Painful diabetic neuropathy (PDN) is the most frequent cause of neuropathic pain and it affects 20% to 25% of all patients with diabetes.1,2 PDN manifests with pain described as burning or cutting, resembling electric shock, cold, or compression. Patients experience hyperalgesia and pain typically occurs at night.3
The natural history of PDN is variable and it has an unpredictable clinical course. Maintaining adequate levels of glycated haemoglobin will slow or even halt the progression of neuropathy.4 Current recommendations for treating PDN5,6 include such antiepileptics as carbamazepine and oxcarbazepine, which belong to the carboxamides.7
In this letter, we wanted to share our experience with eslicarbazepine (ESL) as a second-line treatment in cases of PDN refractory to traditional drugs.
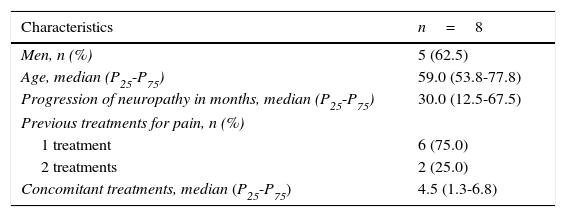
During 2013 we identified 8 patients with PDN whose previous treatments had resulted in poor pain management. In the initial visit, we substituted their previous pain treatment for ESL, gathered demographical data (Table 1), and administered the following questionnaires: DN4 scale, HADS anxiety and depression subscales, Visual Analogue Scale for pain (VAS), the Patient Global Impression-Improvement scale (PGI-I), and the Clinical Global Impression-Improvement scale (CGI-I). In a follow-up consultation conducted 12 weeks after treatment, patients completed the same questionnaires and were asked about any adverse effects.
Demographic data.
| Characteristics | n=8 |
|---|---|
| Men, n (%) | 5 (62.5) |
| Age, median (P25-P75) | 59.0 (53.8-77.8) |
| Progression of neuropathy in months, median (P25-P75) | 30.0 (12.5-67.5) |
| Previous treatments for pain, n (%) | |
| 1 treatment | 6 (75.0) |
| 2 treatments | 2 (25.0) |
| Concomitant treatments, median (P25-P75) | 4.5 (1.3-6.8) |
We subsequently conducted a statistical analysis. We used the Wilcoxon test for quantitative variables and the McNemar test for dichotomous variables. Significance was established at P=.05. All analyses were conducted using SPSS® statistical software, version 19.0.
Regarding previous treatment, 75% of the patients had received pregabalin, 25% amitriptyline, 12.5% clonazepam, and 12.5% fentanyl, either in monotherapy or in combination. At ESL treatment onset, patients were taking a mean of 4.6 drugs simultaneously, with a minimum of one and a maximum of 11 drugs (median: 4.5). No changes were made in concomitant treatment during the follow-up period.
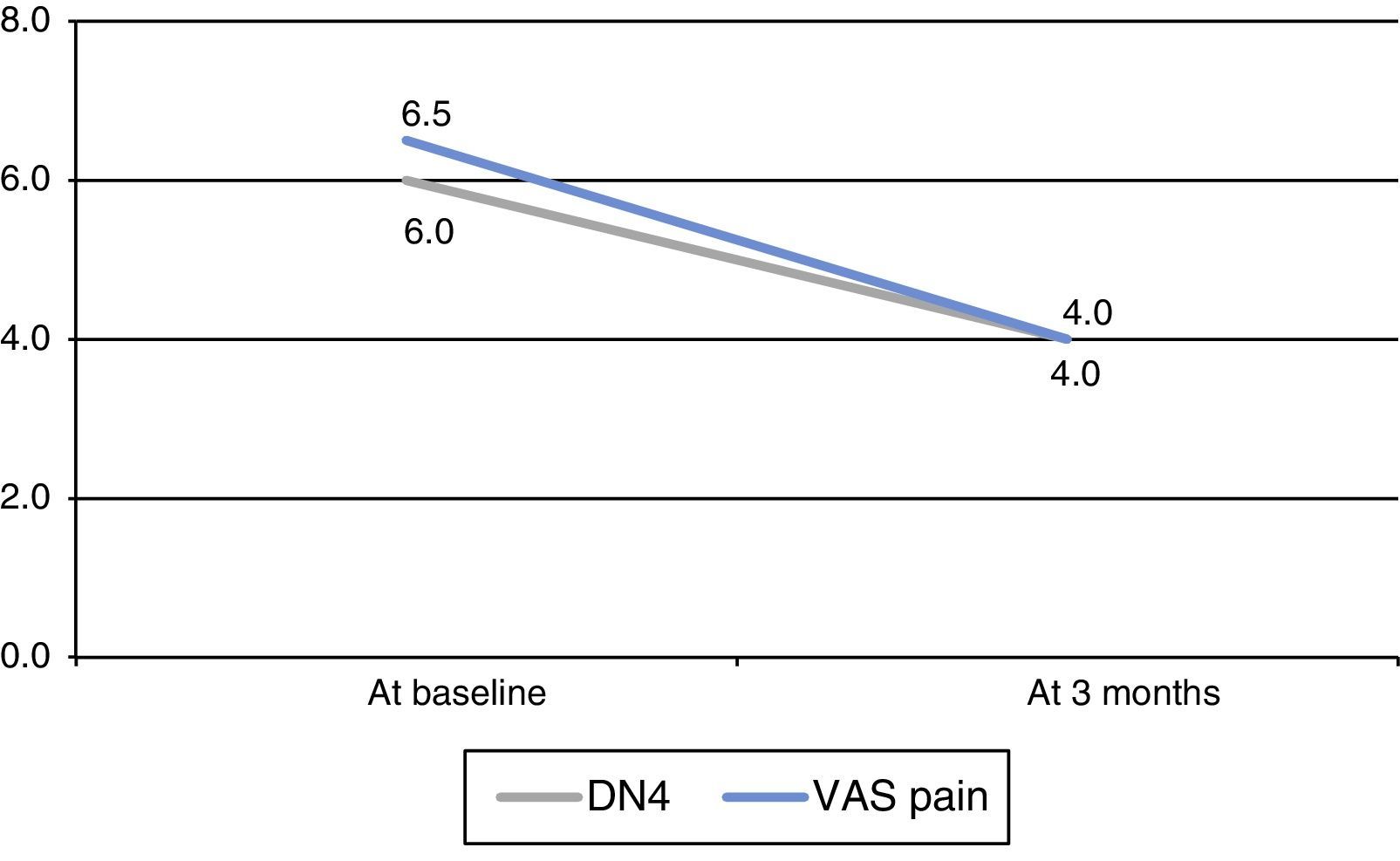
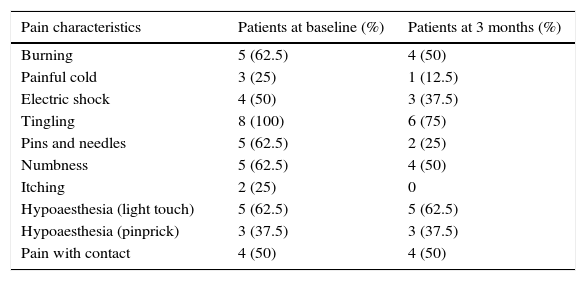
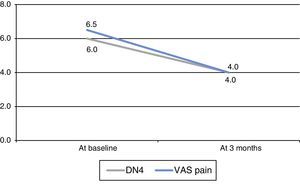
Neuropathic pain, evaluated with the DN4 scale, decreased significantly at 3 months (P=.026, Wilcoxon test) (Fig. 1). We found no significant changes in any of the characteristics of pain measured by this scale (Table 2). We observed a significant decrease in neuropathic pain as measured with the VAS (P=.018, Wilcoxon test) (Fig. 1). Patients’ scores improved on both the anxiety (P=.102, Wilcoxon test) and depression subscales (P=.276, Wilcoxon test) although changes were not statistically significant. Whereas 65.5% of the patients reported feeling ‘much better’ or ‘better’, 87.5% of the clinicians described the patients as ‘much better’ or ‘better’. One patient had to discontinue ESL because of adverse effects (dizziness). At 3 months, 7 patients (87.5%) continued with ESL: 2 dosed at 400mg/day and 5 dosed at 800mg/day.
DN4 scale broken down by item; patients showed significant improvements at 3 months (P=.026).
| Pain characteristics | Patients at baseline (%) | Patients at 3 months (%) |
|---|---|---|
| Burning | 5 (62.5) | 4 (50) |
| Painful cold | 3 (25) | 1 (12.5) |
| Electric shock | 4 (50) | 3 (37.5) |
| Tingling | 8 (100) | 6 (75) |
| Pins and needles | 5 (62.5) | 2 (25) |
| Numbness | 5 (62.5) | 4 (50) |
| Itching | 2 (25) | 0 |
| Hypoaesthesia (light touch) | 5 (62.5) | 5 (62.5) |
| Hypoaesthesia (pinprick) | 3 (37.5) | 3 (37.5) |
| Pain with contact | 4 (50) | 4 (50) |
ESL is a carboxamide marketed in 2010 as an adjuvant treatment for adults with partial seizures with or without secondary generalisation.8 It can be administered in a single daily dose and its advantages include better tolerability, fewer drug interactions, and longer half-life.9–11 In our study, it was administered to patients with PDN as compassionate use.
To date, no studies addressing use of ESL for neuropathic pain have been published. Our literature review returned only one poster presented at the congress of the European Pain Federation (EFIC) in 2013. According to this poster, patients with PDN were treated with ESL for 12 weeks and improvements were not significant compared to those in the placebo group. However, we should point out that this assessment was based on a single numeric scale for pain (NPRS).12
Despite the small sample size, our sample shows that, after 12 weeks, ESL is an effective alternative for managing neuropathic pain in patients with PDN refractory to conventional treatment. ESL demonstrates good tolerability and adherence. We need further studies with larger sample sizes and longer study periods to confirm our data.
FundingThis study has not received financing from any sources.
Please cite this article as: Garcia-Escrivà A, López-Herandez N, Lezcano M, Berenguer L. Experiencia en el tratamiento de la neuropatía diabética dolorosa con acetato de eslicarbazepina. Neurología. 2016;31:639–640.
This study was presented in poster format at the 2014 Annual Meeting of the SEN.