In December 2019, the Chinese City of Wuhan warned of a new disease (COVID-19) caused by infection with a novel coronavirus (SARS-CoV-2).1 Due to its virulence and ease of transmission, the World Health Organization declared the COVID-19 outbreak a pandemic on 11 March 2020.2 Spain has been one of the hardest-hit countries in the world, which has led to a complete reorganisation of healthcare, including neurological care.3
As new cases of COVID-19 have been reported, the clinical description of the disease has expanded to include various respiratory infection syndromes; presence of associated neurological processes and symptoms is very frequent, but the pathophysiological relationship is yet to be established.4,5
Given the high prevalence of COVID-19, the presence of other concomitant infections may simply be coincidental.6 However, given the previously reported neurotropic action of SARS-CoV-2,7 the hyperinflammatory response, and immunosuppression secondary to the infection or the drugs used to treat it,8 we cannot rule out a causal relationship in these patients. In this article, we describe 2 patients with SARS-CoV-2 infection and concomitant central nervous system infection.
Our first patient is a 63-year-old man with history of dyslipidaemia who had suffered a hunting accident years earlier in which he was hit by bird shot in the frontal and maxillary areas. He presented fever and general discomfort of 7 days’ progression. Upon arriving at the emergency department, he presented poor general condition, temperature of 38°C, and tachycardia; the remaining vital signs were normal. The physical examination revealed pronounced psychomotor agitation, which required pharmacological sedation and orotracheal intubation; pulmonary auscultation revealed bilateral basal crackles.
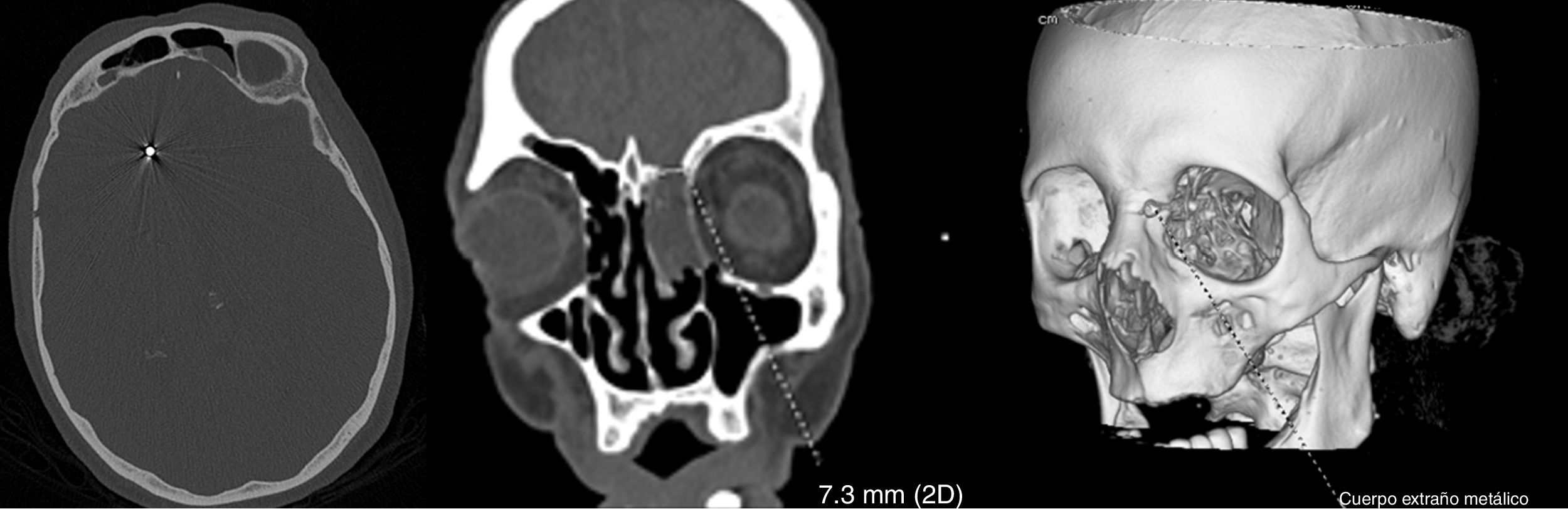
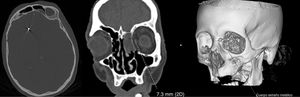
An emergency blood analysis showed leukocytosis (16100cells/mm3) with neutrophilia (89%), lymphocytopaenia (1000cells/mm3), and high levels of fibrinogen (4.72g/L), d-dimer (2645ng/mL), C-reactive protein (5.3mg/L), procalcitonin (0.8ng/mL), and ferritin (401.7ng/mL). A head CT scan showed 2 metallic artefacts in the left nasal region and right frontal lobe, compatible with bird shot (Fig. 1); a chest radiography revealed an interstitial infiltrate in the right lung base and a lumbar puncture yielded turbid cerebrospinal fluid (CSF) with 781leukocytes/μL (predominantly polymorphonuclear, 88%), low glucose levels (3mg/dL), and high protein levels (4.61g/L). Blood cultures and urine pneumococcal and Legionella antigen tests yielded normal results; a PCR test for SARS-CoV-2 returned positive results. CSF microbiology results were positive for Streptococcus pneumoniae. Furthermore, we suspected a CSF fistula; this was confirmed by a facial CT scan.
The patient was admitted to the intensive care unit and started treatment with ceftriaxone, ampicillin, and dexamethasone; after we received the CSF microbiology findings, treatment was reduced to ceftriaxone only, which was maintained for 14 days. We also started treatment with hydroxychloroquine, azithromycin, and lopinavir–ritonavir to treat the coronavirus infection. The patient progressed favourably and was discharged 17 days after admission.
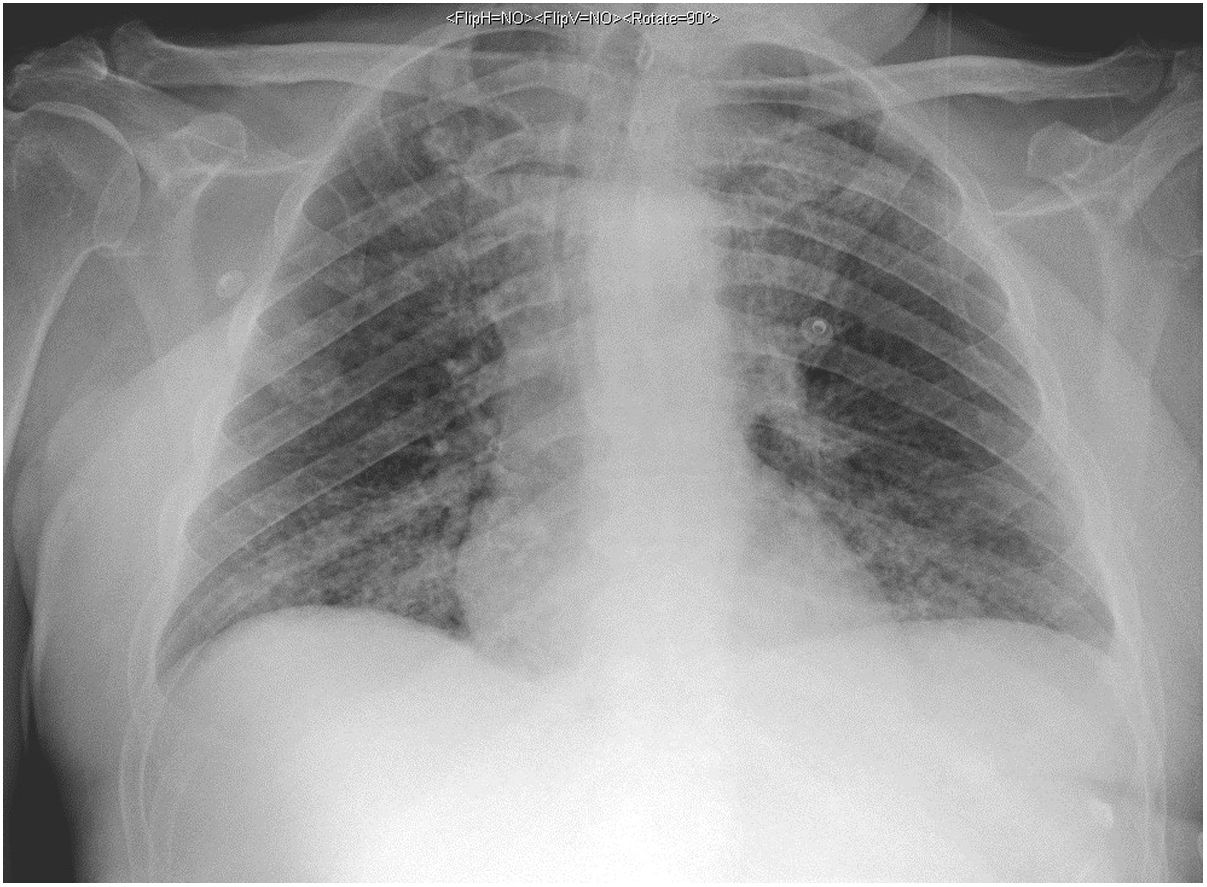
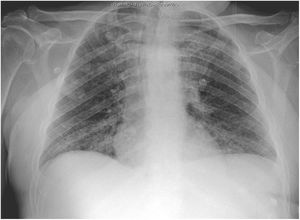
The second patient was a 59-year-old man with vascular risk factors and substance use disorder. He was admitted due to a 3-day history of high fever, cough, and general discomfort. A blood analysis showed leukocytosis (20400cells/mm3) with neutrophilia (87%), lymphocytopaenia (900cells/mm3), and high levels of d-dimer (1500ng/mL), creatinine (2.8mg/dL), sodium (157mmol/L), LDH (761IU/L), CPK (780IU/L), transaminases, C-reactive protein (180mg/L), and ferritin (2900ng/mL), as well as hypoxaemia with hypocapnia. A chest radiography showed interstitial infiltrate in the lung base bilaterally (Fig. 2). We conducted a microbiology study similar to that performed in the first patient, and the PCR test for SARS-CoV-2 yielded positive results.
Level of consciousness deteriorated on day 12 after admission. A head CT scan revealed normal findings, and a lumbar puncture only showed high protein levels (0.66g/dL); microbiology findings were positive for herpes simplex virus 1. Aciclovir was then introduced, but clinical progression was unfavourable and the patient died 5 days later.
Understanding of COVID-19 is progressively increasing, but many aspects remain unclear. Simultaneous infection of the central nervous system in these patients may simply have been coincidental; if not, we are currently unable to identify the pathophysiological mechanism despite the clear temporal association.
Our pathophysiological hypothesis is based on the assumption that immunosuppression secondary to COVID-19 may facilitate central nervous system infection. Furthermore, in the second patient, pharmacological immunosuppression may also have contributed to herpes virus reactivation, considering that infection manifested on day 12 after admission and the patient was receiving high-dose corticosteroids.
Please cite this article as: Romero Cantero V, Moreno Pulido S, Duque Holgado M, Casado Naranjo I. COVID-19 y neuroinfecciones concomitantes. Neurología. 2020;35:332–333.