According to today's most widely accepted international criteria,1–3 diffuse Lewy body disease (DLBD) is an illness that evolves with a spectrum of clinical manifestations, characterized by the presence of cognitive impairment, fluctuations in the state of alertness, sensoriperceptual disorders including visual hallucinations, and Parkinsonism. Over the last few years, the spectral characteristics of this disease have been recognized, with those patients who debut with and have a predominance of cognitive symptoms at one end and with the other end including those individuals in whom Parkinsonian symptoms prevail.4 Both extremes of the disease converge as the disease progresses, as revealed by neuropathological correlation studies.5–7 Moreover, neuropsychiatric symptoms are widely reported by various authors in both groups of patients, albeit little study has been dedicated to the onset and evolution of these symptoms in DLBD. In this article, we present a brief description of a patient who meets the criteria for DLBD and who debuted with neuropsychiatric symptoms (severe depression and visual hallucinations), many years prior to presenting cognitive and Parkinsonism-type symptoms, which has led us to conduct a review of the issue and to suggest that some patients with DLBD may begin with only neuropsychiatric symptoms and persist many years displaying only these symptoms before presenting cognitive impairment and/or Parkinsonism.
A 79-year-old male patient, diagnosed with late-onset, recurring, major depressive disorder, presented a first major depressive episode in December 1994. Ever since onset, he has presented sensoriperceptual alterations in the form of visual hallucinations, with partial response to multiple treatment strategies, leading to hospitalization on several occasions. The initial neuropsychological assessment failed to reveal cognitive impairment (MMSE 29/30, MIS 8/8, GDS 1), although a mild dysexecutive syndrome and mild alterations of visual memory were detected as attributing to the important depressive component without having a major bearing on the basic activities of daily living. In light of the patient's scant clinical improvement, treatment with electroconvulsant therapy (ECT) was initiated, with full remission of the depressive symptoms, although the visual hallucinations persisted. The patient presented recurrence of the depressive syndrome and visual hallucinations on several occasions until January 2003, with a depressive episode including deliria, which required another hospitalization that also led to more ECT sessions given the patient's poor response to treatment (neuroleptics, antidepressants and lithium, among others), presenting confusion that improved by increasing the intervals between ECT sessions until full remission of the symptoms was achieved.
In December 2006, the depressive symptoms became worse, with components of both deliria and confusion (occasional incoherent speech); in addition, the patient presented tremor at rest in the upper right limb, as well as clumsiness in his right hand, difficulty in walking and visual hallucinations that had not remitted in all those years, but had decreased. Diagnosis was oriented toward Hoehn and Yahr's stage II Parkinsonism,8 probably related to neuroleptics (olanzapine). The patient was hospitalized and treated with low-dose haloperidol, which was able to control the patient's deliria. The patient showed clear improvement of his psychiatric symptoms and, as a result, the neuroleptic drugs were discontinued at 3 months; however, the Parkinson-like symptoms not only persisted, but also became worse, with greater functional impairment. Consequently, levodopa at a dose of 50mg every 8h was initiated and he displayed worsening of the visual hallucinations that remitted following withdrawal of l-dopa. In May 2009, the patient once again presented exacerbation of complex visual hallucinations and symptoms of confusion, with persistence of depressive symptoms (sad mood, fatigability, and apathy), as well as worsening of his Parkinsonism, despite withdrawal of the other neuroleptics and being treated solely with clozapine and venlafaxine. At this time, asymmetric Hoehn and Yahr's stage 3 Parkinsonism was observed and the patient scored 70% on the Schwab and England scale of basic activities of daily living.9 The patient presented a total score of 32 points on the unified Parkinson's Disease Scale (UPDRS). The cognitive scales revealed the following: MMSE 22/30 (delayed memory failure), MIS 4/8, blessed: 2/3/4 total=9, GDS 5. This new neuropsychological evaluation concluded that the patient presented cognitive impairment consistent with a mild stage of dementia (CDR 1), with a cortical-subcortical pattern, highlighting a severe deficit in the visual-spatial orientation and dysexecutive areas, compatible with the neuropsychological patterns seen in patients with Parkinson's disease.
Para-clinical studies revealed the following: cranial magnetic resonance: generalized cranial hypotrophy, more accentuated in both the amygdala and the hippocampus; the electroencephalogram was normal. The blood analysis showed all parameters to be normal, including blood testing for syphilis and human immunodeficiency virus, vitamin B12 and folate levels, as well as thyroid hormones. The DATscan exhibited bilateral low uptake in the striatum that was asymmetrical and more noticeable in the right striatum (Fig. 1). In light of the patient's motor impairment, we initiated treatment with low dose dopamine agonist (rotigotine) given his history of worsening visual hallucinations with l-dopa. The patient showed improvement of his Parkinsonism at a dose of 8mg of rotigotine, but his visual hallucinations became worse. As a result, we opted to lower the dose to 6mg/day, at which he has remained stable, with a slight improvement of 30–40% in the Parkinsonism over his previous UPDRS scale score and with few visual hallucinations. We had previously increased the dose of clozapine to 75mg/day; higher doses had increased his depressive symptoms in the past.
This is the case of a 79-year-old male patient presenting a major depression syndrome lasting more than 10 years, with visual hallucinations since the onset of the disease, that had been refractory to many conventional anti-depressant drug strategies and who, from the outset, had presented psychiatric elements of disease that pointed toward a depressive syndrome associated with organic brain disease, such as the presence of visual hallucinations and his poor response to polytherapy with antidepressants and neuroleptics. At that time, the neuropsychological tests showed only a dysexecutive disorder and mild visual symptoms without signs of cognitive impairment, which was attributed to the major depression. Given his poor response to these drugs, he underwent electroconvulsant therapy that showed scant effectiveness and significant side effects (confusional syndromes). The patient exhibited the first neurological symptoms (asymmetrical Parkinsonism) 8 years after the onset of his neuropsychiatric symptoms, initially ascribed to his medication (lithium/olanzapine), but which persisted and progressed over the following months despite having discontinued all the drugs that might cause or worsen his Parkinsonism. Mild cognitive impairment later appeared with cortical–subcortical elements that led us to request a DATscan fluorodopa test in which we could observe low asymmetrical striatal dopaminergic uptake, suggesting presynaptic Parkinsonism. The presence of asymmetric Parkinsonism and the fact that the patient's symptoms continued to progress despite having withdrawn the drugs associated with Parkinsonism, ruled out a possible pharmacological aetiology. The presence of fluctuations in his state of alertness and the visual hallucinations complete the accepted criteria for atypical Parkinsonism, probable diffuse Lewy body disease.10 The patient was neuropsychologically assessed at the onset of his depressive syndrome, displaying a mild dysexecutive syndrome and mild alteration of his visual memory. In a second evaluation carried out 7 years later, these visual and dysexecutive symptoms were seen to have become worse and mild dementia was observed with cortical-subcortical pattern as reported in some cases of DLBD.
DLBD is a spectral disease in which dementia and Parkinsonism are two common forms of clinical expression, according to many publications and international consensus statements, such as the American Lewy Body Dementia Association.11 It has been recognized that in many patients with DLBD the clinical picture begins with profound, rapid dementia with fluctuating elements, in both the cognitive and state of alertness domains, and in others, it debuts with initial Parkinsonism with psychiatric sensoriperceptual manifestations (hallucinations), with variable response to l-dopa. This drug tends to make these symptoms worse. In spite of the fact that many research groups believe that visual hallucinations are an essential symptom of DLBD, there has been little information reported in the medical literature in relation to the onset of DLBD with neuropsychiatric symptoms, such as severe depression and visual hallucinations. One Spanish study reported a group of patients with DLBD with very severe neuropsychiatric symptoms, with a predominance of hallucinations and deliria,12 as observed in our patient; nevertheless, there is little medical information about the course these symptoms follow in patients with DLBD. On the other hand, we are aware of the fact that our patient's diagnostic orientation is based on clinical criteria, as tends to be the case, and that we do not have a neuropathological study available by means of which to make the definitive diagnosis, which limits our observations; nonetheless, the patient meets widely accepted, clinical criteria for DLBD. We present a brain DATscan fluorodopa result compatible with presynaptic Parkinsonism and a neuropsychological study that is consistent with those described in patients with alpha-synucleinopathies. We also wish to point out that, despite the fact that this article presents an isolated case, there are some clinical-pathological correlation studies in which patients debut with neuropsychiatric symptoms and, in a not terribly well-defined period of time, develop the remaining neurological symptoms.13,14
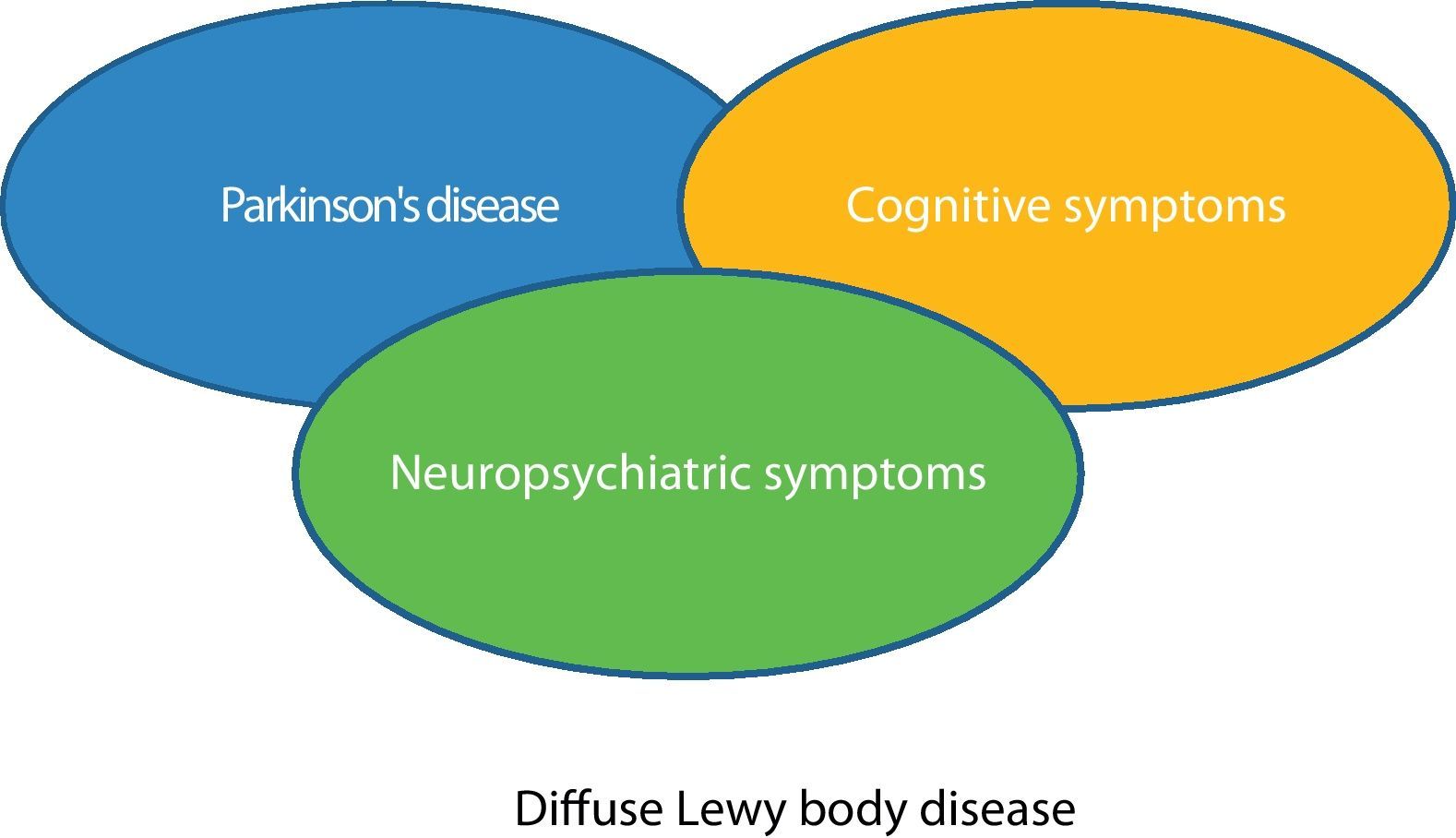
It is possible that visual hallucinations comprise the first clinical manifestations in some patients with DLBD and, after a period of time that has yet to be determined, cognitive and extrapyramidal symptoms appear (Fig. 2). We believe that prospective studies including neuropsychological tests would of interest in patients with unrelated visual hallucinations that cannot be accounted for by any psychiatric disorder or by other known aetiologies (toxic, neoplastic, etc.) and to assess the usefulness of DATscan flurodopa in these patients.
Please cite this article as: Salazar G, et al. Síntomas neuropsiquiátricos en la enfermedad difusa por cuerpos de Lewy: a propósito de un caso. Neurología. 2011;26:499–501.