Epstein-Barr virus (EBV) infections are very frequent in paediatric patients. The main clinical manifestation is infectious mononucleosis (IM).1,2 The age of primary infection varies between different regions: in developed countries, between 80% and 100% of all affected children are aged 3 to 6 years old, and most of them are asymptomatic.1 Once the primary infection has occurred, the virus will remain latent in the human host for life.1,2
In children and adolescents, IM is typically associated with such symptoms as fever, fatigue, general unease, acute pharyngitis, lymphadenopathy, and hepatosplenomegaly. In immunocompetent patients, IM is usually self-limiting and rarely associated with complications.1,2 Around 1% to 18% of patients with IM experience neurological complications; these are more frequent in the acute stage of the disease and rare in the final stage.1–3
In paediatric patients, IM may present with central nervous system involvement (aseptic meningitis, meningoencephalitis, and encephalitis) either as the sole symptom of the disease or before the disease itself develops. Therefore, EBV infection must be regarded as a potential cause of acute meningitis regardless of its associated symptoms.1,4–7 However, other complications have also been described: cerebellitis, cranial nerve palsy, optic neuritis, Guillain-Barré syndrome, hemiplegia, and transverse myelitis.1,3
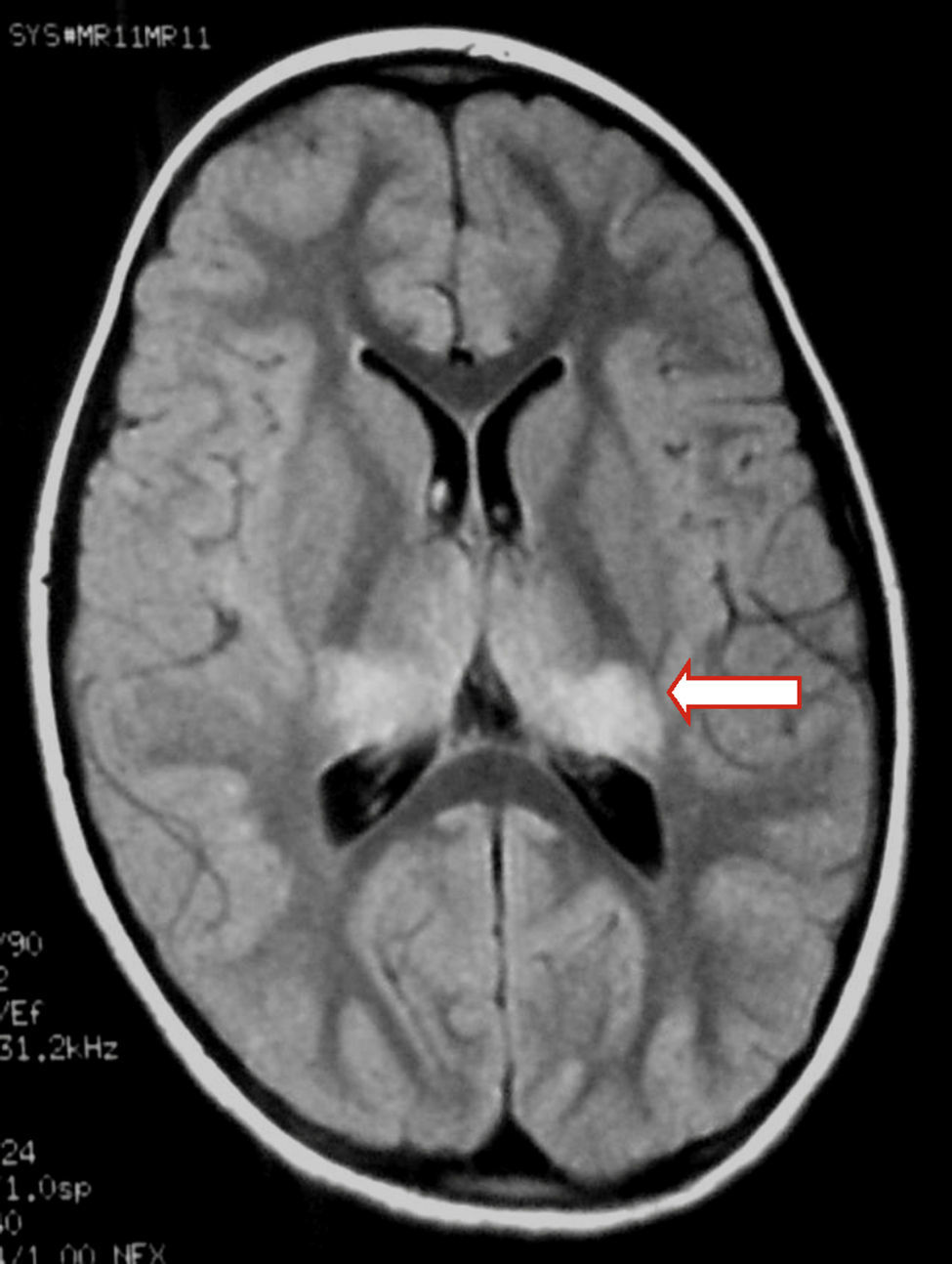
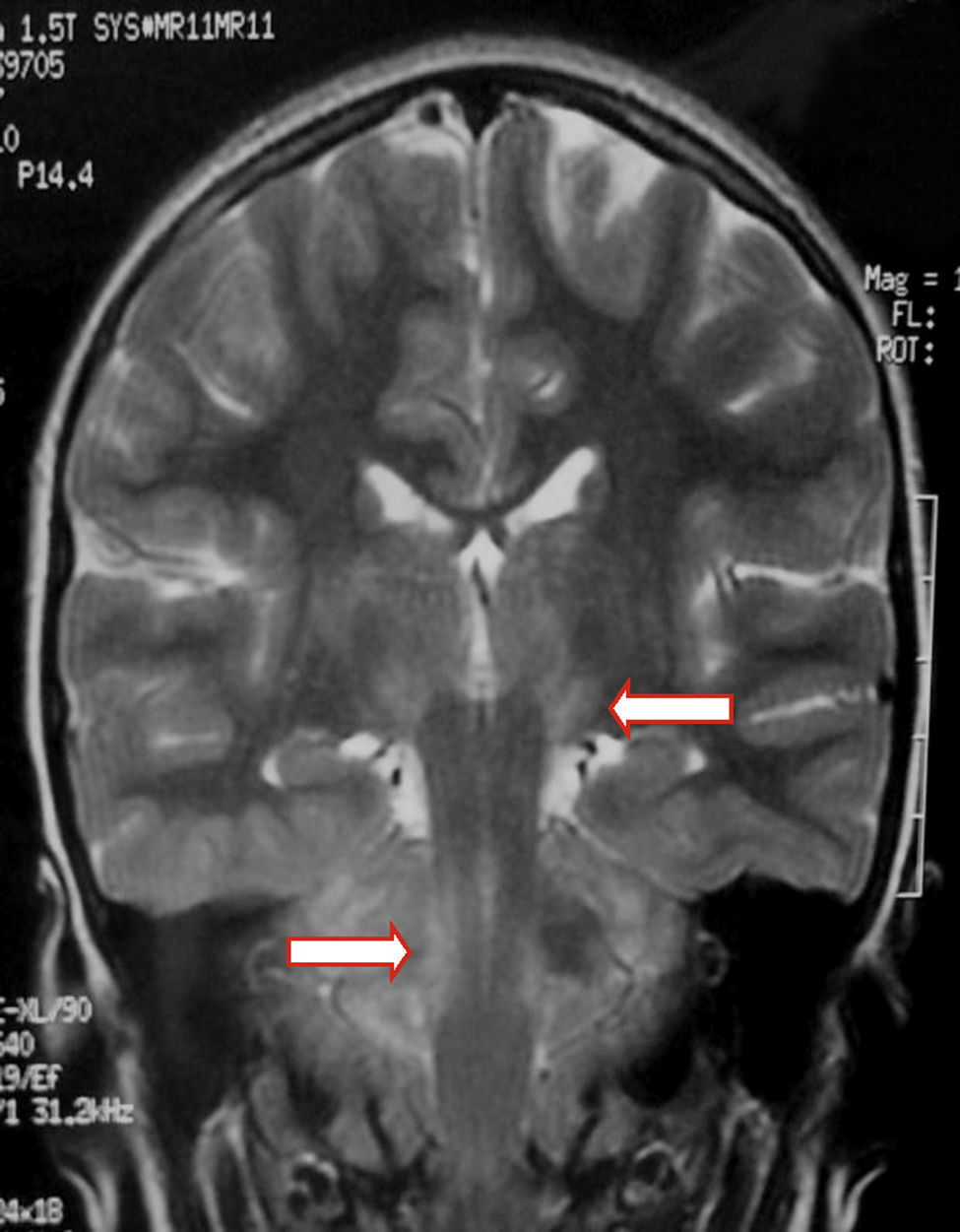
We present the case of a healthy 6-year-old patient with a 20-day history of fever. She was initially diagnosed with pharyngo- and gingivostomatitis. The patient had persistent headache, photophobia, excessive somnolence, and gait abnormalities. She had a normal level of consciousness, generalised hyperreflexia, cerebellar ataxia, and no meningeal signs. A laboratory test revealed leukocytosis (25300leukocytes/mm3; 60% neutrophils) with no activated lymphocytes and no heterophile antibodies (Paul-Bunnell test). A CSF cytochemical test showed 20 red blood cells/mm3, 40 leukocytes/mm3 (60% polymorphonuclear), glucose level of 0.45g/L, and a total protein level of 0.46g/L. The bacteriological study revealed no abnormalities. Polymerase chain reaction (PCR) testing was positive for EBV and negative for enterovirus and herpesviruses 1, 2, and 6. A cranial CT scan revealed no abnormalities and the EEG displayed diffuse cortical and periodic subcortical activity. A brain MRI scan revealed T2-weighted and FLAIR hyperintensities at the level of the thalamus, midbrain, and cerebellum. No contrast uptake or diffusion restriction was seen (Figs. 1 and 2).
Tests for EBV capsid antibodies were positive for IgG and negative for IgM. The results of an additional PCR test for EBV in CSF were positive. Our patient was diagnosed with EBV encephalitis. We started treatment with acyclovir for 21 days and dexamethasone for 72hours. One month after the episode, the patient experienced bilateral loss of vision with normal eye fundus and no response in the visual response potentials test. She was diagnosed with postinfectious retrobulbar optic neuritis and received a bolus of 30mg/kg methylprednisolone daily plus prednisone for 4 weeks; treatment achieved excellent results.
As a general rule, EBV encephalitis has an acute onset, progresses rapidly, and resolves completely.5 Our patient displayed the most frequently described symptoms of EBV encephalitis, namely fever, headache, altered level of consciousness, and gait abnormalities; all these symptoms pointed to encephalitis.8
In patients with typical symptoms of IM, detecting heterophile antibodies is sufficient for diagnosis. However, tests may yield negative results in early stages of the disease, especially in children younger than 4.1,2 Detection of specific EBV antibodies must be limited to those patients with a high clinical suspicion of IM and who test negative for heterophile antibodies or show a severe or atypical form of presentation. These antibodies include IgG and IgM for EBV capsids and IgG for EBV nucleic acid sequences. In serological tests, presence of IgM and IgG antibodies for EBV capsids and lack of IgG for EBV nucleic acid indicates acute or recent infection. IgG EBV capsid antibodies remain in the patient for life and suggest that EBV may be able to reactivate.1,9 We hypothesise that our patient had had a primary EBV infection which could not be confirmed using specific serology tests since they were conducted at a later stage.
CSF tests are essential in patients suspected of having encephalitis. Cytochemical analysis reveals no specific findings and yields normal results in up to 10% of all patients.4 Viral nucleic acid amplification with PCR tests for CSF must be used in appropriate clinical situations to determine the aetiological agent.4,10 EBV can be detected with PCR tests in CSF, although positive results do not necessarily indicate central nervous system infection; latent infected mononuclear cells may lead to false-positive results.10–13 Therefore, test results must be supported by clinical symptoms and serology and imaging findings,4,11,14–16 as in our case.
EEG is a sensitive tool for detecting brain dysfunction and may reveal central nervous system involvement during early stages of the disease. Findings are normally non-specific, as in the case presented here. Severity of the EEG alterations is not correlated with disease severity in the acute phase; however, rapid improvements in EEG tracings frequently point to a good prognosis.3,9
In children with EBV encephalitis, neuroimaging alterations present with varying frequency.1,4,6,17 MRI is considered the imaging technique of choice since it can detect early changes.4 MRI findings are heterogeneous and include diffuse oedema, cortico-subcortical damage, periventricular damage, and basal ganglia involvement. EBV affects all the areas in the brain, including the basal ganglia; prognosis is poorer in the case of limbic or thalamic involvement.6,9,17–19 MR images usually normalise during follow-up.19
EBV is susceptible to acyclovir in vitro. However, the efficacy of acyclovir in vivo is minimal; this drug does not reduce disease severity or duration nor does it alter prognosis. Corticosteroid use is supported for certain complications, especially in post-infectious demyelinating lesions to the central nervous system, as in our case.2,4,6,14,20–22
Prognosis of IM in healthy subjects is excellent; death has only exceptionally been reported.22,23 At present, after a year of follow-up, the patient has recovered completely and shows no neurological, visual, or neuroimaging alterations.
In conclusion, we presented the case of a patient with IM who experienced encephalitis-related central nervous system involvement followed by post-infectious demyelinating manifestations in the form of optic neuritis. Treatment achieved symptom resolution; the patient was completely recovered at one year of follow-up. EBV encephalitis is not frequent in paediatric patients. In a suitable clinical and neuroimaging context, EBV serology tests and PCR tests of CSF help confirm diagnosis.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank our patient's parents for their patience and support to this study.
Please cite this article as: Bazzino Rubio F, Gonzalez Betlza M, Gonzalez Rabelino G, Bello Pedrosa O. Neuritis óptica postencefalitis por virus de Epstein–Barr en niños inmunocompetentes. Caso clínico. Neurología. 2017;32:129–131.