The increasing incidence of trigeminal neuralgia (TN) with age together with population ageing call for reexamination of surgical treatment options for refractory TN in elderly patients.
MethodsRetrospective review of a consecutive series of patients older than 70 who underwent microvascular decompression (MVD) for refractory TN between 1997 and 2015. Outcomes based on the Barrow Neurological Institute pain intensity score (BNI score) and surgical complications were compared to those of patients younger than 70 undergoing MVD in the same period.
ResultsForty patients older than 70 (mean=74.8 years) underwent interventions. At a mean follow-up time of 34 months, 73% of the patients presented complete absence of pain without medication (BNI I) and 85% had good pain control with or without medication (BNI I-III). A comparison of these patients with the 85 patients younger than 70 treated surgically during the same period did not find a significant association between age and achievement of pain control (BNI I-II). However, there was a significant association between age older than 70 and complete pain relief (BNI I; P=.03). The mean hospital stay in patients over 70 was also significantly longer (P=.04), although the postsurgical complication rate was similar to that in younger patients.
ConclusionsElderly patients with refractory TN may benefit from treatment with MVD and the probability of success and surgical risk are comparable to those in younger patients.
El incremento de la incidencia de la neuralgia del trigémino (NT) con la edad junto con el creciente envejecimiento poblacional obligan a valorar las opciones de tratamiento quirúrgico de la NT refractaria en pacientes mayores.
MétodosSe revisó retrospectivamente una serie consecutiva de pacientes mayores de 70 años con NT refractaria tratados mediante descompresión microvascular (DMV) entre 1997 y 2015. Los resultados según la escala de dolor facial del Barrow Neurological Institute (BNI score), así como las complicaciones quirúrgicas, se compararon con los de pacientes menores de 70 años operados durante el mismo período.
ResultadosFueron intervenidos 40 pacientes mayores de 70 años (media 74,8 años). A los 34 meses de seguimiento medio, el 73% de los pacientes presentaba ausencia completa del dolor sin medicación (BNI I) y el 85% tenía un control del dolor sin o con medicación (BNI I-III). Comparando con 85 pacientes menores de 70 años intervenidos en el mismo período no se demostró una asociación significativa entre la edad y la obtención de un control del dolor (BNI I-III), pero sí entre la edad mayor de 70 años y la desaparición del dolor (BNI I; P = 0,03). La estancia media en mayores de 70 años fue significativamente mayor (P = 0,04), aunque la tasa de complicaciones posquirúrgicas fue similar a la de los pacientes más jóvenes.
ConclusionesLas personas de edad avanzada con NT refractaria pueden beneficiarse de un tratamiento mediante DMV con una probabilidad de éxito y unos riesgos equiparables a los de personas más jóvenes.
Trigeminal neuralgia (TN) is a unilateral disorder characterised by paroxysmal, electric shock-like pain of abrupt termination limited to the territory of one or more branches of the trigeminal nerve.1 It is a rare condition, with an estimated annual incidence of 4.3-4.7 cases per 100000 population. It typically appears in adults older than 50. Its incidence has been found to increase with age up to rates of 17.5 and 25.6 cases per 100000 population in patients older than 60 and 70 years, respectively.2–4
Microvascular decompression (MVD) is the only surgical treatment targeting the aetiology of TN; its immediate and long-term effectiveness makes it the treatment of choice for patients with refractory pain.5–9 However, this procedure has been ruled out for elderly patients due to the risk of developing such complications associated with microsurgical treatment of the posterior fossa as cerebellar haemorrhage, cranial nerve damage, or ischaemic events. Age, combined with presence of diseases increasing the anaesthetic risk, has been considered one of the main contraindications of MVD for classical TN. In general terms, the maximum age for indicating MVD is 70.10 Several authors recommend ablation therapy (radiosurgery, percutaneous rhizotomy of the gasserian ganglion) as the first line of treatment in these patients.11–14
We present our experience with a series of consecutive patients aged 70 years and older with refractory classical TN who were treated with MVD and compare outcomes and complications with those experienced by younger patients.
Patients and methodsWe retrospectively gathered data of all patients with classical TN treated with MVD at Hospital General Universitario Gregorio Marañón between January 1997 and June 2015. Data were obtained from medical histories, surgical reports, and outpatient follow-up reports, as well as from telephone interviews with patients who have undergone surgery between 1997 and 2009. Since 2010, the database includes and updates data from all new patients with TN undergoing MVD. All patients included in the database had a diagnosis of classical TN refractory to medical treatment according to the criteria of the third edition of the International Classification of Headache Disorders (ICHD-3 beta)1 and they all had undergone neuroimaging tests, especially magnetic resonance imaging, to rule out presence of TN secondary to other conditions. The treatment protocol of our department establishes MVD as the first surgical option since this technique has greater long-term effectiveness according to the literature and due to our vast experience with this procedure. Percutaneous rhyzotomy and radiosurgery are recommended only for those patients with severe comorbidities that may increase the risk of anaesthetic or surgical complications. Advanced age is not considered a contraindication for MVD.
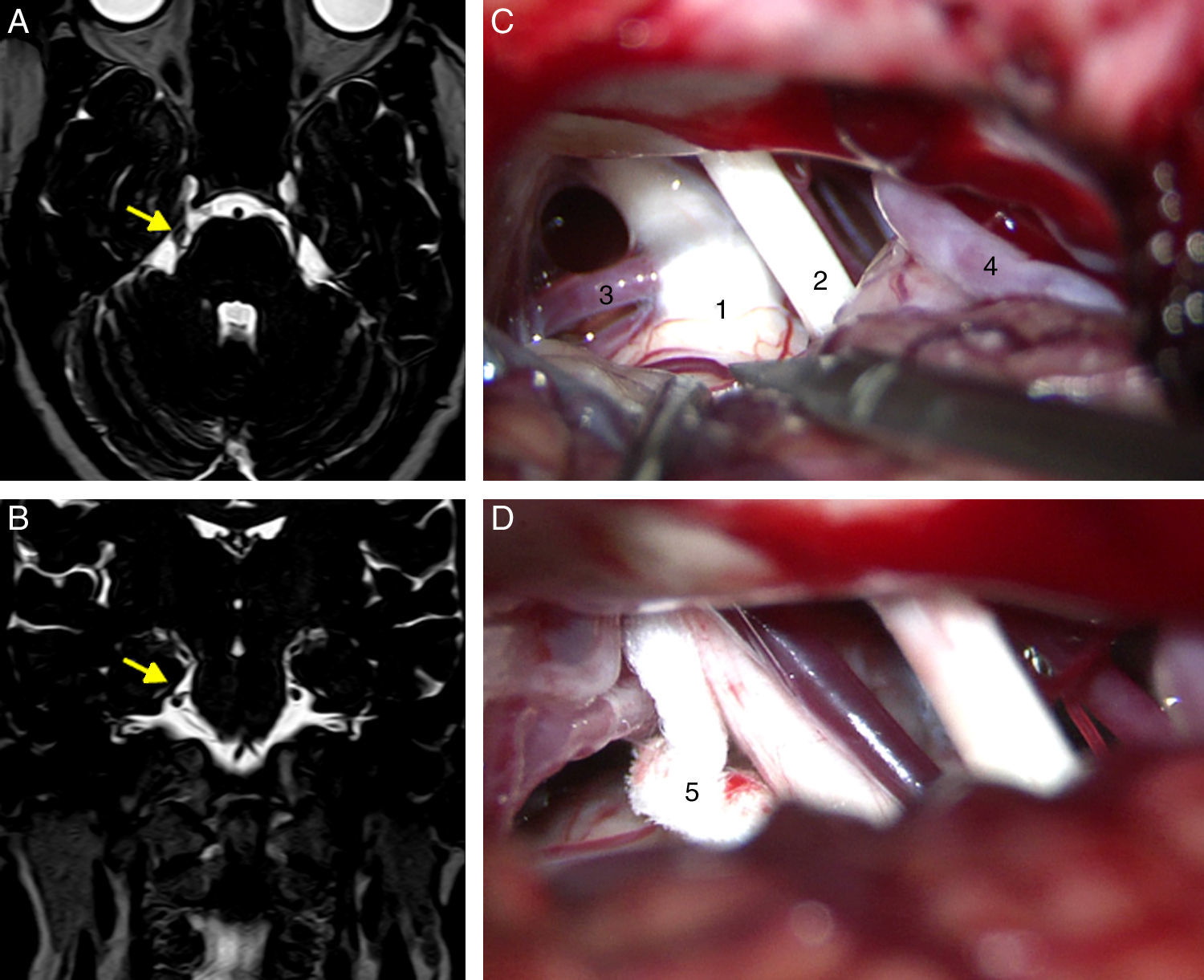
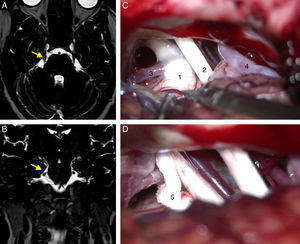
All patients had undergone a preoperative MRI study to rule out tumours, vascular malformations, multiple sclerosis, and other potential causes of TN. Patients undergoing surgery since 2005 had been studied with T2-weighted 3D DRIVE sequences, which enable the detection of neurovascular compression (Fig. 1A and B).
Axial (A) and coronal (B) DRIVE MRI sequences showing a loop in the superior cerebellar artery (SCA), which is in contact with the superomedial aspect of the trigeminal nerve (arrow) near the root entry zone, displacing the nerve. (C) Microsurgical image of the right cerebellopontine angle showing a loop in the SCA which compresses the trigeminal nerve. (D) Decompression of the trigeminal nerve by inserting a piece of Teflon between the offending vessel and the nerve. 1: trigeminal nerve; 2: VII and VIII cranial nerves; 3: SCA; 4: anterior inferior cerebellar artery (AICA); 5: Teflon.
Suboccipital retrosigmoid craniectomy was performed with the patients in either the supine or lateral position, depending on the surgeons’ preferences. We examined the microsurgical anatomy of the cerebellopontine angle to detect vascular compression at the root entry zone or the cisternal segment of the trigeminal nerve. Cases of nerve compression by an artery or vein were treated with MVD following Jannetta's technique (implanting a piece of shredded Teflon between the nerve and the offending vessel)15 (Fig. 1C and D). Those patients in which vascular compression was either not seen or unclear underwent partial sensory rhizotomy of the inferior third of the trigeminal nerve.
The cut-off point for age was set at 70 years; we compared immediate and long-term surgical outcomes in patients older than and younger than 70. Pain relief was quantified using the Barrow Neurological Institute (BNI) pain intensity score for TN. This tool was initially developed to measure the effectiveness of radiosurgery16 although it can also be applied in cases of MVD17–20 (Table 1). No pain without medical treatment (BNI I) was regarded as an excellent surgical outcome, whereas pain relief with medical treatment (BNI I-III) was considered an acceptable outcome. Patients were followed up for a minimum of 6 months.
Barrow Neurological Institute pain intensity score (BNI score).
| I | Excellent: no pain, no medication required |
| II | Good: occasional pain, no medication required |
| III | Acceptable: some pain, adequately controlled with medication |
| IV | Treatment failure: some pain, not adequately controlled with medication |
| V | Poor: severe pain or no pain relief |
Likewise, we compared mean duration of hospital stay and presence of neurological complications and other medical complications in patients aged 70 years and older and in those younger than 70.
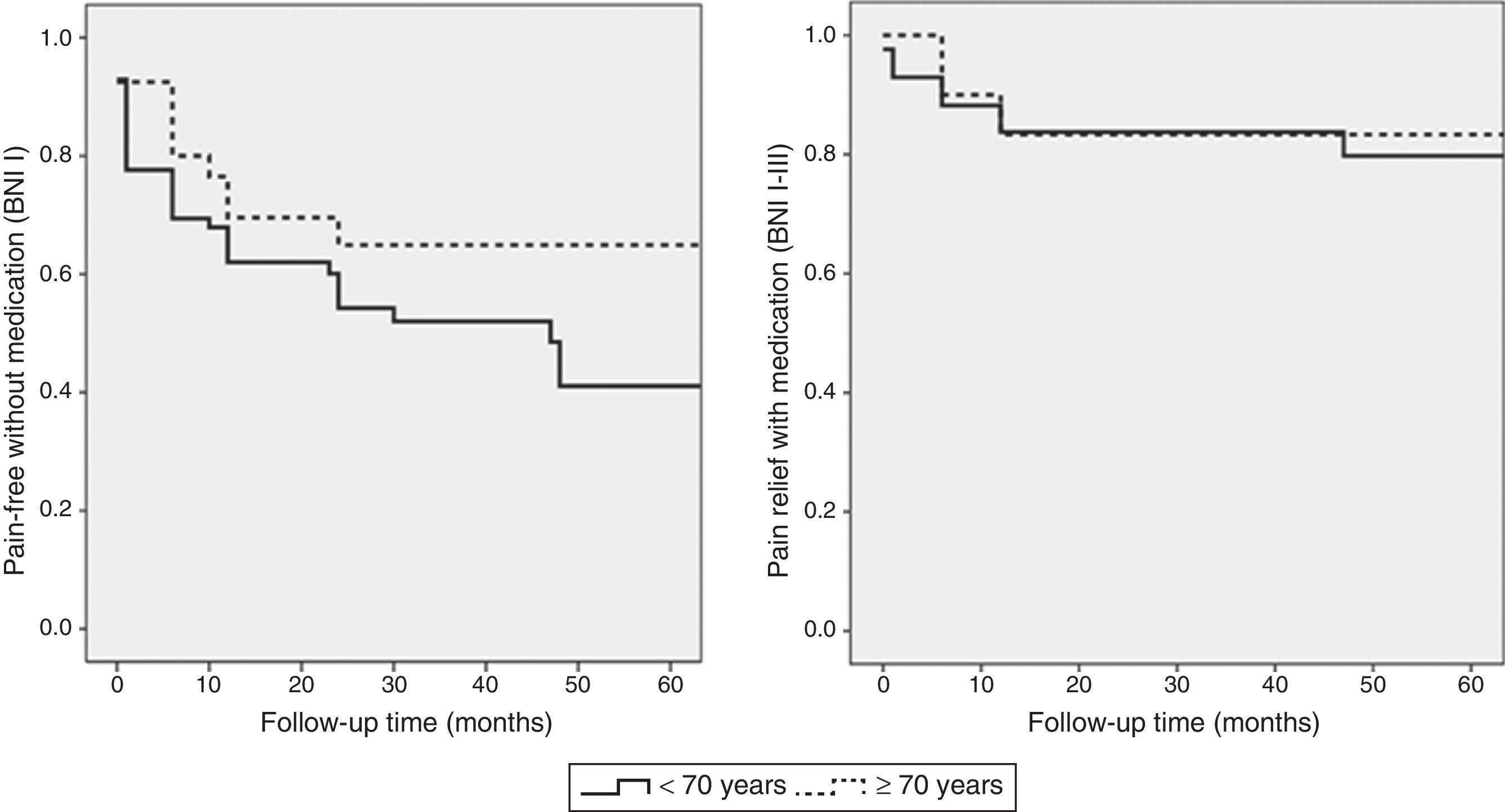
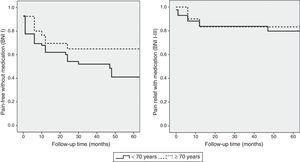
The statistical analysis used a significance level of .05 and a 95% CI for the analysed variables. The association between age and surgery outcomes was estimated using contingency tables and the Fisher exact test. We used binary logistic regression analysis to determine the relative risk ratio and CI of surgery outcomes in each age group. Pain recurrence rates were estimated using the Kaplan–Meier estimator: recurrence of trigeminal pain which could not be controlled with medication (BNI scores IV and V) was regarded as treatment failure. We also estimated pain-free survival time with no pharmacological treatment (BNI score I). Differences in pain recurrence between the 2 groups were analysed using the Mantel–Cox test. Statistical analysis was performed using statistical software SPSS version 15.0.
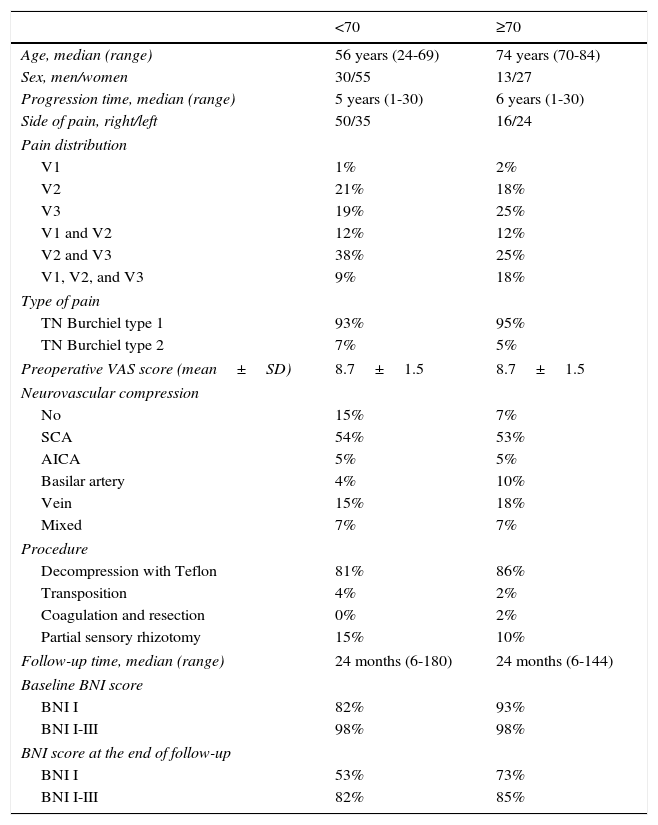
ResultsA total of 125 patients underwent surgery during the study period (43 men and 82 women). Forty of these (32%) were 70 or older, with a mean age of 74.8 (range, 70-84 years). The 85 patients younger than 70 had a mean age of 52.3 (range, 24-69 years). Facial pain was predominantly paroxysmal (Burchiel type I21) in patients older than 70 (95%); in 55% of these patients, pain affected 2 or more territories of the trigeminal nerve, with a mean progression time of 8.2 years. Seventy percent of the patients were receiving 2 or more antiepileptic drugs at the time of surgery; despite treatment, 63% of the patients experienced incapacitating pain affecting speech and oral intake. Four patients (10%) had previously undergone ablation of the gasserian ganglion, without improvement. Examination of the microsurgical anatomy of the cerebellopontine angle revealed vascular compression of the trigeminal nerve in 37 patients (93%; arterial in 68%, venous in 17.5%, and mixed in 7.5%). Thirty-four of the cases underwent MVD with Teflon, one underwent artery transposition, one underwent vein coagulation and resection, and partial sensory rhizotomy was performed in 4. Patients were followed up for a mean of 34 months (range, 6-144). At discharge, 93% of the patients were pain-free without treatment (BNI I). At the end of follow-up, 73% of the patients continued to be pain-free without medication, whereas pain was adequately managed with medication in 85% (BNI I-III). Clinical characteristics of both patient groups are summarised in Table 2.
Patients’ demographical and clinical characteristics.
| <70 | ≥70 | |
|---|---|---|
| Age, median (range) | 56 years (24-69) | 74 years (70-84) |
| Sex, men/women | 30/55 | 13/27 |
| Progression time, median (range) | 5 years (1-30) | 6 years (1-30) |
| Side of pain, right/left | 50/35 | 16/24 |
| Pain distribution | ||
| V1 | 1% | 2% |
| V2 | 21% | 18% |
| V3 | 19% | 25% |
| V1 and V2 | 12% | 12% |
| V2 and V3 | 38% | 25% |
| V1, V2, and V3 | 9% | 18% |
| Type of pain | ||
| TN Burchiel type 1 | 93% | 95% |
| TN Burchiel type 2 | 7% | 5% |
| Preoperative VAS score (mean±SD) | 8.7±1.5 | 8.7±1.5 |
| Neurovascular compression | ||
| No | 15% | 7% |
| SCA | 54% | 53% |
| AICA | 5% | 5% |
| Basilar artery | 4% | 10% |
| Vein | 15% | 18% |
| Mixed | 7% | 7% |
| Procedure | ||
| Decompression with Teflon | 81% | 86% |
| Transposition | 4% | 2% |
| Coagulation and resection | 0% | 2% |
| Partial sensory rhizotomy | 15% | 10% |
| Follow-up time, median (range) | 24 months (6-180) | 24 months (6-144) |
| Baseline BNI score | ||
| BNI I | 82% | 93% |
| BNI I-III | 98% | 98% |
| BNI score at the end of follow-up | ||
| BNI I | 53% | 73% |
| BNI I-III | 82% | 85% |
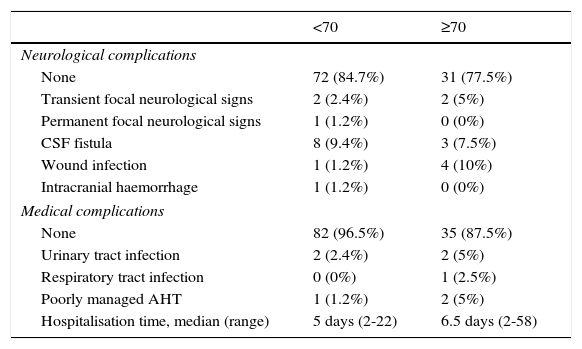
The cut-off point for age was 70 years: we compared data from patients younger than 70 to those aged 70 and older. Means were compared using the Mann–Whitney U test for independent samples; this test showed no differences in progression time between samples. The Fisher exact test showed no association between age and acceptable surgery outcome (BNI I-III) at the end of the follow-up period (P=.6). However, ages 70 and older were associated with higher rates of excellent outcomes (BNI I); these patients usually remained pain-free at the end of follow-up (P=.03). Binary logistic regression analysis showed a statistically significant association between age and excellent surgery outcomes (P=.02). Patients aged 70 years and older were more likely to score BNI I at the end of follow-up (OR 0.4; 95% CI: 0.2-0.9). However, the likelihood of remaining pain-free at the end of the follow-up period was not significantly higher in these patients than in patients younger than 70 (OR 0.7; 95% CI: 0.3-1.9). Kaplan–Meier survival curves (Fig. 2) showed a higher rate of pain-free survival (BNI I), although differences were not statistically significant (log-rank=0.07). Patients aged 70 and older were hospitalised for a mean of 8.8 days whereas hospitalisation times in patients younger than 70 were significantly shorter, with a mean of 6.1 days (P=.04). None of the patients in our series died as a consequence of the intervention (Table 3). Among patients aged 70 years and older, 2 displayed transient focal neurological signs due to traumatic facial and trochlear neuropathy, respectively, 3 had cerebrospinal fluid fistulas, and 4 experienced an infection of the surgical wound. The rates of neurological complications and other types of complications were not significantly higher in patients aged 70 and older (P=.3 and P=.1, respectively).
Surgery complications and mean hospitalisation time.
| <70 | ≥70 | |
|---|---|---|
| Neurological complications | ||
| None | 72 (84.7%) | 31 (77.5%) |
| Transient focal neurological signs | 2 (2.4%) | 2 (5%) |
| Permanent focal neurological signs | 1 (1.2%) | 0 (0%) |
| CSF fistula | 8 (9.4%) | 3 (7.5%) |
| Wound infection | 1 (1.2%) | 4 (10%) |
| Intracranial haemorrhage | 1 (1.2%) | 0 (0%) |
| Medical complications | ||
| None | 82 (96.5%) | 35 (87.5%) |
| Urinary tract infection | 2 (2.4%) | 2 (5%) |
| Respiratory tract infection | 0 (0%) | 1 (2.5%) |
| Poorly managed AHT | 1 (1.2%) | 2 (5%) |
| Hospitalisation time, median (range) | 5 days (2-22) | 6.5 days (2-58) |
Numerous studies have addressed immediate and long-term outcomes of MVD in series of patients followed up for a mean of over 5 years; these studies report initial success rates ranging between 76% and 99%, long-term success rates of 62% to 89%, and relapse rates of 4% to 38%.5–9,22,23 Recently published series report mortality rates below 1%, with rates of severe neurological complications below 5%.8,24 The differences between MVD and percutaneous techniques on the gasserian ganglion have been addressed in several comparative studies, some of which were prospective although not randomised.22,25–27 Initial effectiveness in terms of complete pain control does not differ significantly between percutaneous procedures and MVD. Likewise, radiosurgery achieves a high rate of initial pain relief.28–30 However, the effectiveness of all ablation procedures decreases with time. The rate of persistent pain relief associated with use of ablation techniques ranges between 25% and 60% at 5 years and it is significantly lower than that of MVD.31–33
There is growing interest in MVD for elderly patients. This may be explained by population ageing and the increasing number of people who maintain a good health status until the last decades of life and wish to preserve their quality of life.34 Prevalence of TN starts to increase at the age of 60. If left untreated, this condition may significantly damage patients’ health. MVD has been shown to be superior to radiosurgery and percutaneous ablation techniques in terms of initial and long-term effectiveness; this superiority has been analysed in elderly patients setting the cut-off age at 65 or 70.21,34–40 To date, age has not been proven to be a factor for poor prognosis in patients with TN undergoing surgery, as shown in Table 4. Microsurgical anatomy findings in the cerebellopontine angle in elderly patients are similar to those found in younger patients except for the greater number of compressions secondary to vertebrobasilar dolichoectasia. Tortuous dilated basilar arteries, which may compress the trigeminal nerve, are more frequent among elderly patients and have been associated with presence of arterial hypertension and atherosclerosis.41,42 In our series, 7 patients had vertebrobasilar dolichoectasia; these patients had a mean age of 67 years, and 4 of them were older than 70.
Outcomes and complications of MVD in young and elderly patients in recently published series of surgical patients.
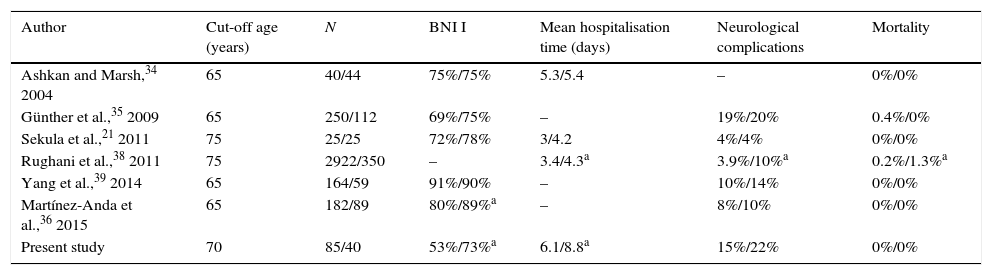
| Author | Cut-off age (years) | N | BNI I | Mean hospitalisation time (days) | Neurological complications | Mortality |
|---|---|---|---|---|---|---|
| Ashkan and Marsh,34 2004 | 65 | 40/44 | 75%/75% | 5.3/5.4 | – | 0%/0% |
| Günther et al.,35 2009 | 65 | 250/112 | 69%/75% | – | 19%/20% | 0.4%/0% |
| Sekula et al.,21 2011 | 75 | 25/25 | 72%/78% | 3/4.2 | 4%/4% | 0%/0% |
| Rughani et al.,38 2011 | 75 | 2922/350 | – | 3.4/4.3a | 3.9%/10%a | 0.2%/1.3%a |
| Yang et al.,39 2014 | 65 | 164/59 | 91%/90% | – | 10%/14% | 0%/0% |
| Martínez-Anda et al.,36 2015 | 65 | 182/89 | 80%/89%a | – | 8%/10% | 0%/0% |
| Present study | 70 | 85/40 | 53%/73%a | 6.1/8.8a | 15%/22% | 0%/0% |
Each column provides data for young patients/elderly patients.
BNI I: no pain and no medication required at the end of follow-up.
As a general rule, success rates and pain-free survival times in elderly patients are similar to those seen in younger patients. In some series, elderly patients have achieved higher rates of complete pain resolution than younger patients.6,36 In our series, patients aged 70 and older achieved better surgery outcomes than younger patients. More specifically, younger patients were less likely to remain pain-free without medication (BNI I) at the end of follow-up, although the probability of achieving acceptable results did not differ significantly between groups. This may be due to the fact that the degree of compression, an accepted prognostic factor, was frequently higher in older patients; in these patients, the microsurgical anatomy of the cerebellopontine angle is easier to examine due to age-associated cerebellar atrophy.8,39,43 Although mean hospitalisation time was significantly higher in patients older than 70, it was not associated with a significant increase in neurological or other complications. As in the case of younger patients, none of the elderly patients of our sample died or experienced permanent neurological sequelae. In fact, most of the published series report similar rates of mortality and neurological sequelae. In a meta-analysis by Sekula et al.,21 no significant differences were found between younger and older patients in terms of risk of neurological impairment, stroke, cerebellar haematoma, and death. Most authors studying the impact of age on surgery for TN conclude that this factor should not be regarded as a contraindication for MVD and recommend analysing each patient's medical status to detect any comorbidities that may increase the risk of anaesthetic or surgical complications.21,37,39,40 Ours is the first study to specifically analyse the effectiveness and safety of MVD in a series of elderly patients with TN in Spain. Although our study's cut-off age was higher than those of other series, and despite the limitations inherent to its retrospective design and the length of the study period, our results show that advanced age is not a factor of poor prognosis in terms of surgery outcomes and complications, and reveal a significantly higher rate of excellent results in patients aged 70 years and older.
Conflicts of interestThe authors have no conflicts of interest to declare. This study has received no funding.
Please cite this article as: Ruiz-Juretschke F, Vargas AJ, Gonzalez-Quarante LH, Gil de Sagredo OL, Montalvo A, Fernandez-Carballal C. Tratamiento microquirúrgico de la neuralgia trigeminal en mayores de 70 años, estudio de eficacia y seguridad. Neurología. 2017;32:424–430.