Thrombotic thrombocytopaenic purpura (TTP) is an infrequent haematological disease characterised by thrombocytopaenia and haemolytic anaemia. Neurological complications are relatively frequent during the development of the disease, and manifest after onset of clinical and analytical haematological symptoms.1 We present the case of a woman with TTP whose neurological complications (recurrent ischaemic strokes) preceded a new episode of TTP activity.
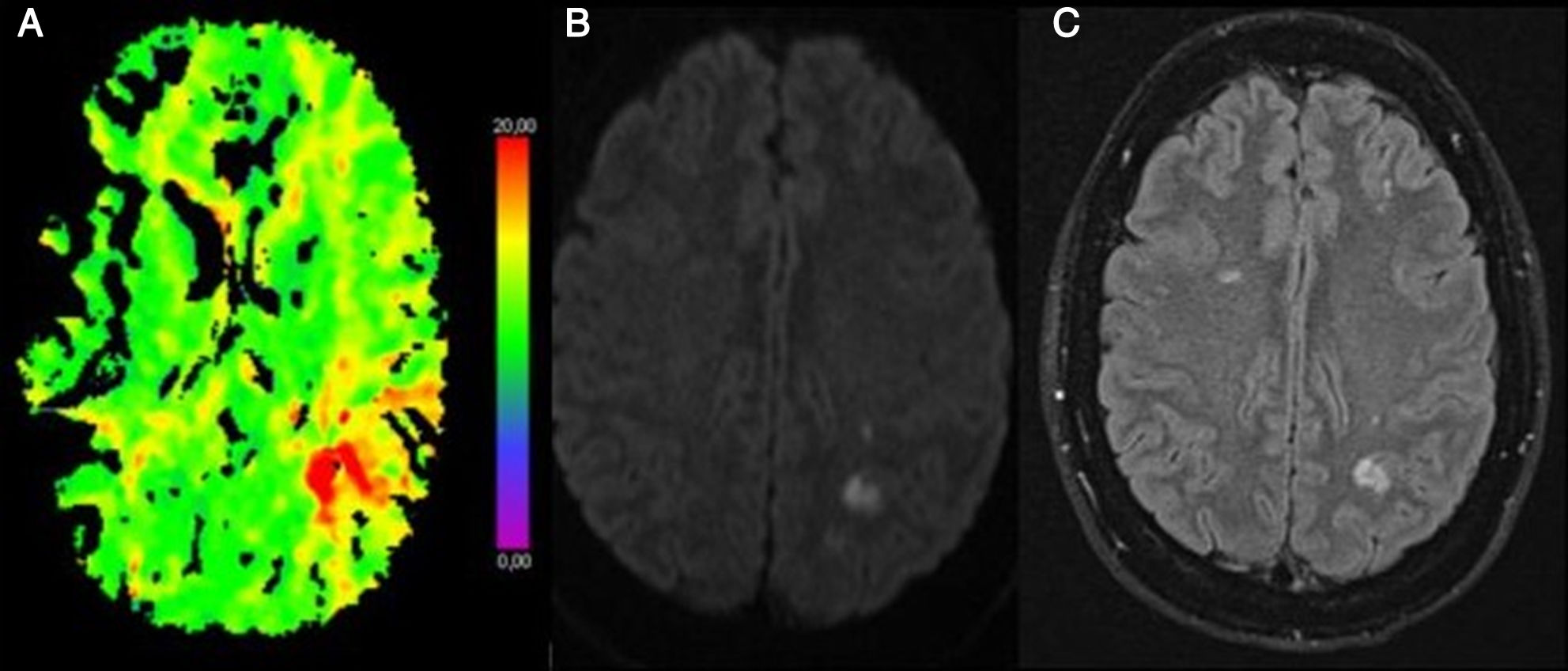
Our patient is a 36-year-old woman diagnosed with TTP in 1998 after presenting multiple haematomas in the context of microangiopathic haemolytic anaemia and thrombocytopaenia. Due to acute-onset dysarthria and weakness in the right limbs, she was admitted to the neurology department with suspected acute ischaemic stroke. The neuroimaging study revealed focal hypoperfusion in the left parietal region, with no filling of the distal M2 segment of the left middle cerebral artery. A follow-up MRI scan revealed multiple acute punctiform ischaemic lesions in the left temporo-parietal region (Fig. 1). The aetiological study revealed no blood alterations (Table 1) or paroxysmal disorders of heart rate (normal Holter ECG and telemetry findings), and a transoesophageal echocardiography ruled out the presence of cardioembolic sources. We diagnosed ischaemic stroke of undetermined cause and recommended antiplatelet treatment (acetylsalicylic acid) at discharge. Fifteen days after the first admission, the patient presented dysaesthesia in the face and right hand. In this instance, neuroimaging studies (CT and MRI) did not reveal acute alterations. The laboratory analysis only revealed the presence of isolated schistocytes (low schistocyte levels might be considered normal in healthy individuals, not suggesting disease), with no other pathological findings (Table 1). Seven days after the second event, she presented symptoms of asthenia with haematomas in the lower limbs. A new analysis revealed thrombocytopaenia and haemolytic anaemia. Matrix metalloproteinase ADAMTS13 activity was 0% (normal activity, 6%-100%), and the anti-ADAMTS13 IgG antibody titre was 80IU/mL (Table 1). After diagnosis of a TTP episode, treatment was started with plasma exchange, corticosteroids, and rituximab, with the patient showing a progressive improvement in both clinical and analytical parameters.
(A) CT perfusion sequence. Time to peak: delay in the posterior territory of the left middle cerebral artery. (B) MRI diffusion-weighted sequence. Cortical and subcortical diffusion restriction in the left parietal region. (C) MRI FLAIR sequence. Left parietal cortico-subcortical lesion (shown in the diffusion-weighted sequences) and bilateral frontal lesions with longer progression times.
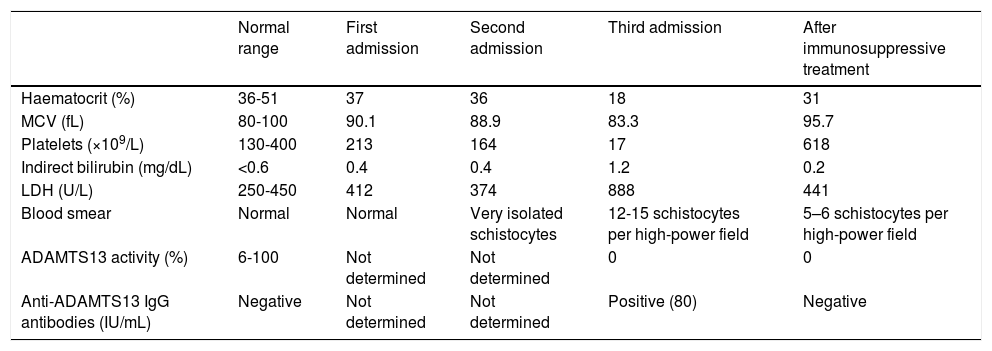
Laboratory findings obtained during the different hospital admissions and after immunosuppressive treatment.
| Normal range | First admission | Second admission | Third admission | After immunosuppressive treatment | |
|---|---|---|---|---|---|
| Haematocrit (%) | 36-51 | 37 | 36 | 18 | 31 |
| MCV (fL) | 80-100 | 90.1 | 88.9 | 83.3 | 95.7 |
| Platelets (×109/L) | 130-400 | 213 | 164 | 17 | 618 |
| Indirect bilirubin (mg/dL) | <0.6 | 0.4 | 0.4 | 1.2 | 0.2 |
| LDH (U/L) | 250-450 | 412 | 374 | 888 | 441 |
| Blood smear | Normal | Normal | Very isolated schistocytes | 12-15 schistocytes per high-power field | 5–6 schistocytes per high-power field |
| ADAMTS13 activity (%) | 6-100 | Not determined | Not determined | 0 | 0 |
| Anti-ADAMTS13 IgG antibodies (IU/mL) | Negative | Not determined | Not determined | Positive (80) | Negative |
LDH: lactate dehydrogenase; MCV: mean corpuscular volume.
TTP is a rare haematological disease whose neurological manifestations occur during the development of the disease (including stroke and transient ischaemic attacks).1 We present a patient whose cerebral ischaemic events preceded the haematological changes typical of active TTP, which is extremely infrequent.2–6 Thrombotic complications of TTP are caused by an immune-mediated phenomenon which causes the inactivation of the ADAMTS13 metalloprotease enzyme, responsible for degrading high-molecular-weight Von Willenbrand factor multimers. Accumulation of those multimers predisposes to platelet aggregation, causing thrombosis at the microvascular level.7,8 Likewise, we cannot rule out that the cerebral ischaemic event triggered the TTP episode, since the literature includes reports of ischaemic stroke-induced coagulation alterations resembling the changes reported in patients with no history of TTP (decreased ADAMTS13 activity in the acute phase).9 Regarding the antithrombotic management of these complications, it should be noted that clopidogrel may act as hapten for anti-ADAMTS13 IgG antibodies in patients with a baseline autoimmune mechanism; therefore, there must be clear justification for its use for preventing new ischaemic events in patients with a history of TTP.10
In conclusion, we should consider TTP as an infrequent cause of stroke in young or middle-aged women, even in the absence of the haematological and analytical changes typical of the disease. In the follow-up of patients already diagnosed with TTP, the appearance of focal neurological signs without abnormal laboratory results at the time of diagnosis should prompt physicians to begin periodic monitoring of platelet and erythrocyte levels. Finally, determining ADAMTS13 activity and anti-ADAMTS13 antibody titres may be useful for early diagnosis.
Please cite this article as: Vicente-Pascual M, Zamora-Martinez C, Amaro-Delgado S. Ictus isquémico como evento predecesor de brote de púrpura trombótica trombocitopénica. Neurología. 2019;34:609–611.
This study was submitted in poster format for presentation at the 68th Annual Meeting of the Spanish Society of Neurology and at the 5th Stroke Competition of the Spanish Society of Neurology.