The classic form of trigeminal neuralgia is usually sporadic (no familial clustering). However, around 2% of all cases of trigeminal neuralgia may be familial. Describing this entity may be useful for diagnosing this process and may also be key to determining the underlying causes of sporadic classical trigeminal neuralgia. We report on cases in a series of 5 families with at least 2 members with classic trigeminal neuralgia, amounting to a total of 11 cases.
Material and methodsWe recorded cases of familial classical trigeminal neuralgia between March 2014 and March 2015 by systematically interviewing all patients with a diagnosis of trigeminal neuralgia who visited the neurology department on an outpatient basis.
ResultsIn our sample, most patients with familial classic trigeminal neuralgia were women. Mean age at onset was 62.9±13.93 years, decreasing in subsequent generations. V2 was the most frequently affected branch. Most of our patients responded well to medical treatment, and surgery was not effective in all cases.
ConclusionsThese family clusters support the hypothesis that classic trigeminal neuralgia may have a genetic origin. Several causes have been suggested, including inherited anatomical changes affecting the base of the skull which would promote compression of the trigeminal nerve by vascular structures, familial AHT (resulting in tortuous vessels that would compress the trigeminal nerve), and mutations in the gene coding for calcium channels leading to hyperexcitability. Classic trigeminal neuralgia may be an autosomal dominant disorder displaying genetic anticipation.
La neuralgia del trigémino clásica es un cuadro habitualmente esporádico, sin asociación familiar. Pero se estima que hasta un 2% de las neuralgias del trigémino podrían ser de tipo familiar. La caracterización de esta entidad es de utilidad para su identificación e incluso podría ser clave para definir las causas subyacentes en la neuralgia del trigémino clásica esporádica. Por esta razón, se aporta una serie de 5 familias en las que al menos existen 2 familiares con este cuadro, constituyendo un total de 11 casos.
Material y métodosSe recogieron casos familiares entre marzo del 2014 y marzo del 2015, interrogando sistemáticamente a los pacientes que acudían a la consulta de Neurología general con el diagnóstico de neuralgia del trigémino.
ResultadosLa neuralgia del trigémino clásica familiar afecta predominantemente a mujeres, la edad media de inicio es de 62,9±13,93 años, es más frecuente la afectación de V2 y la edad de presentación es más temprana en la siguiente generación. La mayoría responde al tratamiento farmacológico. La respuesta al tratamiento neuroquirúrgico no es efectiva en todos los casos.
ConclusionesEstas agrupaciones familiares apoyan la idea de probables implicaciones genéticas en el desarrollo de este cuadro. Se postulan como posibles causas: conformaciones anatómicas heredadas en la estructura de la base del cráneo que facilitarían la compresión del trigémino por estructuras vasculares; HTA familiar responsable de formar vasos tortuosos que comprimirían el nervio trigémino; o alteraciones genéticas en la codificación de canales de calcio que provocarían su hiperexcitabilidad. Se sugiere una forma de herencia autosómica dominante con fenómeno de anticipación.
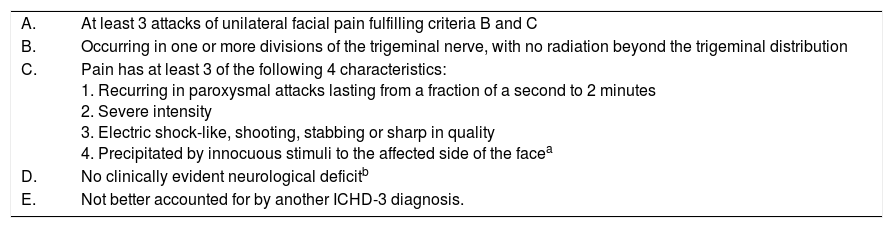
The third edition of the International Classification of Headache Disorders, beta version,1 describes trigeminal neuralgia (TN) as a condition characterised by a very intense, stabbing pain of abrupt onset, lasting seconds or minutes, frequently triggered by exogenous factors and located in one or several branches of the fifth cranial nerve. Table 1 lists the diagnostic criteria. It may also be accompanied by persistent facial pain of moderate intensity, in which case it would be classified as classical trigeminal neuralgia with concomitant persistent facial pain.1
Diagnostic criteria for classical trigeminal neuralgia of the International Classification of Headache Disorders, third edition, beta version.
| A. | At least 3 attacks of unilateral facial pain fulfilling criteria B and C |
| B. | Occurring in one or more divisions of the trigeminal nerve, with no radiation beyond the trigeminal distribution |
| C. | Pain has at least 3 of the following 4 characteristics: 1. Recurring in paroxysmal attacks lasting from a fraction of a second to 2 minutes 2. Severe intensity 3. Electric shock-like, shooting, stabbing or sharp in quality 4. Precipitated by innocuous stimuli to the affected side of the facea |
| D. | No clinically evident neurological deficitb |
| E. | Not better accounted for by another ICHD-3 diagnosis. |
Some attacks may be, or appear to be, spontaneous, but there must be at least 3 that are precipitated in this way to meet this criterion.
Hypoaesthesia or hypoalgesia in the affected trigeminal region always indicates axonal damage. When either is present, there is trigeminal neuropathy and extensive diagnostic work-up is necessary to exclude symptomatic cases. Some patients present hyperalgesia in the painful region, which should not necessarily lead to a diagnosis of trigeminal neuropathy because it may reflect the patient's increased attention to the painful side.
TN usually presents in its classical form, although the symptomatic form, secondary to tumours at the base of the cranium, multiple sclerosis, or neurovascular compression, is not infrequent.2,3 In terms of pain physiopathology, compression of the trigeminal nerve causes focal demyelination of the nerve, which through axonal apposition generates spontaneous impulses with ephaptic conduction to adjacent fibres.4
Despite the limited understanding of familial classic trigeminal neuralgia (FCTN), it may account for 2% of cases; the literature includes reports of an autosomal dominant inheritance pattern.5 Aetiology includes various genetic factors, such as the inheritance of a disorder related to neuronal membrane instability, morphological anomalies of the base of the cranium, or a predisposition to developing premature atherosclerosis.
The analysis of family series is a good model of aetiological research; we present a series of 5 families with FCTN.
MethodsThis is a descriptive study which retrospectively collects familial cases of FCTN. We systematically interviewed patients under follow-up for TN who were attended at our consultation between March 2014 and March 2015 and presented a family history of the disease.
All the identified patients agreed to participate in the study. Information was obtained by reviewing clinical histories, and by telephone interview in the case of a family member not belonging to our hospital's health district (case 4-b).
We retrospectively gathered demographic data and information on age at onset, family relationship, the territory affected, presence of trigger points, neurological examination findings, neuroimaging data, treatment, and clinical outcome. MRI scans were performed at our hospital (with the exception of case 4-b) with a Philips Achieva 1.5T MRI scanner, with Balance and T13D sequences, obtaining thin slices of the posterior fossa.
ResultsAfter interviewing the patients, we identified 5 families with 2 or more members affected by TN.
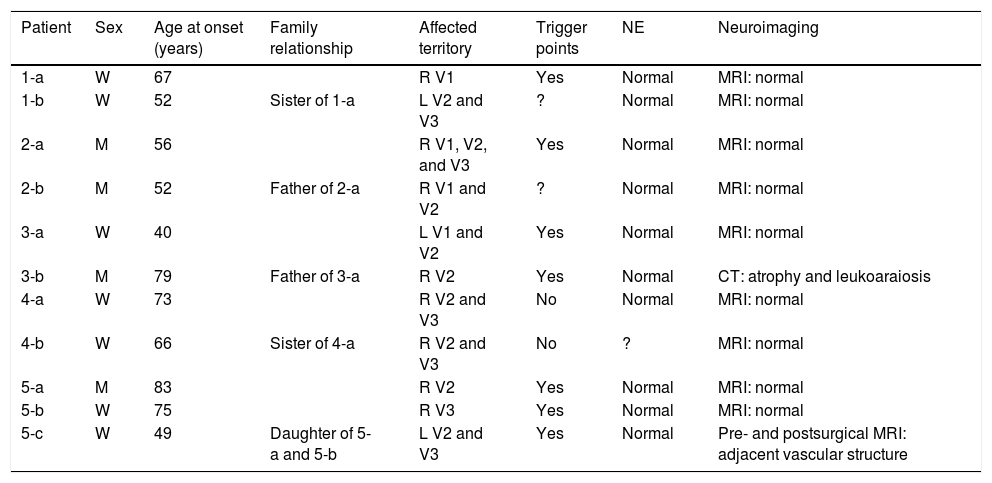
Table 2 includes patients’ demographic data, the clinical characteristics of neuralgia, and clinical and study findings.
Demographic data, characteristics of neuralgia, and examinations.
| Patient | Sex | Age at onset (years) | Family relationship | Affected territory | Trigger points | NE | Neuroimaging |
|---|---|---|---|---|---|---|---|
| 1-a | W | 67 | R V1 | Yes | Normal | MRI: normal | |
| 1-b | W | 52 | Sister of 1-a | L V2 and V3 | ? | Normal | MRI: normal |
| 2-a | M | 56 | R V1, V2, and V3 | Yes | Normal | MRI: normal | |
| 2-b | M | 52 | Father of 2-a | R V1 and V2 | ? | Normal | MRI: normal |
| 3-a | W | 40 | L V1 and V2 | Yes | Normal | MRI: normal | |
| 3-b | M | 79 | Father of 3-a | R V2 | Yes | Normal | CT: atrophy and leukoaraiosis |
| 4-a | W | 73 | R V2 and V3 | No | Normal | MRI: normal | |
| 4-b | W | 66 | Sister of 4-a | R V2 and V3 | No | ? | MRI: normal |
| 5-a | M | 83 | R V2 | Yes | Normal | MRI: normal | |
| 5-b | W | 75 | R V3 | Yes | Normal | MRI: normal | |
| 5-c | W | 49 | Daughter of 5-a and 5-b | L V2 and V3 | Yes | Normal | Pre- and postsurgical MRI: adjacent vascular structure |
CT: computed tomography; L: left; M: man; MRI: magnetic resonance imaging; NE: neurological examination; R: right; V1: first trigeminal branch; V2: second trigeminal branch; V3: third trigeminal branch; W: woman.
Predominantly women were affected by TN in our series, with a woman-to-man ratio of 1.75:1. The mean age at onset was 62.9±13.93 years. The family relationship between patients differed from one family to another, with families 1 and 4 having 2 affected sisters, family 2 a father and son, family 3 a father and daughter, and family 5 the mother, father, and daughter. In families 3 and 5, age at onset was younger in children (40 and 49 years) than in parents (75, 79, and 83 years, respectively).
The area innervated by the second branch of the trigeminal nerve was the most frequently affected; the most frequently affected combination of divisions was V2-V3, representing 36.3% of cases. The right side was most frequently affected, with a ratio of 2.67:1 vs the left side. Most patients presented trigger points. Neurological examination and brain MRI findings were normal, with the exception of case 5-c, in whom contrast administration revealed a small vascular structure running adjacent to the lower part of the trigeminal nerve. Furthermore, patient 4-b reported a fainter, continuous pain around the affected branches, which appears to correspond to classical TN with concomitant persistent facial pain. Relevant data from patients’ personal histories includes arterial hypertension in cases 5-c and 5-b.
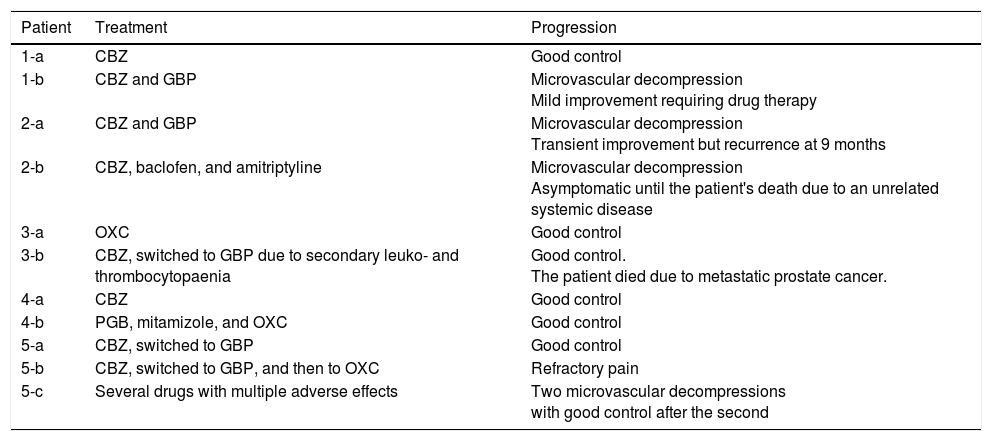
Table 3 includes data on the treatment administered and the progression of pain symptoms.
Treatment and progression.
| Patient | Treatment | Progression |
|---|---|---|
| 1-a | CBZ | Good control |
| 1-b | CBZ and GBP | Microvascular decompression Mild improvement requiring drug therapy |
| 2-a | CBZ and GBP | Microvascular decompression Transient improvement but recurrence at 9 months |
| 2-b | CBZ, baclofen, and amitriptyline | Microvascular decompression Asymptomatic until the patient's death due to an unrelated systemic disease |
| 3-a | OXC | Good control |
| 3-b | CBZ, switched to GBP due to secondary leuko- and thrombocytopaenia | Good control. The patient died due to metastatic prostate cancer. |
| 4-a | CBZ | Good control |
| 4-b | PGB, mitamizole, and OXC | Good control |
| 5-a | CBZ, switched to GBP | Good control |
| 5-b | CBZ, switched to GBP, and then to OXC | Refractory pain |
| 5-c | Several drugs with multiple adverse effects | Two microvascular decompressions with good control after the second |
CBZ: carbamazepine; GBP: gabapentin; OXC: oxcarbazepine; PGB: pregabalin.
All patients were initially treated with monotherapy, with carbamazepine being the most frequently used drug. Cases 1-a, 3-a, 3-b, 4-a, and 5-a achieved satisfactory pain control with monotherapy. Cases 1-b, 2-a, and 4-b required a combination of several drugs to control neuralgia; cases 1-b and 2-a had to receive the combined therapy both before and after surgery. Pain was not controlled pharmacologically in case 2-b, but did resolve with surgery. Case 5-b presented refractory pain; several drugs were tried, with little effect.
Four patients (1-b, 2-a, 2-b, and 5-c) received surgical treatment with microvascular decompression (Janetta procedure). Cases 1-b, 2-b, and 5-c underwent this surgery after the failure of pharmacological treatment, but case 2-a expressed a desire to undergo surgery. In cases 1-b, 2-a, and 2-b, the indication for surgery may be questioned, since not enough drugs were tried before surgery; however, as this is a retrospective series, it is difficult to question the criteria assessed at that time. Surgery was successful in case 2-b, and after a second procedure in case 5-c. Cases 1-b and 2-a showed an initial postsurgical improvement but subsequently relapsed, requiring drug therapy on a continuous basis.
DiscussionWe present 5 families with FCTN, gathered during a period of one year and attended at a general neurology consultation. This is probably one of the largest series of families with classical TN published to date. The family association of TN is poorly understood and no epidemiological data are available on its frequency. In his series, Harris5 found 30 familial cases (some secondary to multiple sclerosis, rather than classical forms) in a total of 1433 patients with TN, amounting to approximately 2% of patients with TN. Therefore, it would seem that FCTN is scarcely detected, probably because history is not analysed sufficiently.
Both in sporadic cases4 and in cases from other family series,5–9 TN has predominantly been observed in women, with an estimated ratio of 1.6:1,7 which is consistent with the results obtained in our series. In contrast, the age at onset described in this series is greater than 44.4 years, the age reported in other studies.10 Also, as in other family series,8,9 neuralgia was predominantly located on the right side of the face. The most frequently affected combination of nerve branches was V2-V3 (36.3%); this is similar to the rate described for sporadic forms (32%) and higher than the 27% reported for FCTN in the review by Fleetwood et al.10
The analysis of family relationships between patients shows an autosomal dominant inheritance pattern, as observed in most family series.6,7,10–12 However, we cannot rule out a possible recessive inheritance pattern in the case of family 5, in which both parents and a daughter were affected. Also noteworthy is the anticipation phenomenon observed in families 3 and 5, which is especially interesting in family 5 as both parents present TN. Harris13 and other authors14,15 also describe this anticipation phenomenon among patients with FCTN. This may be an actual biological mechanism or a bias resulting from earlier diagnosis, since the affected descendants seek medical help earlier.
Savica et al.16 proposed an inherited mutation affecting the calcium channels as a possible aetiology. Furthermore, anomalies of genetic origin at the base of the cranium have been described as a factor possibly favouring neurovascular compression.5,17 Kirkpatrick9 proposed the possibility of an inherited predisposition to developing premature atherosclerosis in the vessels of the posterior fossa, resulting in ectasia and subsequent compression of the trigeminal nerve. The high variability in the distribution of vessels rules out the hypothetical inheritance of a concrete anatomical pattern compressing the nerve as the cause of familial cases. Smyth et al.14 ruled out a direct association between FCTN of vascular aetiology and autosomal dominant inheritance of conditions involving vascular malformation or dysplasia. Familial arterial hypertension is another possible cause of the formation of tortuous vessels as the aetiology of FCTN18; in fact, this possible association was observed in family 5 of our series.
Whatever the cause of trigeminal compression, recurrence following transient improvement after decompression suggests that the vascular origin is only one of the factors involved. This pattern of recurrence after surgery was observed in cases 1-b, 2-a, and 5-c following the first decompression.
ConclusionWith this family series, we aim to raise awareness of the existence of familial TN, despite having first been described by Patrick19 in 1914. The most probable inheritance pattern seems to be autosomal dominant, with a genetic anticipation phenomenon. Compressive causes have been proposed, such as inherited anatomical conformation at the base of the cranium (which would favour vascular compression of the trigeminal nerve) or familial arterial hypertension resulting in tortuous vessels compressing the nerve. Another hypothesis is the presence of alterations in calcium channel–coding genes, which would cause hyperexcitability of the trigeminal nerve. The detection and description of familial cases may help typify the pathophysiological mechanisms involved in TN.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández Rodríguez B, Simonet C, Cerdán DM, Morollón N, Guerrero P, Tabernero C, et al. Neuralgia del trigémino clásica familiar. Neurología. 2019;34:229–233.