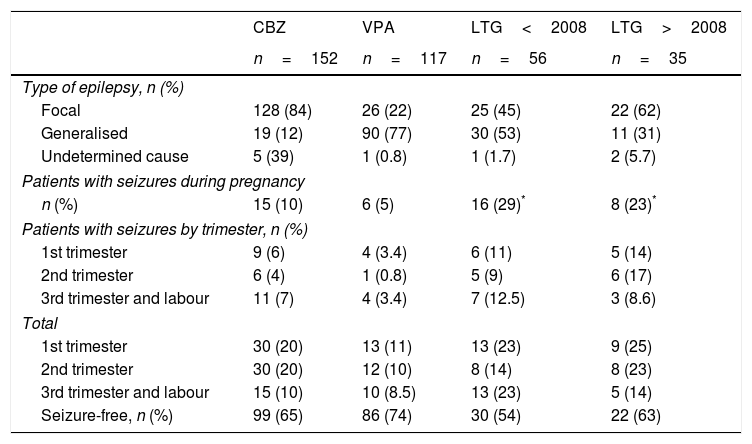
Results for the new antiepileptic drugsA total of 56 patients (21%) from the 2001 to 2007 series received LTG in monotherapy, compared to 35 (24%) in the 2008 to 2015 series, excluding cases of induced abortion and chromosomal disorders. Table 1 shows the distribution of the types of epilepsy, number of seizure-free pregnancies, and seizure frequency per trimester and throughout pregnancy for CBZ, VPA, and LTG.
Type of epilepsy and seizure frequency for patients receiving carbamazepine, valproate, and lamotrigine before and after 2008.
| CBZ | VPA | LTG<2008 | LTG>2008 | |
|---|---|---|---|---|
| n=152 | n=117 | n=56 | n=35 | |
| Type of epilepsy, n (%) | ||||
| Focal | 128 (84) | 26 (22) | 25 (45) | 22 (62) |
| Generalised | 19 (12) | 90 (77) | 30 (53) | 11 (31) |
| Undetermined cause | 5 (39) | 1 (0.8) | 1 (1.7) | 2 (5.7) |
| Patients with seizures during pregnancy | ||||
| n (%) | 15 (10) | 6 (5) | 16 (29)* | 8 (23)* |
| Patients with seizures by trimester, n (%) | ||||
| 1st trimester | 9 (6) | 4 (3.4) | 6 (11) | 5 (14) |
| 2nd trimester | 6 (4) | 1 (0.8) | 5 (9) | 6 (17) |
| 3rd trimester and labour | 11 (7) | 4 (3.4) | 7 (12.5) | 3 (8.6) |
| Total | ||||
| 1st trimester | 30 (20) | 13 (11) | 13 (23) | 9 (25) |
| 2nd trimester | 30 (20) | 12 (10) | 8 (14) | 8 (23) |
| 3rd trimester and labour | 15 (10) | 10 (8.5) | 13 (23) | 5 (14) |
| Seizure-free, n (%) | 99 (65) | 86 (74) | 30 (54) | 22 (63) |
The total number of patients excludes patients who had spontaneous abortions or induced abortions not due to MCMs or chromosomal disorders. Focal epilepsy includes patients with localised seizures, whether partial seizures or secondarily generalised seizures. Generalised epilepsy includes all types of generalised seizures (absence, myoclonic, tonic, tonic–clonic), whether idiopathic, symptomatic, or cryptogenic.
CBZ: carbamazepine; LTG: lamotrigine; VPA: valproate.
We observed differences in the type of epilepsy associated with LTG use between the older series and the more recent series: generalised epilepsy was seen in 53% of patients vs 31% and focal epilepsy in 45% vs 62% (this association has not been analysed in other studies). However, this change in the type of epilepsy was not accompanied by a significant decrease in the number of seizures during pregnancy (29% in the 2001-2007 series vs 23% in the 2008-2015 series), although we did observe a trend towards better seizure control (54% vs 63% of patients were seizure-free). Regarding the number of patients receiving LTG who experienced seizures during each trimester, patients in the present series achieved better seizure control during the third trimester than patients in the original series (14% vs 23% had seizures; P=.29); no significant differences were observed between series for the first and second trimesters (25% and 23% of patients had seizures during the first trimester). Three patients from the 2001 to 2007 series (5%) and 2 from the 2008 to 2015 series (6%) experienced de novo seizures during the second trimester. During the third trimester, by contrast, de novo seizures were reported by 5 patients from the 2001 to 2007 series (9%) and one from the 2008 to 2015 series (3%) (P=.39). In patients receiving the other 2 AEDs, de novo seizures were observed in 3 patients receiving CBZ (2%) during the second trimester and 3 (2%) during the third, and in one patient receiving VPA (0.8%) during the second trimester and another (0.8%) during the third.
Of all patients treated with LTG in monotherapy (111 cases, excluding spontaneous abortions and induced abortions not due to MCMs or chromosomal disorders), 2 had children with MCMs (2%). Table 2 shows the types of malformations observed in these patients’ children: one case of transposition of the great arteries (mother with focal epilepsy; LTG dosed at 300mg/day) and one case of pulmonary stenosis plus ventricular septal defect (mother with focal epilepsy; LTG dosed at 250mg/day).
Types of major congenital malformations associated with use of CBZ, VPA, and LTG in monotherapy, and of LEV in monotherapy and polytherapy.
| Drug in monotherapy, % with MCMs | CNS | Cardiac | Urogenital | Gastrointestinal/orofacial | Other |
|---|---|---|---|---|---|
| CBZ, n=148 5.4% | Spina bifida (1) | ASD (1) VSD (1), coarctation of the aorta (1) | Renal agenesis (1) Hypospadias (1) | Oesophageal atresia (1) | Polydactyly (1) Hip dislocation (1) |
| VPA, n=112 10% | Spina bifida (3), trigonocephaly (1) | ASD (1) Aortic stenosis (1) | Hypospadias (1) Renal agenesis (1) | Cleft lip (1) | Coloboma (1) Hip dislocation (1) Major skin anomalies (1) |
| LTG, n=111 2% | VSD+pulmonary stenosis (1) Transposition of the great arteries (1) | ||||
| LEV, n=50 (mono+polytherapy) 4% | VSD (1) | Talipes equinovarus (1) |
The total number of patients excludes patients who had spontaneous abortions or induced abortions not due to MCMs or chromosomal disorders.
CBZ: carbamazepine; ASD: atrial septal defect; VSD: ventricular septal defect; LTG: lamotrigine; LEV: levetirazetam; MCM: major congenital malformation; CNS: central nervous system; VPA: valproate.
During the original study period, only 4 and 13 patients received monotherapy with LEV and OXC, respectively; we will therefore not analyse these 2 drugs by period, but rather use the total number of patients receiving LEV or OXC during both periods (2001-2015).
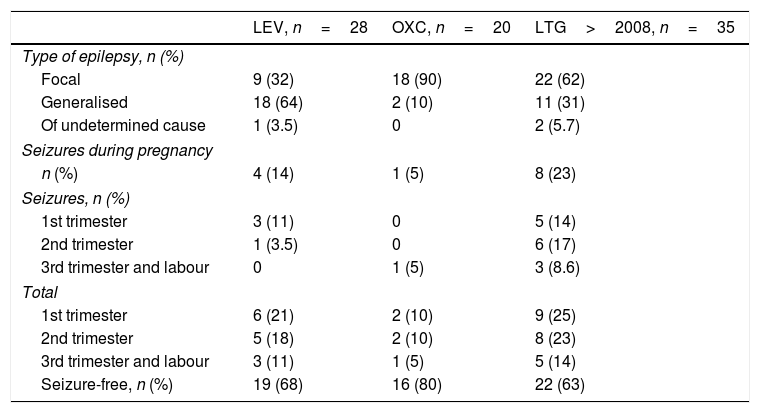
LEV was administered to 57 of the 630 patients registered during the 2001 to 2015 period. Of these, 31 patients received it in monotherapy and 26 in polytherapy. Pregnancy outcomes for patients in monotherapy were 27 live births, 2 spontaneous abortions, one induced abortion due to a chromosomal disorder, and one stillbirth. Patients in polytherapy had 22 live births and 4 abortions. Two patients in monotherapy and none of the patients in polytherapy had children with MCMs: one with ventricular septal defect (dose: 1000mg/day) and the other with talipes equinovarus (dose: 1500mg/day). Polytherapy with LEV was most frequently associated with LTG (9 patients), followed by OXC (6), VPA plus TPM (2), CBZ (3), clobazam (2), and 3 AEDs (3). Table 3 shows the type of epilepsy, number of seizure-free pregnancies, and seizure frequency per trimester and throughout pregnancy in patients receiving monotherapy. Of the 28 LEV monotherapy patients who carried the pregnancy to term or aborted due to chromosomal disorder, 18 (64%) had generalised epilepsy and 9 (32%) had focal epilepsy; the type of epilepsy was undetermined in one case. Four patients treated with LEV (14%) had convulsive seizures during pregnancy and 19 (68%) remained seizure-free throughout pregnancy. Seizures predominantly occurred during the first trimester (3 cases, 10%), decreasing throughout pregnancy (no seizures during the third trimester).
Type of epilepsy and seizure frequency for levetiracetam, oxcarbazepine, and lamotrigine after 2008.
| LEV, n=28 | OXC, n=20 | LTG>2008, n=35 | |
|---|---|---|---|
| Type of epilepsy, n (%) | |||
| Focal | 9 (32) | 18 (90) | 22 (62) |
| Generalised | 18 (64) | 2 (10) | 11 (31) |
| Of undetermined cause | 1 (3.5) | 0 | 2 (5.7) |
| Seizures during pregnancy | |||
| n (%) | 4 (14) | 1 (5) | 8 (23) |
| Seizures, n (%) | |||
| 1st trimester | 3 (11) | 0 | 5 (14) |
| 2nd trimester | 1 (3.5) | 0 | 6 (17) |
| 3rd trimester and labour | 0 | 1 (5) | 3 (8.6) |
| Total | |||
| 1st trimester | 6 (21) | 2 (10) | 9 (25) |
| 2nd trimester | 5 (18) | 2 (10) | 8 (23) |
| 3rd trimester and labour | 3 (11) | 1 (5) | 5 (14) |
| Seizure-free, n (%) | 19 (68) | 16 (80) | 22 (63) |
The total number of patients excludes patients who had spontaneous abortions or induced abortions not due to MCMs or chromosomal disorders. Focal epilepsy includes patients with localised seizures, whether partial seizures or secondarily generalised seizures. Generalised epilepsy includes all types of generalised seizures (absence, myoclonic, tonic, tonic–clonic), whether idiopathic, symptomatic, or cryptogenic.
LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine.
A total of 40 patients received OXC: 22 in monotherapy and 18 in polytherapy. Pregnancy outcomes were 20 live births and 2 spontaneous abortions for monotherapy patients, and 17 live births and one induced abortion due to MCMs (spina bifida; OXC+VPA) for polymedicated patients. Of the 20 monotherapy patients who carried the pregnancy to term, 18 (90%) had focal epilepsy. Only one patient (5%) experienced seizures, which occurred during the third trimester. Sixteen patients (80%) remained seizure-free throughout pregnancy. No significant differences were observed in the number of seizures between trimesters.
The patients treated with LEV were found to have generalised epilepsy more frequently and to achieve better seizure control than those receiving LTG: 14% of patients receiving LEV had seizures during pregnancy vs 29% of patients receiving LTG in the older series and 23% in the more recent series. There were statistically significant differences in seizure frequency during pregnancy between patients receiving LTG and those receiving VPA, while no significant differences were found between patients treated with VPA and those treated with LEV. According to the analysis of seizure frequency by trimester, one of the 28 patients receiving LEV (3.5%) experienced de novo seizures during the first trimester, compared to 8 of 86 patients receiving LTG in the first study period (14%) and 3 of 35 patients receiving LTG in the more recent series (8.6%).
DiscussionThe percentages of patients with generalised epilepsy and focal epilepsy in our study are similar in both periods analysed and consistent with the percentages observed in the EURAP registry.6 However, other studies (UK, US)7,8 have not analysed this variable, or report results that differ from ours (focal epilepsy in 48% of patients in the Australian registry vs 58% in the present study).9 Such differences are very likely due to methodological reasons: the EURAP registry gathers data recorded by neurologists, whereas other registries gather patient-reported data.10 Data on the type of epilepsy is important, given that patients with generalised epilepsy are known to be more likely to remain seizure-free during pregnancy than those with focal epilepsy.11–14
Our study shows a change in the pattern of LTG treatment, with fewer patients with generalised epilepsy receiving the drug in the more recent period (31%) than in the first (53%). This may be due to the fact that this drug is not as effective for generalised myoclonic epilepsy (as reported in 2007 by the SANAD study).15 However, and contrary to what may be expected, no significant changes were seen in seizure control throughout pregnancy. We only observed a trend towards better control of seizures in general (54% seizure-free patients in the older series vs 63% in the more recent series) and in the number of de novo seizures during the third trimester (3% vs 9%), although these differences were not statistically significant.
According to the results of the Australian registry,9 classic AEDs are more effective for managing epilepsy in pregnant women than the new AEDs (LTG, LEV, and TPM) in the whole series as well as in the group of patients treated since 2008. That study also found classic AEDs to be more effective in the treatment of focal or generalised epilepsy than the new AEDs. The Australian registry9 shows no differences in seizure occurrence during the year before pregnancy between patients with active epilepsy receiving classic AEDs and those receiving new AEDs. However, the new AEDs were found to control seizures significantly less in the group of patients with inactive epilepsy during the year before pregnancy. According to the results from both the Australian9 and the EURAP13 registries, LTG was responsible for these differences, as it achieves worse seizure control during pregnancy. In our study, no significant differences were found in the percentage of patients treated with LTG and experiencing seizures between the first (2001-2007) and the second study periods (2008-2015). According to Reisinger et al.,14 seizures worsen during the second trimester when blood levels of LTG decrease from preconception levels by >35%. Sabres et al.16 report that thorough follow-up of LTG plasma levels combined with dose adjustment to maintain adequate levels results in similar rates of seizure control to those associated with other AEDs. Our study did not determine LTG plasma levels or dose increases in successive trimesters to analyse whether LTG doses were adjusted. However, we assume that doses were adjusted each trimester as of 2008, due to better understanding of the pharmacokinetic properties of the drug. We need further studies with larger samples, follow-up on doses and plasma LTG levels, and analysis of seizure occurrence before pregnancy to determine whether seizure control improves with this drug, given its good safety profile regarding the risk of MCMs or cognitive/behavioural disorders in newborns.6,17
Our study found a decrease in the use of CBZ, and to a lesser extent of VPA, in the second study period. Use of LEV increased significantly. Interestingly, 64% of patients treated with this drug had generalised epilepsy. There were no significant differences in the percentage of patients with generalised epilepsy who received LEV or VPA (77% of patients treated with VPA had generalised epilepsy). However, we did find differences between VPA and LTG (P=.001) in both study periods; LTG is less frequently used to treat generalised epilepsy. Further studies are necessary to confirm these results, since our series included only a small number of patients receiving LEV.
Vajda et al.9 compare use of LEV to that of VPA, finding no significant differences between these 2 AEDs in terms of seizure control. However, that study provides no data on the percentage of patients with generalised epilepsy who received LEV or VPA, or on seizure occurrence in these patients during the year before pregnancy. Other studies directly or indirectly analysing seizure frequency in patients receiving LEV show seizure occurrence rates no higher than those associated with the use of classic AEDs.8,9,18
In our study, the percentage of children born with MCMs was similar in both periods.2 We observed a lower number of polymedicated patients giving birth to children with MCMs (7.6% in the more recent series vs 12% in the older series), probably due to a slight decrease in the number of patients treated with VPA or the use of lower doses.19,20 Two of the 28 patients receiving monotherapy with LEV had children with MCMs. This striking finding may be attributed to chance, given that a literature review conducted by Chaudhry et al.21 reports an incidence of MCMs of 2.2% (27/1213) in children of mothers taking LEV.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank the SEN's Epilepsy Study Group for its funding and its contribution to the EURAP registry, and Drs A. Cobos and S. Quintana for their help with statistical analysis.
Professionals participating in the EURAP registry (and not listed as authors of this study), ordered by number of patients included: E. Pastor Millán, Hospital Universitario Virgen de las Nieves, Granada; M.D. Castro Vilanova, Hospital Universitario Severo Ochoa, Madrid; I. García Morales, Hospital Ruber Internacional and Hospital Clínico Universitario San Carlos, Madrid; M. Codina and J.L. Becerra, Hospital Germans Trias i Pujol, Badalona; I. Garamendi Ruiz H., Hospital Universitario Cruces, Baracaldo; E. López Gomariz, Hospital Lluis Alcanyis de Xàtiva, Valencia; M.L. Galiano, Centro de Especialidades de Moratalaz, Madrid; J. Galán, Hospital Universitario de Valme, Seville; P. Fossas, Hospital de Mataró, Barcelona; T. García and N. Muelas, Hospital Universitari i Politècnic La Fe, Valencia; G. Sansa, Hospital Parc Taulí, Sabadell; D. Sopelana, Hospital Universitario de Albacete, Albacete; C. Cabeza, Hospital Virgen de la Salud, Toledo; J. Macarrón, Yagüe, Complejo Asistencial Universitario de Burgos, Burgos; R. Ribacoba, Hospital Álvarez Buylla, Asturias; A. Molins, Hospital Universitari Dr. Josep Trueta, Gerona; M. Veciana, Hospital Comarcal de Sant Boi, Barcelona; E. Comas, Hospital Universitari Sagrat Cor, Barcelona; L. Morlas, Hospital Universitario de Getafe, Madrid; A. Arribas, Hospital Comarcal de l’Alt Penedes, Vilafranca del Penedès; M. Amorin, Hospital Comarcal del Noroeste, Murcia; J. Poza, Hospital Nuestra Señora de Aranzazu, San Sebastián; M.J. Imaz and L. García-Esteve, Hospital Clinic, Barcelona.
More information about the professionals who cooperated in this study is provided in Appendix A.
Please cite this article as: Martinez Ferri M, Peña Mayor P, Perez López-Fraile I, Escartin Siquier A, Martin Moro M, Forcadas Berdusan M, et al. Estudio comparativo del uso de fármacos antiepilépticos durante el embarazo en un periodo de 12 años. Eficacia de los nuevos fármacos lamotrigina, levetiracetam y oxacarbamacepina. Neurología. 2018;33:78–84.
Part of this study was presented at the Annual Meeting of the Catalan Society of Neurology (May 2015).