Rivastigmine, a treatment for mild to moderate Alzheimer disease (AD), is the first cholinesterase inhibitor to be available in the transdermal format. We aim to describe user experience and satisfaction with the rivastigmine patch, as well as any clinical changes perceived in patients.
MethodsObservational, cross-sectional, multicentre study with 239 investigators and 1851 informal caregivers of patients with mild to moderate AD. Patients were treated with transdermal rivastigmine patches for ≥6 months and had previously received high doses of oral rivastigmine.
ResultsMean caregiver age was 59.8±14.4years and 70.9% were women. They spent 10.0±7.1hours per day providing care and 79.8% lived with the patient. Patch instructions were described as easy to follow by 97.1% of the caregivers and 92.1% of them rated patch application as easy or very easy. The most commonly cited disadvantage was adhesion problems (26.8%). Discontinuation of treatment was due to cutaneous reactions in most cases. Overall, 76.5% of the caregivers were satisfied or very satisfied with transdermal treatment and 77.4% considered that its interference with daily activities was minimal or null. The patch was preferred to oral treatment by 94.3% of caregivers. Clinical Global Impression of Change ratings improved according to 61.3% of the caregivers and 53% of the investigators. Few caregivers reported medication forgetfulness.
ConclusionsMost caregivers of patients with mild to moderate AD preferred the transdermal format of rivastigmine to the oral format. Caregivers also reported overall satisfaction, ease of use, and reduced impact on daily activities for transdermal rivastigmine format, in addition to patient improvement compared to their condition under the previous treatment.
Rivastigmina, tratamiento para la enfermedad de Alzheimer (EA) leve-moderada, es el primer inhibidor de la colinesterasa administrable por vía transdérmica. Se describen la experiencia de uso y la satisfacción de los cuidadores informales con los parches de rivastigmina, así como el cambio clínico percibido en los pacientes con su empleo.
MétodosEstudio observacional, transversal y multicéntrico, con 239 investigadores y 1851 cuidadores informales de pacientes con EA leve-moderada, tratados con rivastigmina transdérmica durante ≥6 meses, y que previamente recibían dosis altas de rivastigmina por vía oral.
ResultadosLa edad media de los cuidadores fue 59,8±14,4 años (70,9% mujeres). El 79,8% compartía domicilio con el paciente, dedicando 10,0±7,1h/día a su cuidado. Para la mayoría de los cuidadores las instrucciones del parche fueron comprensibles (97,1%) y la aplicación fácil o muy fácil (92,1%). La principal dificultad mencionada fueron problemas de adhesión (26,8%). En general, los abandonos del tratamiento se produjeron por reacciones cutáneas. El 76,5% de los cuidadores estuvieron satisfechos o muy satisfechos con el parche y el 77,4% consideró que interfería poco o nada en las actividades diarias propias. El 94,3% prefirió la vía transdérmica respecto a la vía oral. La Impresión Clínica Global de Cambio fue mejor en algún grado para el 61,3% de los cuidadores y para el 53% de los investigadores. Se notificaron pocos olvidos de la medicación.
ConclusionesLa mayoría de los cuidadores de pacientes con EA leve-moderada prefirieron la vía transdérmica de rivastigmina a la vía oral, notificando satisfacción global, facilidad de uso e impacto reducido en sus actividades diarias propias con la ruta transdérmica, así como mejora de los pacientes respecto al tratamiento anterior.
Treatment for mild to moderate Alzheimer disease (AD) is based on oral acetylcholinesterase inhibitors (AChEI) such as rivastigmine, donepezil, and galantamine.1 These drugs have been shown to exert a dose-dependent beneficial effect on cognitive function.2,3 However, the incidence of adverse events also increases with the dose—especially when there is no prior dose adjustment period—and with the peak plasma concentrations resulting from oral administration.4 The first transdermal treatment for AD has now been approved: a rivastigmine patch5 that continuously releases the drug over the 24hours after application.6 Dosed at 9.5mg/day, this patch has an effect equivalent to that of higher doses of oral rivastigmine, and it is associated with fewer adverse gastrointestinal effects.7 It has also been shown that patients treated with high doses of oral rivastigmine may change directly to the transdermal patch dosed at 9.5ml/day with no need for a prior adjustment period and without experiencing further adverse effects.8
Treatment for patients with AD is generally managed by caregivers.9 As a result, their assessment of treatment efficacy and the workload generated by the treatment may have a decisive effect on efforts to improve treatment compliance and ensure that the patient obtains the greatest benefit from the drugs prescribed.10 Earlier studies have reported a better user experience, higher satisfaction, and less interference in the carer's daily activities with transdermal rivastigmine than with oral rivastigmine.11,12
The purpose of our study was to report on user experience with high doses of transdermal rivastigmine among informal caregivers of patients with mild to moderate AD. Likewise, we gathered data about caregiver satisfaction and preferences regarding the transdermal route, any improvements perceived in patients after the change from oral treatment, and treatment adherence. At the same time, we analysed user experience, satisfaction, and any perceived improvements in patients treated transdermally as reported by healthcare professionals treating patients with AD.
Patients and methodsThis cross-sectional multi-centre observational study was carried out across Spain. During a 6-month period, informal caregivers were recruited consecutively during routine visits to different participating neurology, psychiatry, or geriatric care clinics. Inclusion criteria were as follows: (a) caring for a patient with mild to moderate AD (defined as a score ≥10 and <26 on the Mini-Mental State Exam [MMSE]) who was on stable treatment during ≥6 months with the highest approved dose of transdermal rivastigmine (9.5mg/day), and who previously had received high doses of oral rivastigmine (9–12mg/day); (b) having cared for the patient for at least 1 year; (c) being responsible for managing the patient's medications, and (d) being able to provide accurate information about the study variables. We excluded caregivers of patients with severe AD (MMSE<10) or dementia of other causes, paid caregivers, and those considered by researchers to be unable to participate in the study. The decision to use MMSE rather than other AD assessment scales, and the scoring ranges employed to classify patients as having mild-to-moderate or severe AD, reflect the latest guidelines published by the National Institute for Health and Clinical Experience (NICE) for rivastigmine treatment in AD patients.13
During the same inclusion visit, and after receiving the caregiver's written informed consent statement, researchers proceeded to take down data. The main variable, the caregiver's user experience with transdermal rivastigmine, was measured using an ad hoc questionnaire. As secondary variables, researchers recorded the caregiver's demographic and care arrangement data (including time as patient's caregiver, daily hours dedicated to care activities, number of daily medications, and time spent preparing and administering treatment). The researcher's user experience and the caregiver's and the researcher's satisfaction with treatment were documented using ad hoc questionnaires similar to the one used for the main variable. Overall caregiver satisfaction was evaluated quantitatively and qualitatively; caregivers rated satisfaction between 1 (very satisfied) and 5 (very unsatisfied). Doctor satisfaction, whether overall or satisfaction with patch efficacy and tolerability, was scored from 1 (no satisfaction) to 10 (maximum satisfaction). Caregiver preference for treatment was evaluated using the question “What form of rivastigmine treatment do you prefer?” Caregivers were able to choose between ‘oral’ and ‘transdermal’. Improvements in the patient, whether perceived by caregivers or doctors, were evaluated using the Clinical Global Impressions-Change scale (CGI-C).14 This questionnaire consists of a single question comparing the patient's general states of health under the current and the previous treatments. There are 7 possible responses ranging from ‘much better’ to ‘much worse’. Treatment adherence was evaluated using a Morisky adherence scale.15 This questionnaire includes 2 questions to measure forgetfulness and commitment to providing care, and 2 others to determine whether treatment was interrupted at any time and if the respondent understands the long-term benefits of the medication. A single suboptimal answer served to classify the respondent as showing poor adherence. Since this questionnaire was originally designed for use by patients, we adapted it for use by caregivers and to specifically address transdermal rivastigmine treatment. We also added a question to determine the frequency with which caregivers forgot to administer treatment. Researchers followed the Declaration of Helsinki and Good Clinical Practice guidelines at all times. The study was approved by the Clinical Research Ethics Committee at Hospital Universitari de Bellvitge (L’Hospitalet de Llobregat, Barcelona).
Statistical analysis was completed using SAS statistics software for Windows, version 9.2. The analysis included all carers who met selection criteria and for whom there were valid data for the main variable. Substitution methods were not employed for missing data. Discrete qualitative and quantitative variables were described as absolute frequencies (n) and relative frequencies (percentages). Continuous quantitative variables were described as the mean±standard deviation (SD). The chi-square test was used to assess differences in the CGI-C scale scores given by informal caregivers and doctors.
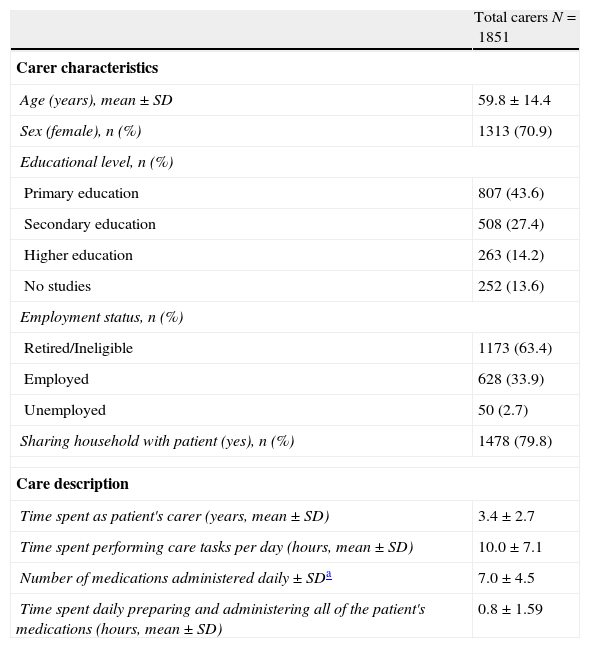
ResultsPopulation characteristicsThe study recruited a total of 1975 informal caregivers. Of these caregivers, 89 were excluded due to not meeting selection criteria, and 35 were excluded because they lacked experience with transdermal rivastigmine. In the end, 1851 caregivers and 239 doctors from 198 centres were included. Table 1 summarises the characteristics of both the caregivers and the care they provide.
Sociodemographic data from informal caregivers in the study; description of the care they provide to their patients.
| Total carers N=1851 | |
| Carer characteristics | |
| Age (years), mean±SD | 59.8±14.4 |
| Sex (female), n (%) | 1313 (70.9) |
| Educational level, n (%) | |
| Primary education | 807 (43.6) |
| Secondary education | 508 (27.4) |
| Higher education | 263 (14.2) |
| No studies | 252 (13.6) |
| Employment status, n (%) | |
| Retired/Ineligible | 1173 (63.4) |
| Employed | 628 (33.9) |
| Unemployed | 50 (2.7) |
| Sharing household with patient (yes), n (%) | 1478 (79.8) |
| Care description | |
| Time spent as patient's carer (years, mean±SD) | 3.4±2.7 |
| Time spent performing care tasks per day (hours, mean±SD) | 10.0±7.1 |
| Number of medications administered daily±SDa | 7.0±4.5 |
| Time spent daily preparing and administering all of the patient's medications (hours, mean±SD) | 0.8±1.59 |
SD: standard deviation.
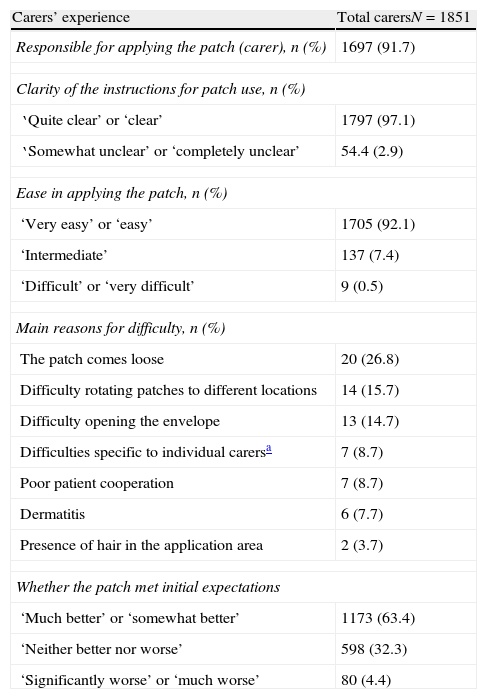
Table 2 displays the most important findings regarding caregivers’ user experience for transdermal rivastigmine. The most common areas for patch application were the chest, arms, and dorsal-lumbar region (data not shown). Most caregivers (94.7%) reported changing patches daily.
Carers’ experience with using high-dose transdermal rivastigmine as treatment for Alzheimer disease.
| Carers’ experience | Total carersN=1851 |
| Responsible for applying the patch (carer), n (%) | 1697 (91.7) |
| Clarity of the instructions for patch use, n (%) | |
| ‘Quite clear’ or ‘clear’ | 1797 (97.1) |
| ‘Somewhat unclear’ or ‘completely unclear’ | 54.4 (2.9) |
| Ease in applying the patch, n (%) | |
| ‘Very easy’ or ‘easy’ | 1705 (92.1) |
| ‘Intermediate’ | 137 (7.4) |
| ‘Difficult’ or ‘very difficult’ | 9 (0.5) |
| Main reasons for difficulty, n (%) | |
| The patch comes loose | 20 (26.8) |
| Difficulty rotating patches to different locations | 14 (15.7) |
| Difficulty opening the envelope | 13 (14.7) |
| Difficulties specific to individual carersa | 7 (8.7) |
| Poor patient cooperation | 7 (8.7) |
| Dermatitis | 6 (7.7) |
| Presence of hair in the application area | 2 (3.7) |
| Whether the patch met initial expectations | |
| ‘Much better’ or ‘somewhat better’ | 1173 (63.4) |
| ‘Neither better nor worse’ | 598 (32.3) |
| ‘Significantly worse’ or ‘much worse’ | 80 (4.4) |
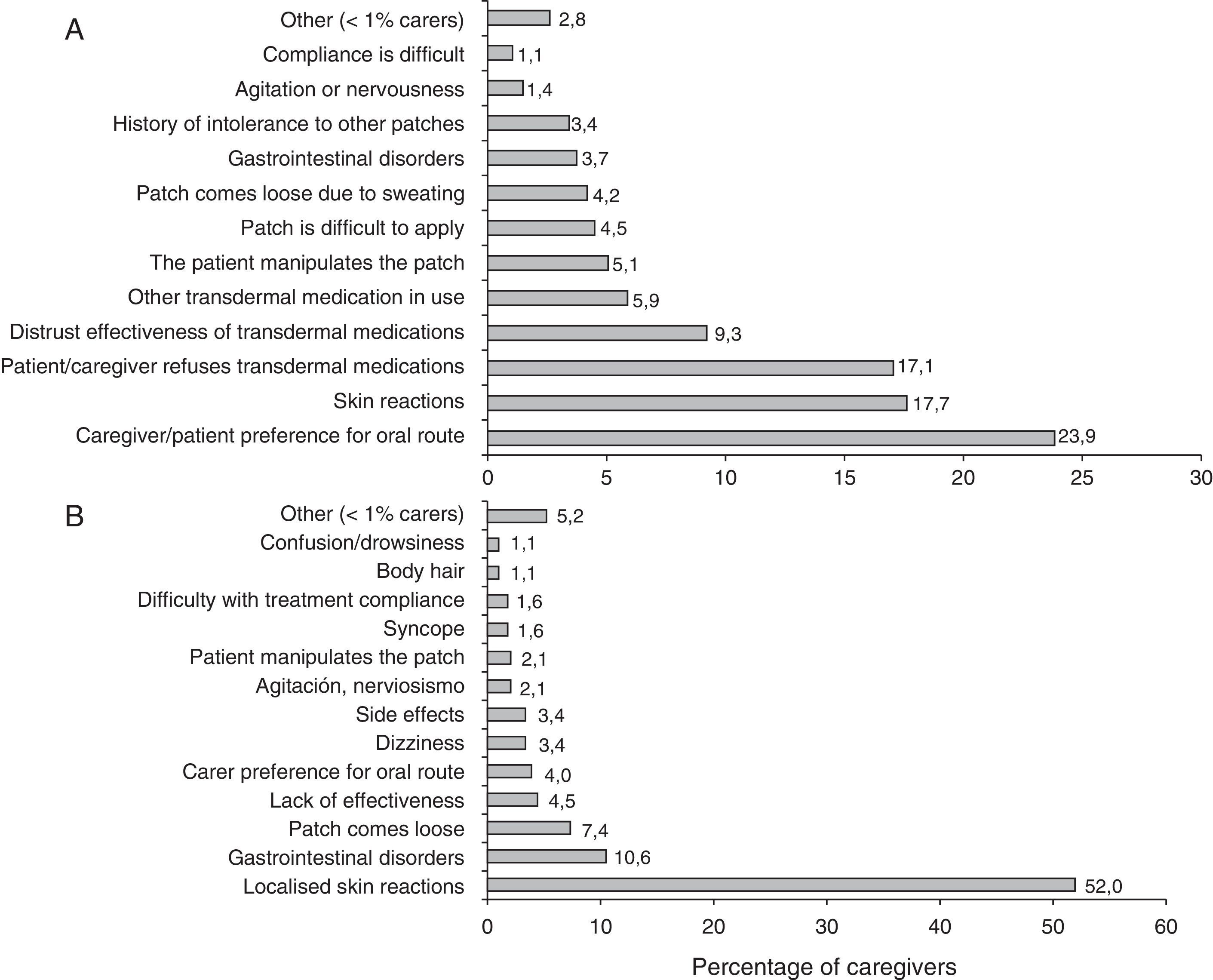
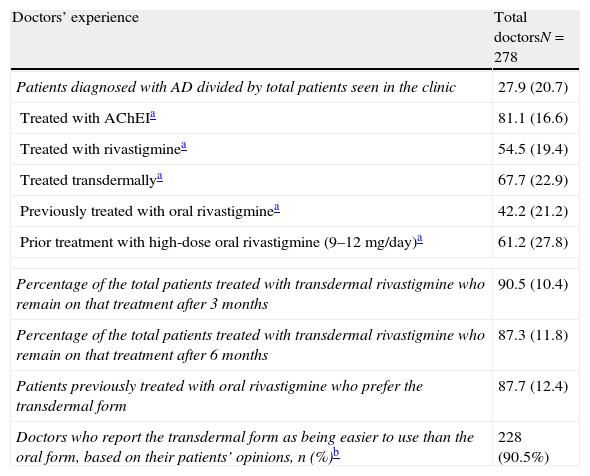
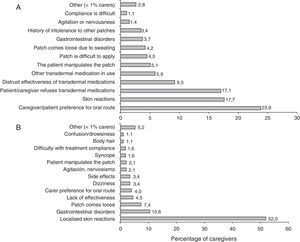
Researchers’ experience is summarised in Table 3. More than half of the patients treated with rivastigmine received it in transdermal form. Most patients in this subgroup remained on the same treatment 6 months after having started it. The majority of the researchers recorded that patients and caregivers preferred transdermal treatment and found it easier to use than the oral form. The main reasons cited by patients for leaving or not beginning transdermal treatment are summarised in Fig. 1.
Doctors’ experience with high-dose transdermal rivastigmine as treatment for Alzheimer disease based on results in their clinics.
| Doctors’ experience | Total doctorsN=278 |
| Patients diagnosed with AD divided by total patients seen in the clinic | 27.9 (20.7) |
| Treated with AChEIa | 81.1 (16.6) |
| Treated with rivastigminea | 54.5 (19.4) |
| Treated transdermallya | 67.7 (22.9) |
| Previously treated with oral rivastigminea | 42.2 (21.2) |
| Prior treatment with high-dose oral rivastigmine (9–12mg/day)a | 61.2 (27.8) |
| Percentage of the total patients treated with transdermal rivastigmine who remain on that treatment after 3 months | 90.5 (10.4) |
| Percentage of the total patients treated with transdermal rivastigmine who remain on that treatment after 6 months | 87.3 (11.8) |
| Patients previously treated with oral rivastigmine who prefer the transdermal form | 87.7 (12.4) |
| Doctors who report the transdermal form as being easier to use than the oral form, based on their patients’ opinions, n (%)b | 228 (90.5%) |
All items show the mean percentage (standard deviation) except where otherwise stated.
AD: Alzheimer disease; AChEI: acetylcholinesterase inhibitors.
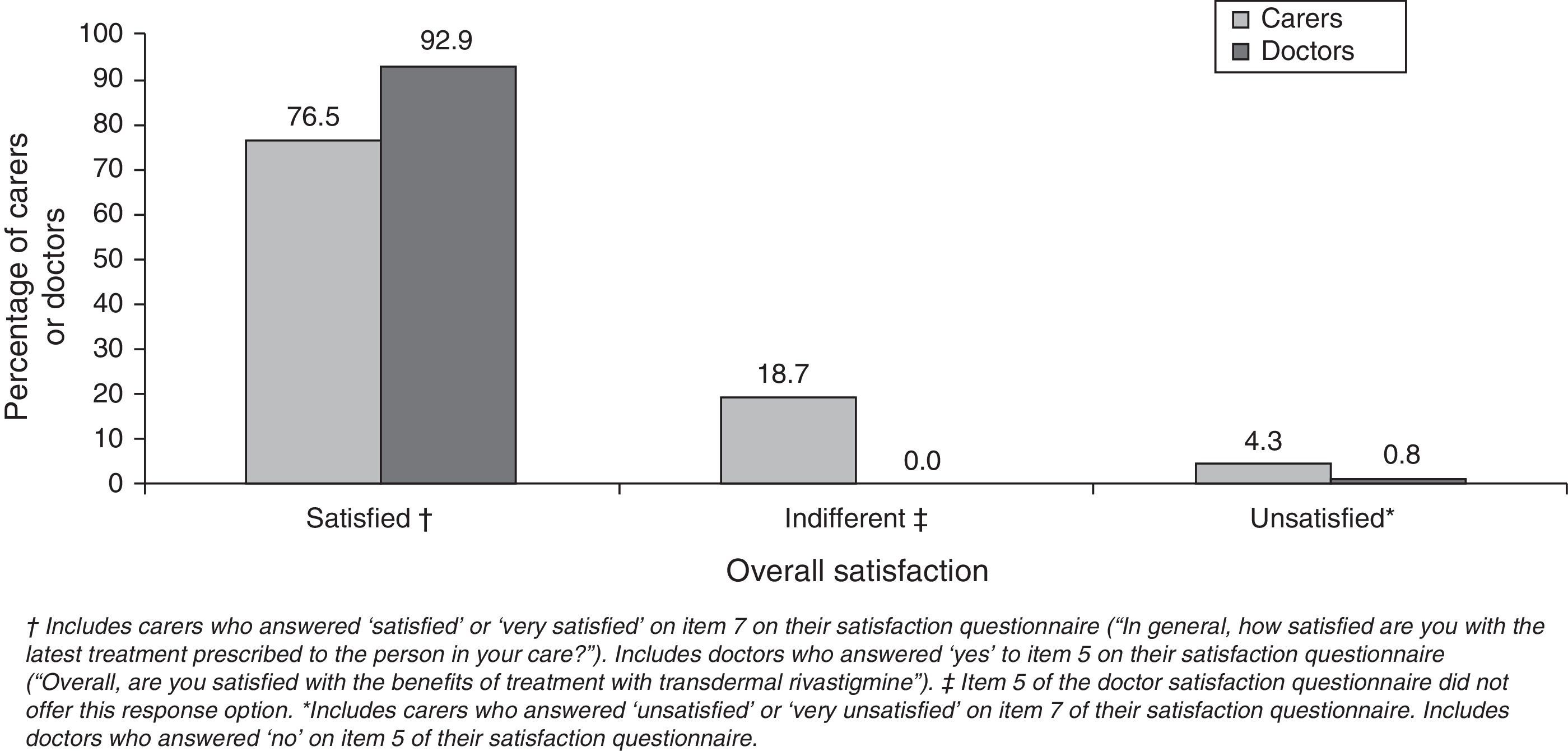
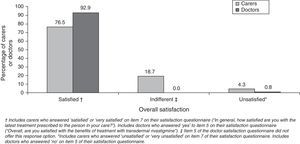
Most caregivers and doctors expressed overall satisfaction with transdermal treatment (Fig. 2). Caregivers scored their satisfaction level at 2.6±0.4 points. Most caregivers rated using the patch as easy or very easy (92.8%) and assigned a similar rating to following the treatment schedule (94%). However, only 65% rated the patch as easy for patients to apply by themselves. Most caregivers indicated that administering transdermal treatment interfered little or not at all in their daily routine (77.4%) or the patient's daily routine (78.6%). Assessment of caregiver preference for rivastigmine routes of delivery showed that this group clearly favoured the transdermal form (94.3%). Among doctors, 90% had medium-to-high hopes for the rivastigmine patch before beginning to use it; approximately 92% declared that the product delivered the results they had expected. Almost all of the professionals (90.9%) rated the treatment as easy based on the opinions expressed by their patients. Clinical efficacy earned a mean score of 7.4±1.33 points. Cutaneous tolerability had a mean score of 7.0±1.48 points; gastrointestinal, 8.8±0.96; and overall tolerability, 8.1±0.93.
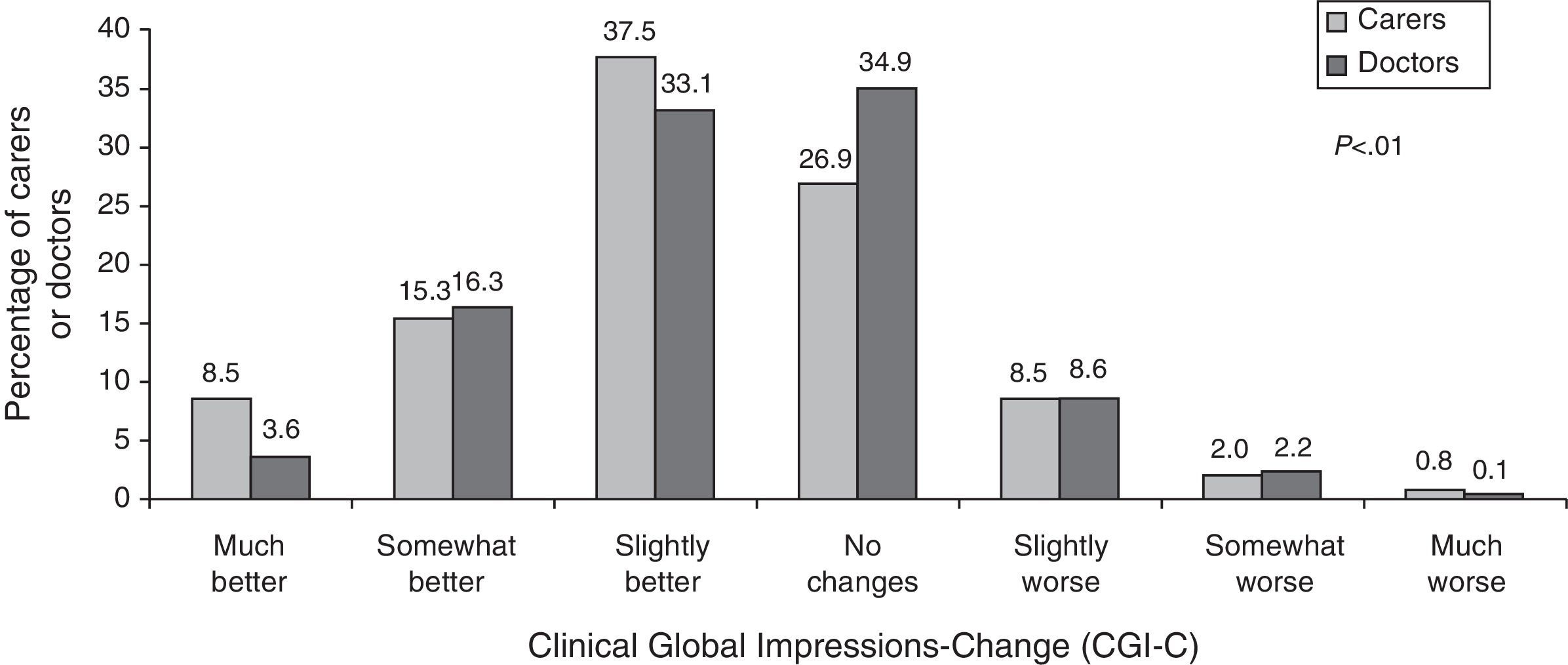
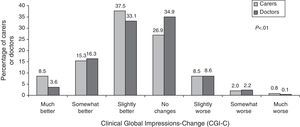
CGI-C scaleCaregivers’ and doctors’ opinions on the patient's state of health under the new treatment compared to the previous treatment are shown in Fig. 3. In general, both groups considered that patients had remained stable or shown improvement, although informal caregivers perceived significantly greater degrees of improvement than doctors did.
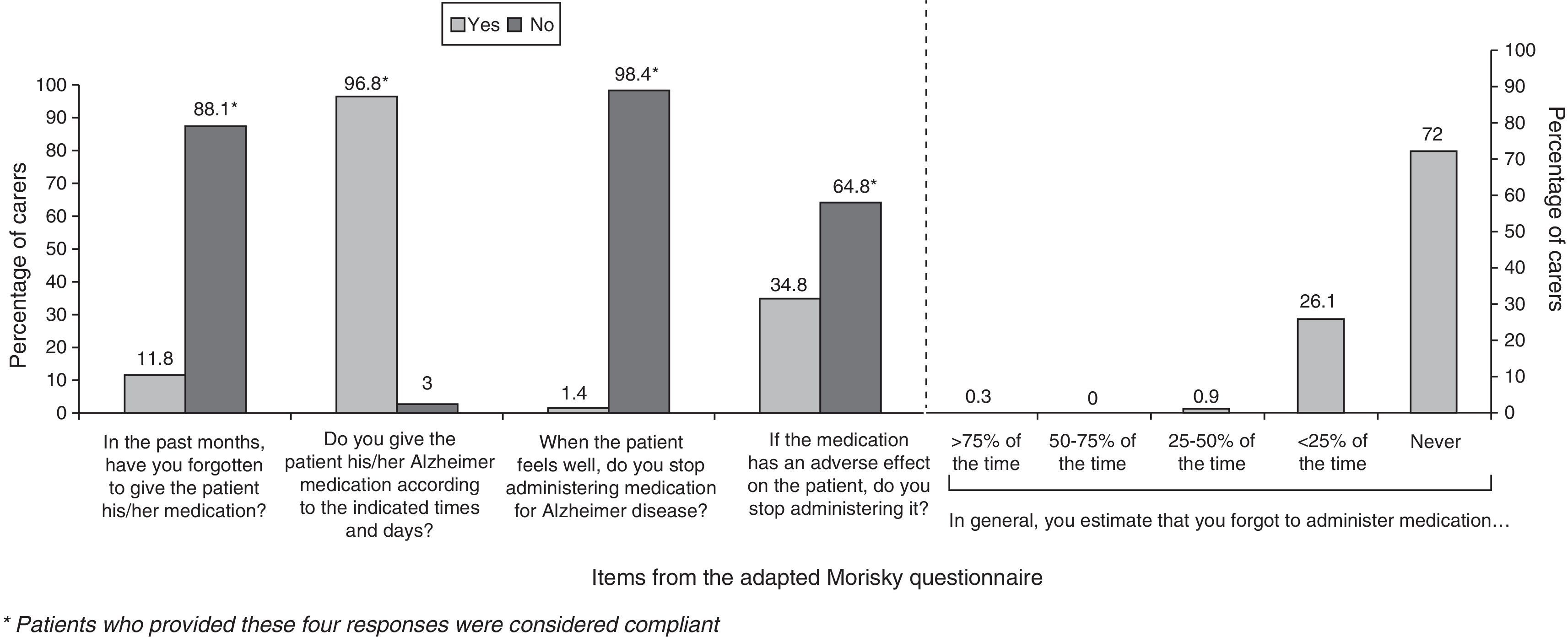
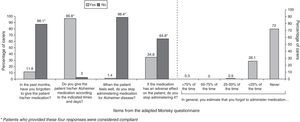
ComplianceFifty-seven per cent of the caregivers were categorised as compliant. The factor with the greatest impact on treatment compliance was the tendency among caregivers to stop administering the drug if patients experienced adverse events. The forgetfulness rate was low when patients were treated with rivastigmine patches. Results from the adherence questionnaire are shown in Fig. 4.
DiscussionThe characteristics of the caregivers assessed in this study coincide with those found by another recent study in our region that examined carers of patients treated with AChEI.16 Most were women about 60 years of age, with a primary education and actively employed. Roughly 80% shared a household with their patients and spent a mean of 10hours daily caring for them.
Generally speaking, caregivers expressed satisfaction with transdermal treatment and considered that instructions were easy to follow and patches were easy to apply. Concurring with these results, a recent randomised study12 found that 93% of patients (or their caregivers) who had been receiving high doses of oral rivastigmine and who switched directly to high transdermal doses found the patches easy or very easy to use; 95.4% found the treatment schedule easy or very easy to follow. In our case, ease of use as perceived by caregivers was also consistent with ease of use reported by doctors.
Most of the caregivers expressed satisfaction with transdermal treatment, as was the case in the randomised study mentioned above.12 In that study, 90.7% of patients/caregivers who switched from high-dose oral rivastigmine to a transdermal form were satisfied with the patches; in contrast, 79.1% of patients who continued with oral treatment were satisfied (P=.0001). In our study, doctors expressed a higher degree of overall satisfaction than carers (92.9% vs. 76.5%; P<.0001). This discrepancy may be explained by a number of factors. On the one hand, the question used to evaluate overall satisfaction differed between these groups, and each had different possible responses. On the other hand, the factors that determine satisfaction with treatment probably differ between caregivers and health professionals. For caregivers, satisfaction tends to depend on whether the treatment is user friendly and how it affects activities of daily life.7,11,17 It is quite unlikely that a health professional would rate these considerations as more important than treatment efficacy or safety on a satisfaction questionnaire. Caregivers were not optimistic that their patients would be able to self-administer their patches but we should point out that if AD patients were able to do so, it would decrease the burden on their caregivers. This could be a decisive factor for satisfaction in this group, considering that a large percentage of the carers in this study were actively employed.
The study showed that CGI-C scores reported by both doctors and informal caregivers of AD patients are similar. When these scores are compared to objective measuring instruments, however, we see that caregivers tend to overestimate neurological decline in their patients.18 In this study, most caregivers reported improvement among patients after switching from oral to transdermal rivastigmine, and they were significantly more likely to do so than doctors (61.4% vs. 53.1%; P<.0001). Nevertheless, no objective measurements of patient decline were performed that would enable us to assess these perceptions, nor did we evaluate the correlation between their opinion and the doctors’ opinion. In contrast, some of the caregivers did not perceive any differences in the status of the patient with respect to the previous treatment. Caregivers are not always conscious of the fact that efficacy of an AD treatment often manifests as stabilisation of the patient's state.19 This lack of improvement perceived by some caregivers in our study may affect their level of satisfaction with the patch. It suggests that clear communication between doctor and caregiver is needed in order to create realistic expectations for the treatment.
Patients treated with oral AChEI generally display low persistence. A retrospective20 study estimated that only 30.8% of patients remained in treatment with AChEI 6 months after having started; use of low doses was cited as a risk factor for stopping treatment. In our case, persistence with transdermal rivastigmine reported at the 6-month mark was 87.3%. This considerably higher figure may be partially due to the fact that all patients received high doses of rivastigmine. Nevertheless, the inherent advantages of the transdermal route are also likely to have had a strong impact.
The most frequent reason for stopping treatment was local skin reaction. Although these are the most frequent reactions caused by treatment with transdermal drugs, they tend to be mild to moderate in the case of rivastigmine patches.21,22 Cutaneous reactions can be lessened by changing patches daily, and almost all caregivers in the study complied with this guideline. In addition, simple patient care routines, such as removing the patch carefully and using emollients and topical corticosteroids, decrease the impact of skin reactions.23 In addition, there were few cases of stopping transdermal treatment due to gastrointestinal disorders or other adverse effects, which confirms the good tolerability of this route.
Despite the high percentage of treatment permanence, we observed only moderate treatment compliance (57%). We did not record compliance for the patients’ previous period of oral treatment. Based on published evidence, however, it would be reasonable to think that compliance with transdermal treatment would be higher, or at least that causes of poor compliance would be more easily avoidable than in oral treatment. In an earlier prospective observational study in patients with poor adherence to oral treatment,24 researchers found that treatment compliance increased as patients switched from oral to transdermal treatment. When the percentage of patch users was at 64.8%, treatment compliance had reached 73.6%.24 This figure is higher than that obtained by our study. In part, the difference could be due to the way compliance was assessed. In the prospective study, patients were considered compliant if they forgot <20% of their doses and took their medication as prescribed >80% of the time.
Researchers have observed that patients taking tablets were more likely to forget their dose than patients using patches.24 This study shows a low rate of forgotten doses; in fact, most of the caregivers classified as non-compliant made a conscious decision not to adhere to treatment. More than a third temporarily suspended the patches when patients experienced adverse events. The same cause of treatment non-compliance has been reported among patients taking oral medications for chronic illnesses that resemble AD in that treatment benefits are not immediately apparent.25 This may indicate that caregivers for patients with AD will require additional training about the long-term benefits of treatment so that patients’ experience with the transdermal route will yield optimal results.
In conclusion, most informal caregivers preferred transdermal rivastigmine to oral rivastigmine. Caregivers reported few incidents of forgetting doses and they indicated that the patch was easy to use and apply with little impact on the carer's daily routine. Most caregivers expressed satisfaction with transdermal treatment, and like the doctors, they perceived overall improvement in patients who had switched from the previous treatment. Studies report positive user experience results, acceptance of the transdermal form, and acceptable tolerability of the rivastigmine patch at high doses (9.5mg/day) in patients with AD who had previously been treated with high oral doses (9–12mg/day). One implication of these data is that it may be beneficial to market rivastigmine patches with higher doses (13.3mg/day), as has been confirmed by more recent randomised trials.26
FundingThis study received financial support from Novartis Farmacéutica, S.A.
Conflicts of interestJavier Ricart and Basilio Hernández are employed by Novartis Farmacéutica, SA. Ramón Reñé has no conflicts of interest to declare with regard to this study.
Please cite this article as: Reñé R, Ricart J, Hernández B, en representación de los investigadores del Estudio Experience. Experiencia de uso y satisfacción con rivastigmina transdérmica en cuidadores de pacientes con enfermedad de Alzheimer de leve a moderada previamente tratados con rivastigmina oral a dosis altas. Neurología. 2014;29:86–93.