Eslicarbazepine acetate (ESL) is a new antiepileptic drug (AED) and an analogue to carbamazepine (CBZ) and oxcarbazepine (OXC). In this study, we evaluate initial therapeutic response to ESL and events in the change from CBZ and OXC.
MethodsWe evaluated 61 patients with a broad spectrum of drug-resistant epilepsies in a cross-sectional study. The switch from CBZ and OXC to ESL was carried out in a single night at ratios of 1:1.3 and 1:1mg respectively.
ResultsThe most common form of epilepsy was temporal lobe epilepsy (62.3%). The most common aetiology was mesial temporal sclerosis (26.2%). Mean follow-up time was 4.7±3.2 months. In 40 patients with a minimum follow-up period of 3 months, monthly median seizure frequency dropped by 63.6% (P<.001) and a reduction of 80% or more was recorded in 30%. Adverse events (AEs) occurred in 54%; all appeared during the titration phase. They were more frequent at doses in excess of 800mg (73.9% vs. 47.4%; P=.042). The most common AE was dizziness (34.4%), which was commonly associated with VPA, LTG and/or LCS consumption (19.2% vs. 45.7%; P=.031). The retention rate at 3 months was 75.4%. A total of 25 patients replaced CBZ or OXC treatment with ESL; any AEs were transient (69.2% for CBZ and 33% for OXC; P=.073). At 3 months after the treatment change, median seizure frequency had decreased by 20% (P<.075).
ConclusionsESL is effective in the treatment of focal epilepsies and its early retention rate is >70%. AEs occurred during the titration phase and corresponded to associated AEDs. A rapid change from CBZ and OXC to ESL treatment can be safely performed.
La eslicarbazepina (ESL) es un nuevo fármaco antiepiléptico (FAE) análogo de la carbamazepina (CBZ) y la oxcarbazepina (OXC). Analizamos su respuesta terapéutica inicial y el paso desde CBZ y OXC.
MétodosEvaluamos en un estudio transversal a 61 pacientes con un amplio espectro de epilepsias farmacorresistentes. El cambio desde CBZ y OXC se realizó en una noche con una equivalencia de 1:1,3 y 1:1mg.
ResultadosLa epilepsia más frecuente fue la de lóbulo temporal (62,3%). La etiología más frecuente la esclerosis mesial (26,2%). El seguimiento medio fue de 4,7±3,2 meses. En 40 pacientes con seguimiento mínimo de 3 meses, la frecuencia mediana de crisis se redujo un 63,6% (p<0,001), siendo en un 30% la reducción ≥ 80%. Los efectos adversos (EA) aparecieron en el 54,3% siempre durante la fase de titulación, siendo más frecuentes a dosis superiores a 800mg (73,9% vs. 47,4%; p=0,042) y el más importante el mareo (34,4%), siendo más habitual en asociación con VPA, LTG y/o LCS (19,2% vs. 45,7%; p=0,031). La tasa de retención temprana a los 3 meses fue del 75,4%. En 25 pacientes se cambió desde CBZ o OXC, siendo los EA transitorios (69,2% y 33%; p=0,073). Pasados 3 meses del cambio, la frecuencia mediana de crisis había disminuido un 20% (p<0,075).
ConclusionesLa ESL es eficaz en el tratamiento de epilepsias focales, con una tasa de retención temprana > 70%. Los EA tienen lugar durante la fase de titulación y según los fármamcos antiepilépticos asociados. El cambio rápido desde CBZ y OXC puede realizarse de forma segura.
Eslicarbazepine acetate (ESL) is a new antiepileptic drug derived from carbamazepine (CBZ) and oxcarbazepine (OXC). It acts by stabilising the inactivated state of voltage-gated sodium channels (VGSC).1 Designed to prevent production of toxic epoxide metabolites and for metabolism to S-licarbazepine primarily by hydrolysis, ESL demonstrates a greater tolerability and efficacy than CBZ and OXC.2
The utility of ESL has been tested in single daily doses of 800 or 1200mg as an adjuvant treatment in adult patients with drug-resistant focal epilepsy.3–6 Open phases of clinical trials have corroborated this treatment's sustained therapeutic effect and its positive impact on patients’ quality of life and mood.3,7–9
ESL was first marketed in Spain in February 2011, and apart from its authorisation studies, published experience with the drug remains scarce. Furthermore, there is only limited information on whether patients who did not respond to CBZ or OXC, or who experienced adverse events (AE), might benefit from ESL, and how to switch from those drugs to ESL.10
On this subject, Phase III trials found that improvements were the same regardless of the antiepileptic drug used concomitantly with ESL. However, adverse effects were more frequent with CBZ. According to these trials, patients taking OXC had to be excluded since that drug has the same metabolites as ESL.4,5,11,12
For these reasons, this study is intended to analyse our epilepsy unit's initial experience using ESL and substituting CBZ or OXC with ESL.10 To this end, we provide an exhaustive follow-up on our patients, emphasising changes in seizure frequency, appearances of AEs, and interactions with other antiepileptic drugs.
Subjects and methodsThis observational, descriptive, cross-sectional study included, in consecutive order, the first patients with drug-resistant epilepsy to have received adjuvant treatment with ESL in our epilepsy unit. As per ILAE criteria, patients were considered to have drug-resistant epilepsy if they did not achieve seizure freedom after being treated with 2 well-tolerated and appropriately chosen antiepileptic drugs (either as monotherapies or in combination) during a minimum period of 3 times the longest pre-treatment interseizure interval in the preceding year, or 12 months, whichever was longer.13
Seizure frequency was measured in standard periods of 4 weeks (seizures per month).
Types of epilepsy and epileptic syndromes were classified according to the ILAE criteria,14,15 using information obtained from neuroimaging studies (3Tesla MRI or cranial computed tomography) and electroencephalography results (video-EEG or simple EEG).
ESL was started at a dose of 400mg/day and raised to 800mg/day over a 1 to 2 week period. Depending on the patient's response, it was later raised to 1200mg/day. To maintain the full concentration of the monohydroxy derivatives S- and R-licarbazepine, we opted for an OXC:ESL exchange ratio of approximately 1.1mg.10 Keeping in mind the equivalence of CBZ to OXC, we obtained an approximate CBZ:ESL equivalence of 1:1 to 1:1.3mg16 up to a maximum ESL dose of 1600mg/day. Treatment was substituted directly in a single night, first in patients hospitalised in our epilepsy unit. As our growing experience showed the switch to be safe, treatment was also substituted in outpatients.
We recorded antiepileptic drugs associated with ESL for each patient.
Patients were examined at the 1-month, 3-month, and 6-month points and alerted us by telephone if any AEs appeared. During their visits, patients were asked scripted questions about whether any physical or psychiatric AEs had presented. AEs were classified as transient if they resolved spontaneously, upon reducing the ESL dose, or upon changing the time of administration. Persistent AEs were those that did not remit after a dose adjustment, or which resulted in suspending ESL treatment.
Seizure frequency was measured using a seizure diary from the first day of treatment with ESL. Efficacy was measured as the change in the number of seizures in a standard 4-week period and according to variations in the percentages of patients in each of the following categories: seizure-free (no seizures during follow-up); decrease ≥80% in seizure frequency; decrease ≥50% in seizure frequency; decrease ≥30% in seizure frequency; no changes; and increase in seizure frequency. Since follow-up time was short (less than 1 year), patients were only considered seizure-free if they had experienced no seizures in a follow-up period of more than 3 months. Patients were only included in the study if their seizure frequency was greater than or equal to 1 seizure/month in the full year prior to recruitment. Patients who did not attain seizure freedom were evaluated by calculating the change in the number of seizure-free days.
A scheduled analysis was also performed in the first month, including a serum electrolyte study, blood count, liver function test, and in indicated cases, levels of adjuvant antiepileptic drugs. We evaluated only those abnormal results that fell in the pathological range and were not present in earlier tests.
Criteria for suspending treatment were an increase in the number of seizures despite dose adjustment, appearance of permanent or severe AEs, and/or finding significant changes in laboratory results.
Statistical studyStatistical analysis was completed using SPSS version 17.0 for Windows. Quantitative variables are described as the mean±standard deviation or the median (interquartile range [IR]). Qualitative variables are given as percentages. Normal distribution for continuous variables was tested using the Kolmogorov-Smirnov test. Univariate analysis was performed using the chi-square test or Fisher's exact test for dichotomous variables (odds ratio [OR] with a 95% confidence interval [CI]). Changes in seizure frequency and number of seizure-free days before and after beginning ESL treatment were evaluated using the Wilcoxon rank-sum test for paired data since these variables did not have a normal distribution. Values of P<.05 were considered statistically significant.
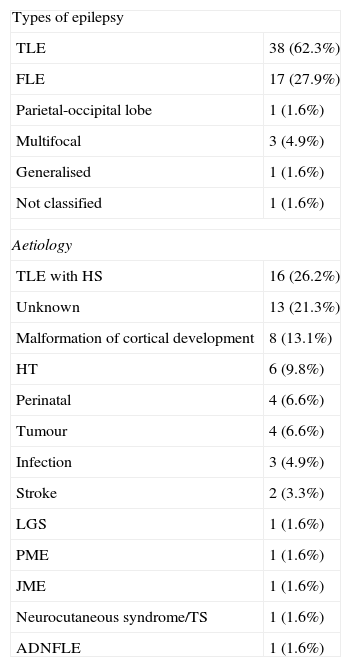
ResultsBaseline characteristics of the study populationThe study population consisted of 19 men (31.5) and 42 women (68.8%) with a mean age of 43.6±14.4 years. The most frequent type of epilepsy was temporal lobe epilepsy (TLE) at 62.3%. The most frequent aetiologies in the cohort were mesial sclerosis (26.2%), malformations of cortical development (13.1%) and head trauma (9.8%) (see Table 1).
Baseline characteristics of the study population (n=61).
| Types of epilepsy | |
| TLE | 38 (62.3%) |
| FLE | 17 (27.9%) |
| Parietal-occipital lobe | 1 (1.6%) |
| Multifocal | 3 (4.9%) |
| Generalised | 1 (1.6%) |
| Not classified | 1 (1.6%) |
| Aetiology | |
| TLE with HS | 16 (26.2%) |
| Unknown | 13 (21.3%) |
| Malformation of cortical development | 8 (13.1%) |
| HT | 6 (9.8%) |
| Perinatal | 4 (6.6%) |
| Tumour | 4 (6.6%) |
| Infection | 3 (4.9%) |
| Stroke | 2 (3.3%) |
| LGS | 1 (1.6%) |
| PME | 1 (1.6%) |
| JME | 1 (1.6%) |
| Neurocutaneous syndrome/TS | 1 (1.6%) |
| ADNFLE | 1 (1.6%) |
ADNFLE: autosomal dominant nocturnal frontal lobe epilepsy; FLE: frontal lobe epilepsy; TLE: temporal lobe epilepsy; JME: juvenile myoclonic epilepsy; PME: progressive myoclonic epilepsy; TS: tuberous sclerosis; HS: hippocampal sclerosis; LGS: Lennox–Gastaut syndrome; HT: head trauma.
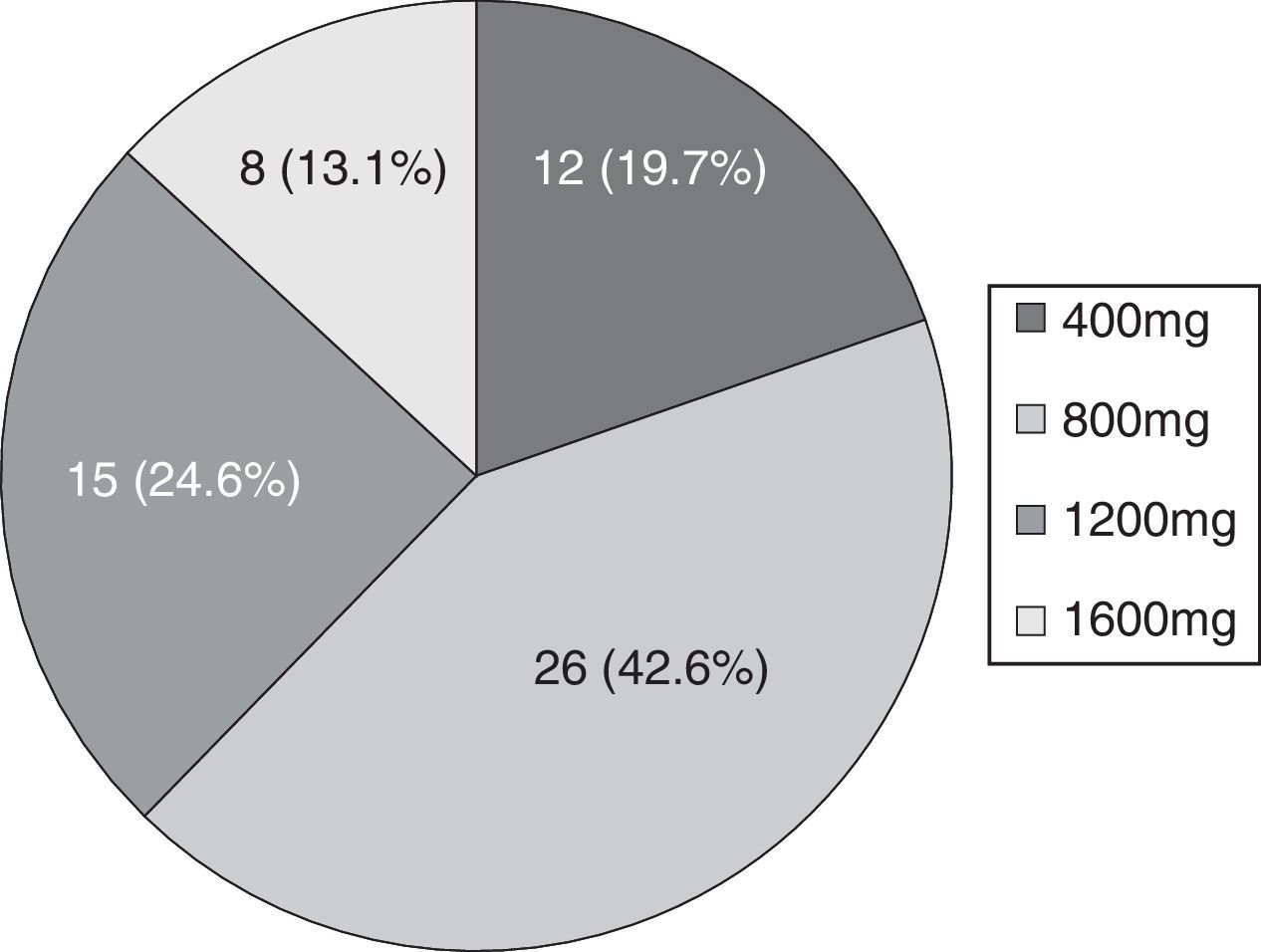
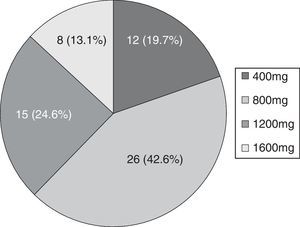
Mean seizure frequency prior to ESL treatment was 5.5 seizures/month (IR 1.13–30). The maximum dose reached in 42.6% of patients was 800mg (see Fig. 1).
Change in seizure frequency with eslicarbazepineThe mean follow-up period for patients taking ESL was 4.7±3.2 months.
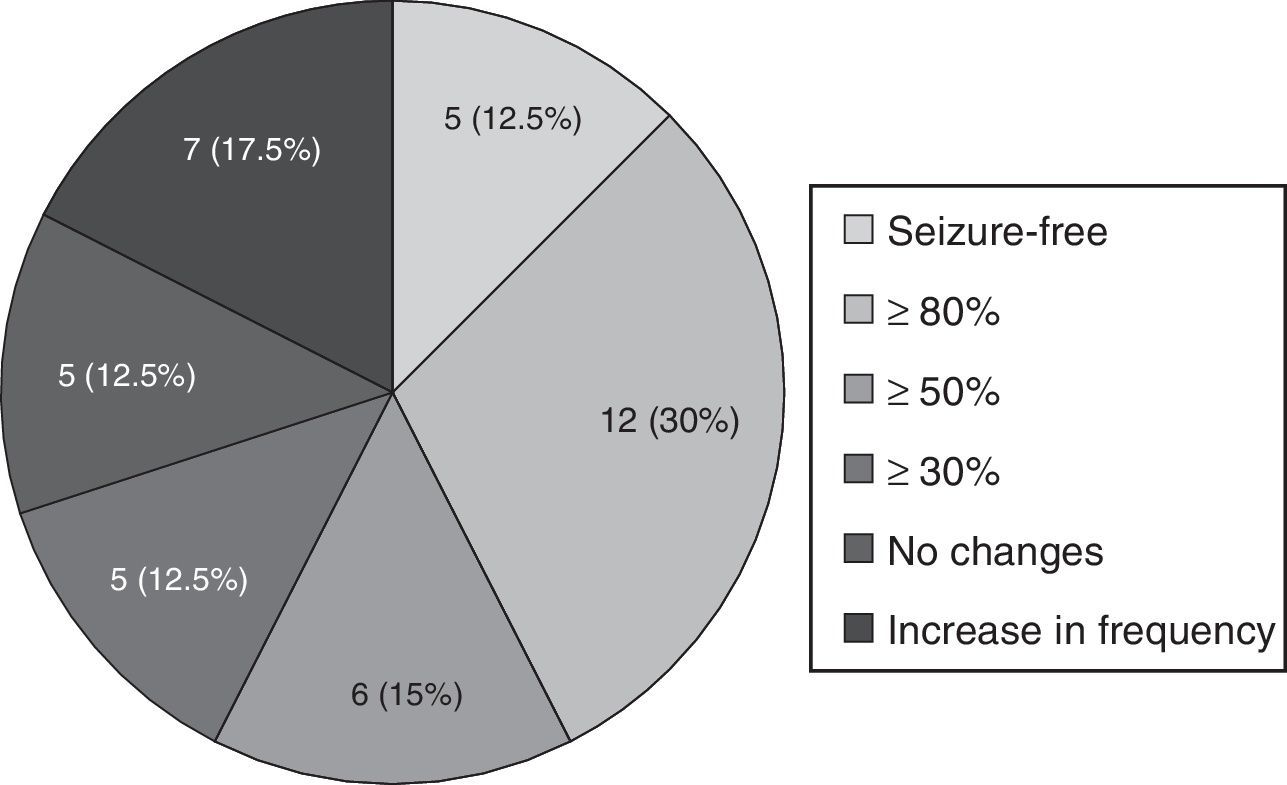
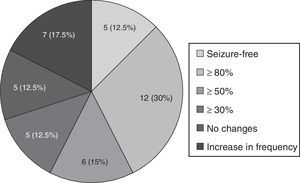
Forty patients completed the minimum required follow-up period of 3 months; in this group, mean follow-up time was 6.53±2.49 months. The mean reduction in monthly seizures was 63.6% (from 5.5 [IR 1.5–30] to 2 [IR 1–5.37]; P<.001). Five patients (12.5%) achieved seizure freedom; 12 (30%) experienced a decrease in seizure frequency of at least 80%; and 6 (15%) experienced a decrease of at least 50%, as shown in Fig. 2. Another 5 patients (12.5%) presented no changes and 7 (17.5%) experienced an increase in seizure frequency. In patients who did not achieve seizure freedom, seizure-free days increased from 5.09 (IR 0.9–18.7) to 9.33 (IR 4.3–18.7), P=.018.
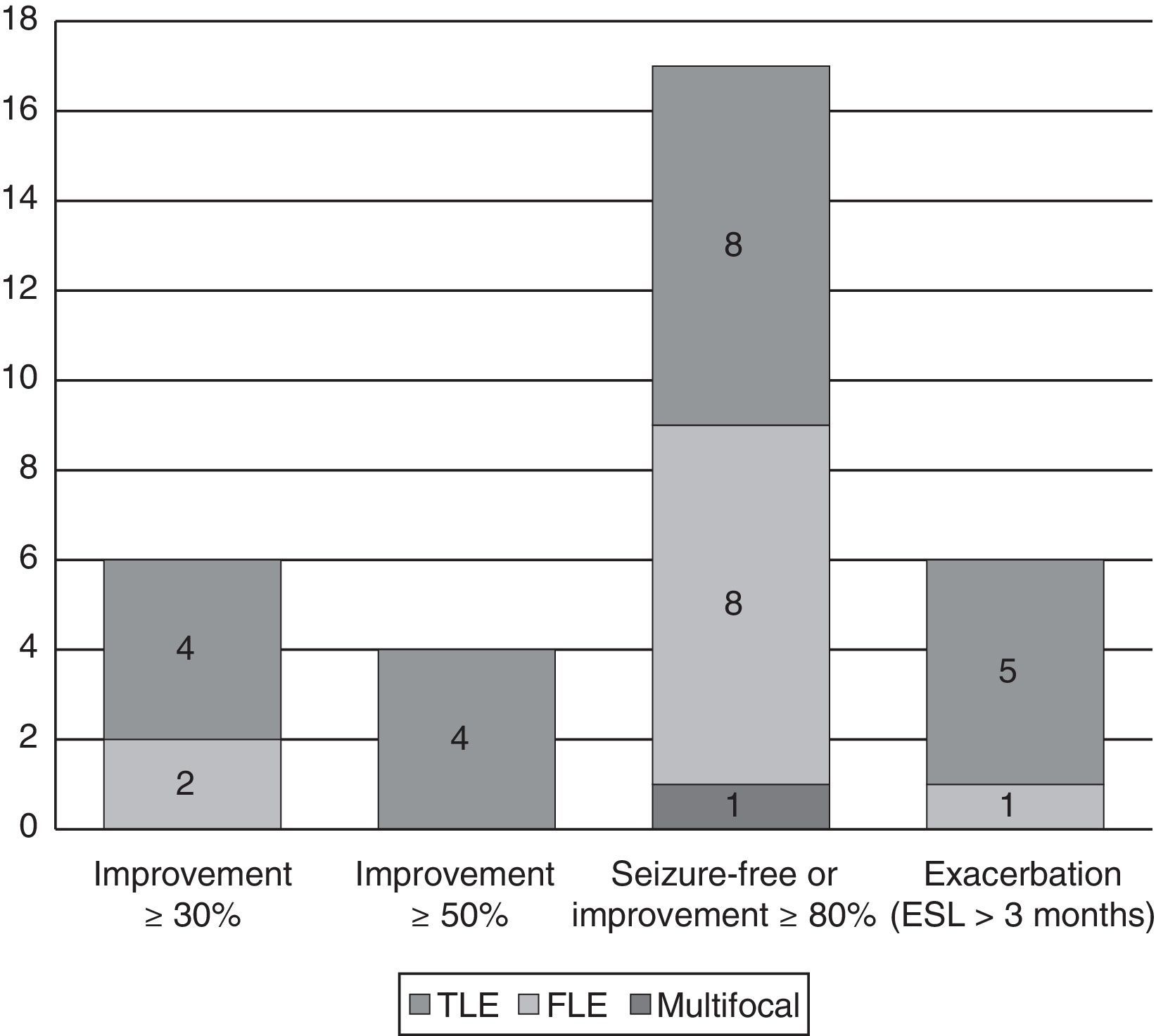
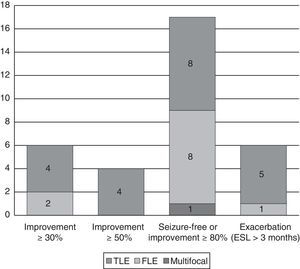
Changes in seizure frequency by epilepsy typeEpilepsy types with significant improvementOf the 17 patients (42.5%) who achieved seizure freedom or a decrease in seizures of more than 80%, 11 (64.7%) had symptomatic epilepsy (7 with TLE and 4 with frontal lobe epilepsy [FLE]); epilepsy was secondary to trauma in 4, to hippocampal sclerosis in 3, to dysplasia in 1, to cerebrovascular damage in 1, and to central nervous system infection in 2. There was also 1 case of multifocal epilepsy due to a pachygyria-type neuronal migration disorder. In the remaining 5 patients, the cause was unknown.
Epilepsy types with exacerbationThe 7 patients whose seizure frequency increased included 1 case of juvenile myoclonic epilepsy that had initially and erroneously been diagnosed as focal epilepsy; 1 case of autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE); and 5 of TLE (one due to hippocampal sclerosis, 1 to dysplasia, and 3 of unknown causes). In another 6 patients who experienced an increase in seizure frequency, ESL was suspended before the 3-month mark. This group included 1 case of progressive myoclonic epilepsy with frontal seizures, 1 case of cryptogenic Lennox–Gastaut syndrome (LGS) with multifocal seizures, 2 cases of FLE (1 secondary to perinatal injury and the other, cryptogenic), and 2 cases of TLE (1 due to hippocampal sclerosis; the other, insular and cryptogenic) (see Fig. 3).
Laboratory resultsAbnormal laboratory results that had not been present in previous studies were detected in 11 patients (18%). These results included 4 cases of hyponatraemia (6.6%) (range of 128–132 mEq/l) in which none of the patients was taking CBZ or OXC; augmented liver enzymes in 4 cases (6.6%) (glutamic-oxaloacetic transaminase range, 42–110 and gamma-glutamyl transpeptidase range, 68–331); and high cholesterol (range, 207–235mg/dl) and triglycerides (range, 221–359mg/dl) in another 3 cases. In no cases was ESL suspended for those reasons. Lastly, one patient experienced resolution of the hyponatraemia he experienced with OXC (from 128 to 138mmol/L); another maintained the same level of hyponatraemia, 133mmol/L, as with the previous OXC treatment.
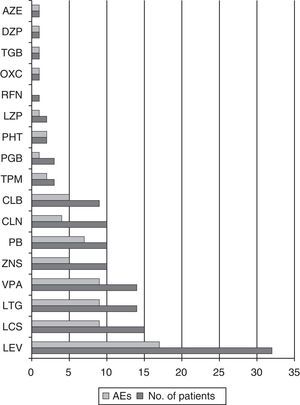
Antiepileptic drugs associated with eslicarbazepineThe antiepileptic drugs most frequently used in association with ESL were LEV in 32 patients (52.5%), LCS in 15 (24.6%), LTG in 14 (22.9%), and VPA in 14 (22.9%). The median number of adjuvant antiepileptic drugs was 2 (IR 1–3); most patients were treated with either 1 (29.5%) or 2 (32.8%). Of the 17 patients with seizure freedom or an 80% decrease in seizure frequency, 8 were taking LEV (47%), 7 LCS (41.2%), 6 VPA (35.3%), and 4 LTG (23.5%).
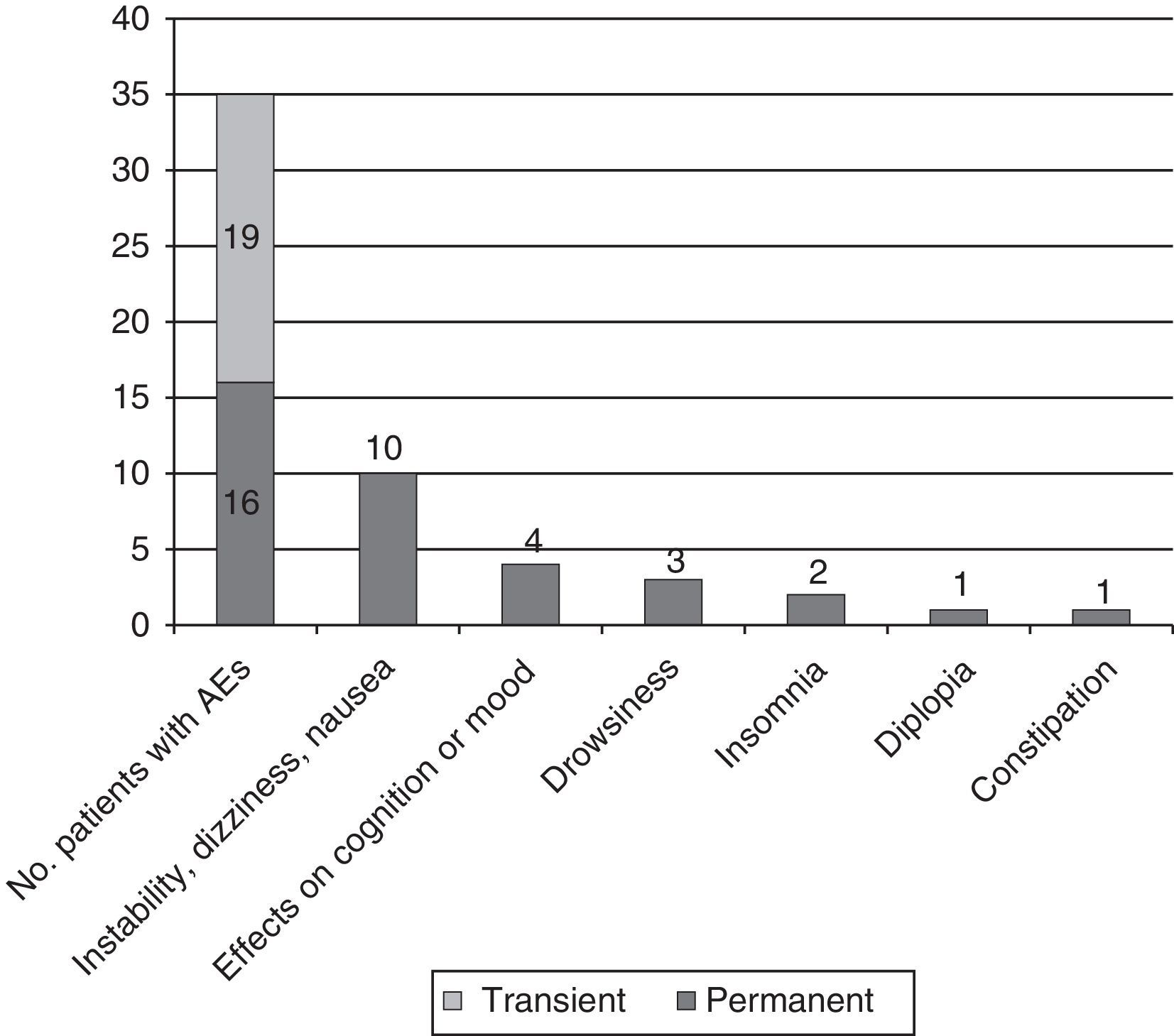
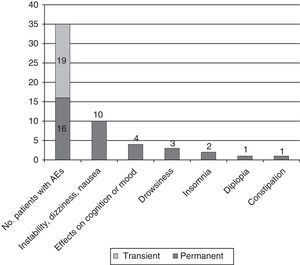
Adverse events: association with doses of eslicarbazepine and adjuvant antiepileptic drugsAEs were recorded in 35 patients (57.4%); the most common were instability, dizziness, and nauseas, experienced by 21 patients (34.4%); cognitive and mood changes in 6 (9.8%; 3 [4.9%] with depressed mood and 3 [4.9%] with anxiety/irritability, including 1 experiencing difficulty concentrating); and sleep disorders in 10 (16.4%; 7 cases [11.5%] of drowsiness and 3 cases [4.9%] of insomnia). Cases of insomnia improved by moving the ESL dosing time forward.
We should also mention 2 cases of exanthematic cutaneous reactions (1 severe reaction appearing as a rash with angiooedema and 1 mild case of pruriginous erythema).
AEs appearing during the dose titration period were transient in 54.3% of cases and persistent in the rest. In 6 cases (9.8%), they motivated the decision to suspend treatment (4 due to dizziness and nausea; 2 due to rash) (see Fig. 4).
Treatment was also suspended in another 8 patients due to lack of effectiveness or increased seizure frequency; the final retention rate was 75.4%.
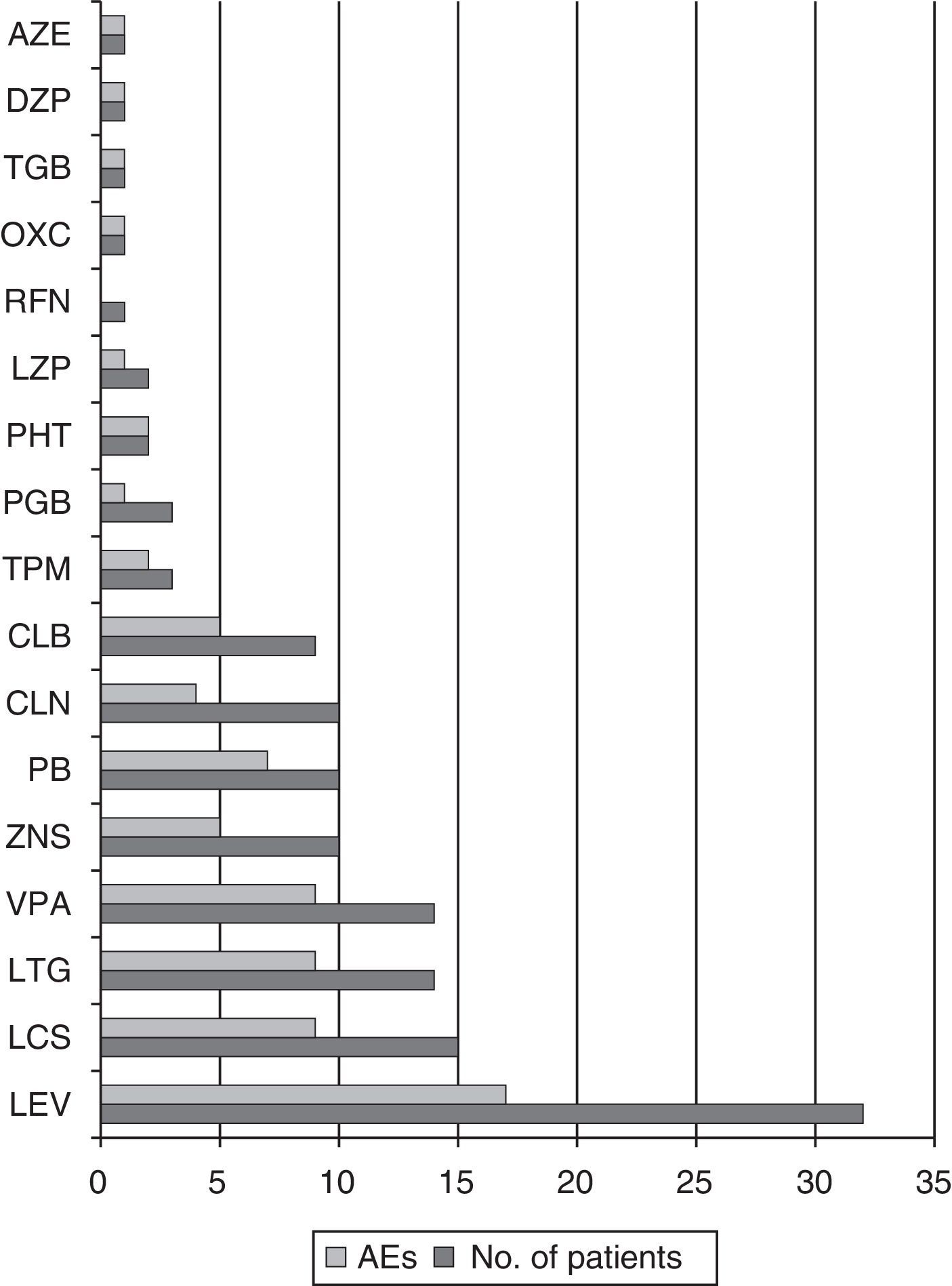
Of the patients taking ESL dosed at 400mg, 41.7% presented AEs; this percentage was 50% among those taking an 800mg dose, 80% among those taking an 1200mg dose, and 62.5% of those on a 1600mg dose. A total of 73.9% of the patients on doses higher than 800mg presented AEs, while 47.4% of those taking doses of 800mg or less experienced AEs (OR 3.15; CI 95%, 1.02–9.72; P=.042). Among the most commonly used antiepileptic drugs, PB elicited AEs in 70% of the patients, VPA in 64.3%, LTG in 64.3%, LCS in 60%, and LEV in 53.1% (see Fig. 5).
We observed no statistically significant differences in percentage of AEs according to the adjuvant antiepileptic drug in the univariate analysis. However, patients treated with VPA, LTG, and/or LCS demonstrated a greater tendency towards presenting instability, dizziness, and nausea (45.7% vs 19.2%, OR 3.5; CI 95%, 1.09–11.5; P=.031). Of the 6 patients whose complaints had to do with cognition or mood, 4 were treated with LEV, and in this group, 2 reported depressed mood, 1 reported nervousness and irritability, and 1, difficulty concentrating.
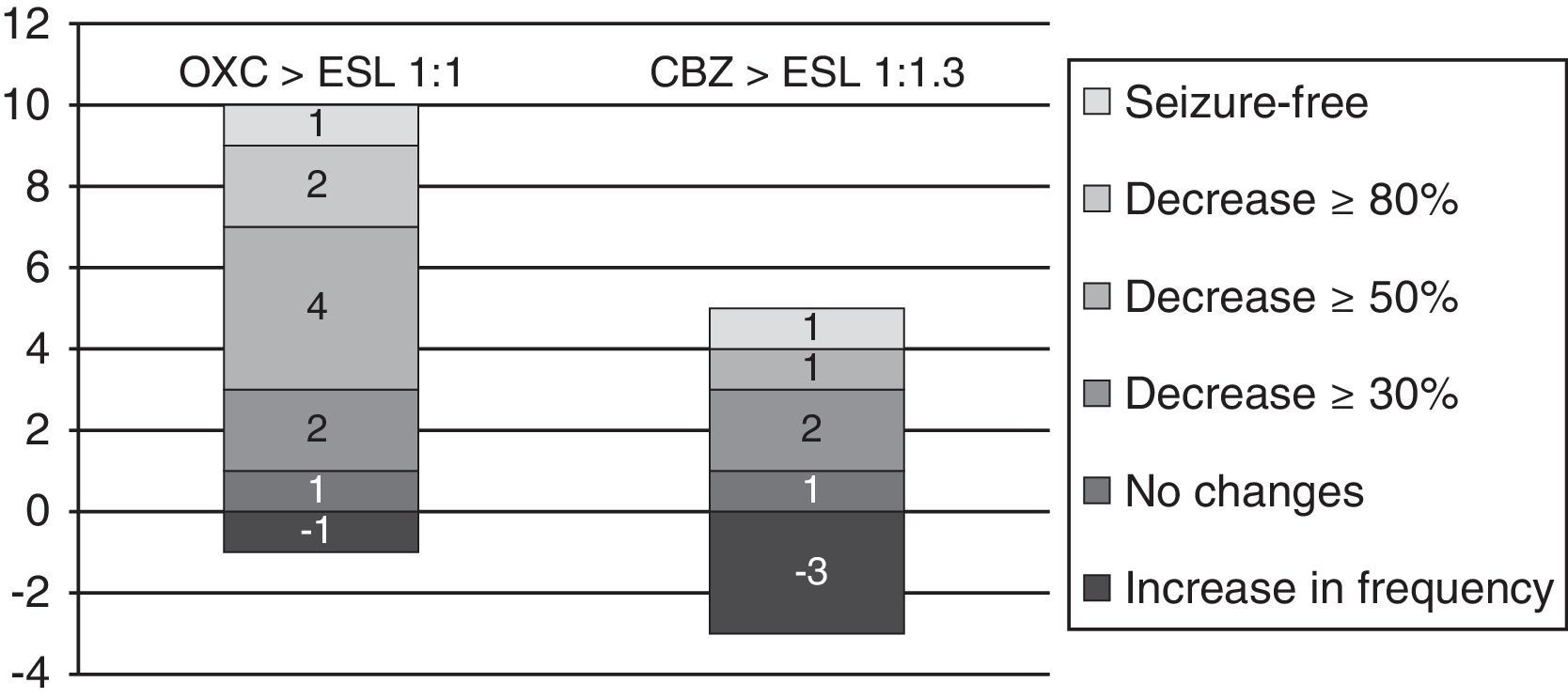
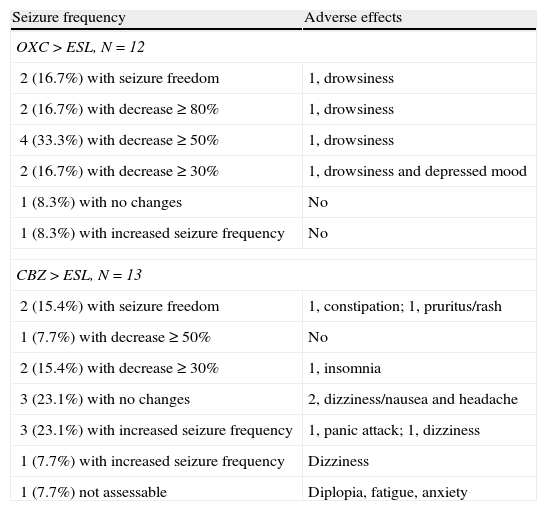
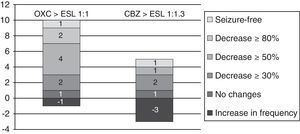
From oxcarbazepine/carbamazepine to eslicarbazepineTwelve patients (19.7%) switched from oxcarbazepine to eslicarbazepine and 13 patients (21.3%) changed from carbamazepine to eslicarbazepine according to the protocol described here. Of this total, 19 patients were monitored during at least 3 months. In this group, the median decrease in monthly seizures was 20% (from 2.5 [IR 1.5–8] to 2 [IR 0.5–5]; P<.075).
By groups, 11 of the 12 patients who switched from OXC to ESL had a follow-up period of at least 3 months; in these patients, median decrease in seizures per month was 66.7% (from 1.5 [IR 1.5–8] to 0.5 [IR 0.5–4.5]; P=.017). One patient (9%) achieved seizure freedom; 2 (18%) presented a decrease in seizures of ≥80%; 4 (36.4%) presented a decrease of ≥50%; 1 (9%) experienced no changes; and 1 (9%) experienced an increase in seizure frequency.
Likewise, 8 of the 13 patients who switched from CBZ to ESL were monitored longer than 3 months. In these 8 patients, the median number of monthly seizures increased by 33.3% (from 2.25 [1.25–5.38] to 3 [1.25–26-5]; P=.93). One patient (12.5%) remained seizure-free; 1 (12.5%) experienced a decrease ≥50% in the number of seizures; 1 (12.5%) had no changes; and 3 (37.5%) experienced an increase in seizure frequency (see Fig. 6).
After the switch, 4 of the patients formerly on OXC experienced AEs (drowsiness in all cases, with symptoms of depression also present in one case). AEs also affected 8 patients previously treated with CBZ (4 cases of dizziness and nausea, 2 of anxiety, 1 of insomnia, 1 of constipation, and 1 of pruritic rash). AEs were present in 69.2% and 33% of each group, respectively (OR 4.5; CI 95%, 0.84–24.18; P=.073). In all cases, AEs were transient. On the other hand, 2 patients reported that the AEs they experienced with OXC (dizziness and drowsiness) improved with the switch to ESL (see Table 2).
Variation in seizure frequency and appearance of AEs after switching from OXC/CBZ to ESL at equivalent doses of 1:1mg and 1:1.3mg respectively in all patients.
| Seizure frequency | Adverse effects |
| OXC>ESL, N=12 | |
| 2 (16.7%) with seizure freedom | 1, drowsiness |
| 2 (16.7%) with decrease≥80% | 1, drowsiness |
| 4 (33.3%) with decrease≥50% | 1, drowsiness |
| 2 (16.7%) with decrease≥30% | 1, drowsiness and depressed mood |
| 1 (8.3%) with no changes | No |
| 1 (8.3%) with increased seizure frequency | No |
| CBZ>ESL, N=13 | |
| 2 (15.4%) with seizure freedom | 1, constipation; 1, pruritus/rash |
| 1 (7.7%) with decrease≥50% | No |
| 2 (15.4%) with decrease≥30% | 1, insomnia |
| 3 (23.1%) with no changes | 2, dizziness/nausea and headache |
| 3 (23.1%) with increased seizure frequency | 1, panic attack; 1, dizziness |
| 1 (7.7%) with increased seizure frequency | Dizziness |
| 1 (7.7%) not assessable | Diplopia, fatigue, anxiety |
Patients switching from OXC to ESL (mean follow-up time of 5.25±2.3) had a 100% retention rate; the rate was 69.2% for patients switching from CBZ to ESL (mean follow-up of 3.96±3.02) (OR 1.4; CI 95%, 1.01–2.08; P=.096). The differences between follow-up periods between these 2 groups and with respect to the patient total were not statistically significant.
DiscussionThis study showed a high percentage of seizure reduction and seizure freedom (63.6% and 12.5%, respectively) during the initial period of using ESL as an adjuvant treatment for drug-resistant focal epilepsies with different aetiologies. This does not rule out the possibility of a subsequent exacerbation due to the patient acquiring tolerance to the drug. However, data from extension studies show similar results, with a decrease in seizure frequency in 40.6% to 58% of patients at one year of follow-up.7–9 One of this study's limitations is that its definition of seizure freedom identifies patients who were seizure-free during more than 3 months and who had experienced more than one seizure per month during the entire preceding year. However, the short follow-up period does not allow us to rule out recurrences in periods exceeding one year, as would also be required by the official ILAE definition.13
Additionally, based on observations of specific cases of off-label use, ESL seems to demonstrate a similar effect to that of CBZ, involving exacerbation of idiopathic types of epilepsy with absence and myoclonic seizures. There was also an increase in seizure frequency in one patient with ADNFLE.17
AEs were mostly mild or moderate, and also transient, during the titration phase, and they were dependent on the final ESL dose. As was described in authorisation studies, the most common AEs were instability and dizziness (34.4%) and drowsiness (11.5%). Regarding other recorded AES, such as insomnia (4.9%), anxiety (4.9%), and depressed mood (4.9%),6–9 we should state that in open-phase studies, patients improved significantly in the areas of depression, quality of life, and cognitive function on the Montgomery-Åsberg and QOLIE-31 scales.12,18–21 Our study did not use these scales. We also recorded 2 cases of exanthematic allergic reactions, one of which affected a patient who had previously been taking CBZ.22 The withdrawal rate due to AE (WDAE) was 9.8%, which is within the upper limit of the extension studies in which rates ranged from 3.5% to 11.4%.7–9
Despite the very low frequency of hyponatraemia in pivotal studies and long-term open observation studies,4,5,10–12,23,24 we did observe decreased sodium levels in 6.6% of our patients, although no values were below 125mmol/L. As a result, this condition did not motivate discontinuing the drug, except in one case in which treatment was associated with dizziness and instability. Furthermore, one patient's sodium concentration normalised after the switch from OXC to ESL. Although these cases of hyponatraemia are rarely clinically relevant, as is the case with CBZ and OXC treatment, laboratory testing is recommended.
ESL may be effective in antiepileptic drug resistance with a similar action mechanism on voltage-gated sodium channels, since VGSCs in these patients may have altered protein subunits and therefore decreased sensitivity to some specific antiepileptic drugs.25 In these patients, VGSCs may respond better to different sodium-channel antagonists, such as ESL. Murine models also yield evidence that ESL may delay the appearance of induced activation or kindling,26 an effect that does not occur with CBZ. While only a small patient sample was analysed after the switch from a previous treatment with CBZ or OXC, we did observe significant improvement in seizure control and fewer AEs among patients changing from OXC to ESL. The data we present here have a practical impact, since they show that a direct, one-night transition from OXC to ESL in a 1:1 proportion may be beneficial. Although ESL has a half-life of 20 to 24hours and 4 to 5 days are needed to achieve stable doses of its metabolites in the blood, the total concentration of monohydroxy derivatives (S- and R-licarbazepine) does not change when a patient switches from OXC. Significant loss of efficacy is therefore not a concern. Furthermore, the higher percentage of S-licarbazepine, which crosses the blood-brain barrier in a 2:1 ratio compared to R-licarbazepine, indicates that efficacy of ESL may be greater.10 There were 2 cases in which AEs improved (dizziness and drowsiness) after switching from OXC to ESL. This fact may be linked to data showing that unmetabolised OXC, which makes up approximately 20% of the dose in blood, is more neurotoxic than ESL or CBZ.27,28
In contrast, no significant changes were observed after switching from CBZ to ESL at a 1:1.3 equivalence ratio. The explanation could be due to differences in CBZ and ESL metabolism (especially with regard to the oxidative metabolism of CBZ, which generates an epoxide metabolite, unlike the enzymatic conjugation occurring with ESL).
In addition, we have discovered a surprisingly higher incidence of AEs in patients switching from CBZ to ESL, despite the theoretical decrease in toxic epoxide metabolites.
The final retention rate of patients taking ESL was high (75.4%) and reached 100% in patients previously on OXC vs 69.2% in patients previously treated with CBZ.
Although the analogous chemical structures and similar VGSC blocking mechanisms between CBZ/OXC and ESL seem to indicate that the switch can be performed directly in a single night, this process has not been studied in randomised trials comparing a gradual, one-week transition to a rapid, one-night switch. This being the case, questions remain about which patient groups treated with CBZ may benefit from a rapid switch and which will not, especially when patients are on polytherapy. One relevant factor is the degree to which self-induction of metabolism varies among patients on long-term CBZ treatment. A rapid switch may provoke AEs in patient groups with more pronounced self-induction, which is accompanied by decreased tolerance. This may partially explain the lower retention rate observed among patients in our sample who switched from CBZ to ESL.
Another aspect that was not specifically evaluated was the safety of this rapid switch. As stated before, ESL will take 4 to 5 days to reach a therapeutic dose. Therefore, abrupt suspension of CBZ may theoretically entail loss of protection for some 24 to 48hours. Nevertheless, the total effect of the decreasing CBZ metabolites may be added to that of the increasing monohydroxy derivatives (S- and R-licarbazepine) without any loss of efficacy, as we see in patients switching from CBZ to OXC. In this study, the shorter follow-up times and lower retention observed in patients switching from CBZ was also due in part to more discontinuation of treatment due to increased seizure frequency. However, these factors lie outside the scope of the present article and should be examined in depth by other studies.
Some of our patients were treated with doses that were higher than drug leaflet recommendations. The underlying premise was that if tolerance is good, administering higher doses is not only possible, but also desirable in order to achieve seizure freedom in very drug-resistant types of epilepsy. At the same time, despite the fact that administering a single daily dose is more comfortable, using 2 daily doses is a reasonable option because it is compatible with other antiepileptic drugs. Moving the dosing schedule forward is also an option in patients with sporadic insomnia.
Regarding other antiepileptic drugs used in association with ESL, LEV has a good adverse effect profile. In contrast, PB, VPA, and drugs with a similar action mechanism on VGSCs, such as LCS or LTG, present a higher frequency of such AEs as instability, dizziness, and nausea.
In conclusion, in a preliminary experience with ESL in patients with drug-resistant epilepsy, we observed a significant decrease in seizure frequency and a good tolerability profile. AEs were dose-dependent and reflected the action mechanisms of adjuvant antiepileptic drugs. Treatment elicited slight decreases in blood sodium level, and doctors had to pay close attention to the appearance of skin reactions. We determined that a direct, one-night switch from OXC to ESL at equivalent or slightly lower doses was safe and effective, but that switching from CBZ was both less effective and more difficult. This may call for a more gradual approach to drug switching. We do not recommend using ESL in association with antiepileptic drugs with similar profiles, such as LCS or LTG, as this practice is linked to a higher frequency of AEs. Associating ESL with LEV seems to be beneficial in this respect.
Conflicts of interestThe authors have no conflicts of interest to declare.
This article was presented as an oral communication at the 63rd Annual Meeting of the Spanish Society of Neurology. It has not received funding from any public or private institutions.
Please cite this article as: Massot A, Vivanco R, Principe A, Roquer J, Rocamora R. Estudio postautorización de la eslicarbazepina en el tratamiento de epilepsias farmacorresistentes: resultados preliminares. Neurología. 2014;29:94–101.