The COVID-19 pandemic is changing approaches to diagnosis, treatment, and care provision in multiple sclerosis (MS). During both the initial and peak phases of the epidemic, the administration of disease-modifying drugs, typically immunosuppressants administered in pulses, was suspended due to the uncertainty about their impact on SARS-CoV-2 infection, mainly in contagious asymptomatic/presymptomatic patients. The purpose of this study is to present a safety algorithm enabling patients to resume pulse immunosuppressive therapy (PIT) during the easing of lockdown measures.
MethodsWe developed a safety algorithm based on our clinical experience with MS and the available published evidence; the algorithm assists in the detection of contagious asymptomatic/presymptomatic cases and of patients with mild symptoms of SARS-CoV-2 infection with a view to withdrawing PIT in these patients and preventing new infections at day hospitals.
ResultsWe developed a clinical/microbiological screening algorithm consisting of a symptom checklist, applied during a teleconsultation 48 h before the scheduled session of PIT, and PCR testing for SARS-CoV-2 in nasopharyngeal exudate 24 h before the procedure.
ConclusionThe application of our safety algorithm presents a favourable risk-benefit ratio despite the fact that the actual proportion of asymptomatic and presymptomatic individuals is unknown. Systematic PCR testing, which provides the highest sensitivity for detecting presymptomatic cases, combined with early detection of symptoms of SARS-CoV-2 infection may reduce infections and improve detection of high-risk patients before they receive PIT.
La pandemia por SARS-CoV-2 está condicionando los abordajes diagnósticos, terapéuticos y asistenciales establecidos en esclerosis múltiple (EM). Durante las fases inicial y pico de la epidemia, los fármacos modificadores del curso de la EM caracterizados por ser inmunosupresores administrados en pulsos (TIP), vieron pospuesta su administración debido a la incertidumbre sobre su influencia en la infección, principalmente en contagiosos asintomáticos/presintomáticos. El objetivo de este trabajo es presentar un algoritmo basado en criterios de seguridad que permita reanudar los TIP durante la fase de desescalado.
MetodosSe elabora un algoritmo, cuya estructura se sustenta en la experiencia clínica en EM de los autores y en una revisión bibliográfica del conocimiento acumulado, que facilita la detección de contagiosos asintomáticos, presintomáticos o con síntomas leves de SARS-Cov-2, con el objetivo de evitar la administración de TIP y contagios por contacto prolongado en hospital de día (HdD).
ResultadosAlgoritmo con doble filtro clínico-microbiológico consistente en la aplicación telemática de un listado de comprobación de síntomas y después realización de PCR para SARS-CoV-2 en exudado nasofaríngeo, a las 48 y 24 horas antes del TIP programado respectivamente.
ConclusiónConsiderando el balance beneficio-riesgo, la aplicación del algoritmo resultaría ventajosa pese a que no se conoce la proporción real de asintomáticos/presintomáticos contagiosos. La realización sistemática de PCR, como test con mayor sensibilidad en la fase presintomática de la infección, en combinación con un sistema de detección precoz de síntomas, reduciría contagios y favorecería la identificación de pacientes con riesgo antes de su exposición a TIP.
The COVID-19 pandemic that began in early 2020 represents a challenge for healthcare systems worldwide. All healthcare levels have been affected, from emergency and intensive care departments to hospital and outpatient healthcare services. This situation has led to an urgent reorganisation of medical care and a change in the direct relationship with the patient.
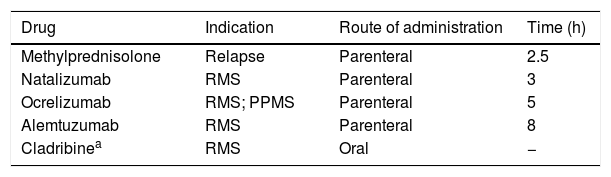
Multiple sclerosis (MS) units have been no exception, and telemedicine has become a very useful strategy to reduce in-person consultations and limit the risk of infection. However, many interventions performed at MS units require the physical attendance of patients; these include the parenteral (and oral) administration of pulse immunosuppressive therapy (PIT), especially at day hospitals (Table 1). Using immunosuppressants while maintaining adequate safety measures represents a true challenge and a test of the Spanish healthcare system’s capacity for adaptation.
Drugs used in pulse immunosuppressive therapy for multiple sclerosis.
| Drug | Indication | Route of administration | Time (h) |
|---|---|---|---|
| Methylprednisolone | Relapse | Parenteral | 2.5 |
| Natalizumab | RMS | Parenteral | 3 |
| Ocrelizumab | RMS; PPMS | Parenteral | 5 |
| Alemtuzumab | RMS | Parenteral | 8 |
| Cladribinea | RMS | Oral | − |
PPMS: primary progressive multiple sclerosis; RMS: recurrent multiple sclerosis.
Given the current lack of scientific evidence, we must consider immunosuppressed patients as a high-risk population in the event of SARS-CoV-2 infection.1 However, prior experience with such other coronaviruses as SARS-CoV and MERS-CoV and the limited data available on SARS-CoV-2 infection in immunosuppressed patients do not currently suggest more severe symptoms in the event of infection.2 In any case, this situation poses a dilemma regarding the risk-benefit ratio of PIT.
Administration of PIT during the initial and peak stages of the pandemic was influenced by the lack of information on the prevalence of contagious, asymptomatic/presymptomatic carriers of the virus and the limited access to diagnostic tests. On the positive side, adherence to lockdown measures among MS patients in general (as in the general population), and immunosuppressed patients (considered a high-risk population) in particular, may have reduced the number of infections; this situation may change during the de-escalation phase.
During these stages, given the uncertainty about the consequences of SARS-CoV-2 infection in patients receiving PIT and the recommendations of several expert groups, the strategy used by many MS units has been to postpone the onset and administration of PIT, awaiting the end of the pandemic, in order to minimise the risk of infection of patients visiting hospital.3–5
Considering that lockdown measures are now being eased, and that the available experience does not allow us to expect an end to the pandemic in the short term, the strategy of delaying administration of PIT indefinitely is not acceptable due to the high risk of MS reactivation6–8; therefore, a change of strategy is needed.
To do this, we developed an algorithm based on safety criteria, the main aim of which is to detect asymptomatic, presymptomatic, and mildly symptomatic patients with SARS-CoV-2 infection before scheduled PIT sessions.
Objectives- 1
To avoid administering PIT to an unidentified infected patient/contagious asymptomatic patient (with active infection) or presymptomatic patient (in the incubation period).
- 2
To reduce the risk of infection of patients receiving PIT at day hospitals due to prolonged contact with an unidentified infected individual or contagious asymptomatic/presymptomatic individual.
- 3
To avoid these 2 situations in patients with mild symptoms suggestive of active SARS-CoV-2 infection.
The algorithm is based on our own clinical experience in the management and treatment of MS, the knowledge acquired during the COVID-19 pandemic, and a literature review of studies published on each topic during the pandemic.
We present an analysis of the rationale underpinning the inclusion of each objective and its fulfilment in the algorithm.
Objective 1: to avoid administering PIT to an unidentified infected patient/contagious asymptomatic patient (with active infection) or presymptomatic patient (in the incubation period).
- -
No clinical or experimental information or evidence is available on whether PIT may facilitate or exacerbate progression of SARS-CoV-2 infection or whether, to the contrary, it has no influence at all. For example, some studies with infected post-transplant immunosuppressed patients report that infection was not more severe than in the general population; however, the results are controversial.9 Several registries have been created to gather experience and increase understanding of this question.
- -
An empirical approach to the situation, based on the clinical experience with other diseases, would recommend avoiding administration of PIT to asymptomatic/presymptomatic infected patients.10
Objective 2: to reduce the risk of infection in patients receiving PIT at a day hospital due to prolonged contact with an unidentified infected individual/contagious asymptomatic/presymptomatic individual.
- -
Although the prevalence of contagious, asymptomatic/presymptomatic carriers of the virus is unknown, cases of infection in the presymptomatic stage/incubation period have been reported.11–13 Experience from reported cases seems to indicate that, in mild cases, transmission of infection mainly occurs in the first week after symptom onset, from 1 to 2 days before to 5–6 days after. Patients with more severe symptoms are thought to be more contagious, and for a longer period.14,15
- -
Restricting the number and duration of visits to healthcare facilities to decrease the risk of SARS-CoV-2 infection16,17 is not applicable to patients requiring PIT administration at day hospitals. However, patients may benefit from physical distancing measures and appointment scheduling.
- -
Considering that prolonged sessions are frequently necessary for PIT administration, the risk of susceptible individuals (other patients and healthcare staff) being infected by contagious asymptomatic/presymptomatic individuals should be reduced by avoiding their presence at day hospitals.
Objective 3: to avoid these 2 situations in patients with mild symptoms suggestive of active SARS-CoV-2 infection.
- -
Before the pandemic, the administration of PIT in patients with banal viral infections, even with mild symptoms (low-grade fever, headache) was already delayed in clinical practice. There is even greater justification for applying this reasoning to patients with mild symptoms, who should not attend a day hospital until infection with SARS-CoV-2 is ruled out.
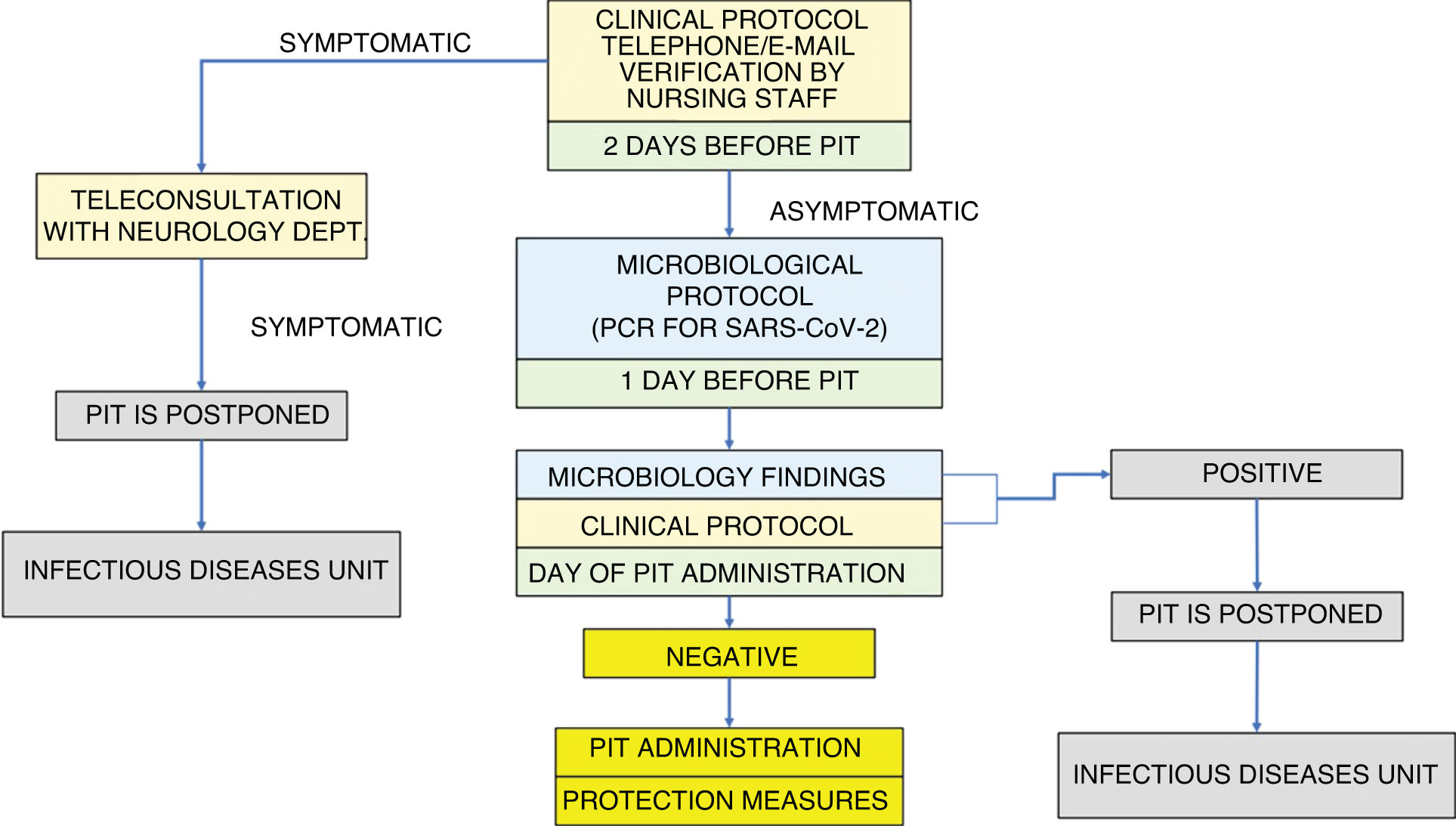
Our algorithm is structured around the application of a double screening: first a clinical and then a microbiological screening (Fig. 1).
Absence of symptoms compatible with SARS-CoV-2 infection is verified by telephone or e-mail, using a symptom checklist, for all patients scheduled to receive PIT. As nursing staff are responsible for administering PIT, and given their significant role in MS units, they are probably the best qualified professionals to conduct this check. The clinical protocol is applied 48 h before and on the day of PIT session (Fig. 1).
Symptom checklist:
- -
Are you experiencing or have you experienced the following symptoms over the past 2 weeks?
▪Throat pain
▪Persistent cough
▪Diarrhoea
▪Anosmia
- -
Have you been in contact with anybody with confirmed COVID-19 over the past month?
- -
Does any member of your family have fever or any symptom of infection?
Outcomes after the application of the clinical protocol:
▪Symptomatic patient/suspected active infection: if infection is medically confirmed, PIT is postponed. Assessment by the infectious diseases unit
▪Asymptomatic patient: application of the microbiological protocol
- -
1) The day before PIT (24 h before):
▪PCR test for SARS-CoV-2 in nasopharyngeal exudate
- -
2) The day scheduled for the PIT session: verification of microbiology findings:
▪Negative result: patient may pass to the treatment room with protection measures (surgical mask and hand sanitiser)
▪Positive result: PIT is postponed. Assessment by the infectious diseases unit
Our protocol proposes a double screening, with initial clinical telephone verification as triage for patients scheduled to receive PIT, with the aim of identifying mildly symptomatic patients. This enables us to channel patients to the systems established for clinical management of SARS-CoV-2 infection and to avoid appointments or treatments with potentially contagious patients.18
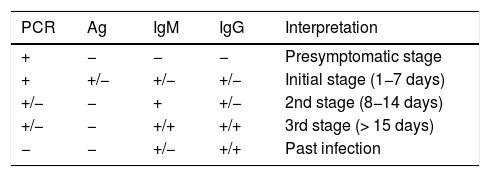
The exact sensitivity and specificity of PCR tests for SARS-CoV-2 in asymptomatic and presymptomatic individuals is unknown, but different studies seem to suggest that this is the test with the highest diagnostic yield in this stage (Table 2).19 One study suggests that jointly performing a PCR test and a SARS-CoV-2 IgM ELISA test significantly increases sensitivity (up to 98.6%) in very early stages in symptomatic and asymptomatic patients.20
Interpretation of diagnostic tests in all stages of SARS-CoV-2 infection.
| PCR | Ag | IgM | IgG | Interpretation |
|---|---|---|---|---|
| + | − | − | − | Presymptomatic stage |
| + | +/− | +/− | +/− | Initial stage (1−7 days) |
| +/− | − | + | +/− | 2nd stage (8−14 days) |
| +/− | − | +/+ | +/+ | 3rd stage (> 15 days) |
| − | − | +/− | +/+ | Past infection |
Ag: viral antigen detection test; IgG: IgG antibody detection test; IgM: IgM antibody detection test; PCR: polymerase chain reaction.
Modified from: Carlos III Health Institute.19
In Spain, testing for SARS-CoV-2 infection frequently involves at least 2 types of diagnostic tests: PCR and rapid antibody tests.21 The latter is able to detect active infection of several days of progression, especially from the 7th day, so it would not be useful for detecting asymptomatic or presymptomatic cases. Availability of routine serology tests that discriminate between IgM and IgG antibodies would enable us more precisely to diagnose the stage of infection. The degree and duration of immunity after infection remain unknown.
The COVID-19 pandemic has given rise to unprecedented situations that represent a challenge for care provision at MS units. During the early and peak stages of the pandemic, delaying the onset and administration of scheduled PIT sessions was inevitable given the epidemiological context of high transmissibility, with an unknown percentage of contagious, asymptomatic/presymptomatic patients (which is currently subject to study) and limited availability of diagnostic tests. Clinical management of this situation was highly complex, as the administration of most of these treatments cannot be interrupted due to a high risk of MS reactivation with relapses and increased disability. In fact, with the aim of postponing doses or increasing intervals between sessions, non-validated biomarkers have been used in patients with MS.22
In these circumstances, the indefinite suspension of PIT administration is not a reasonable option, as the pandemic is not expected to end in the short term. Furthermore, once the peak of the pandemic seemed to have been overcome, measures were introduced to ease the lockdown of the general population. If this process does not meet the recommendations of the healthcare authorities, the risk of infection will be higher. In this context, the objective of our algorithm is to increase the safety of PIT administration, which would reduce the risk for asymptomatic or presymptomatic patients and the risk of infection for other immunosuppressed patients.
There are currently no specific recommendations from clinical or official guidelines for this type of patients; therefore, we propose that this safety algorithm be applied in clinical practice.
Our proposal does present some limitations. The algorithm currently only includes PCR tests, whose results are only useful in the moment before PIT administration. Many PITs create a state of persistent immunosuppression during which patients are susceptible to infection; therefore, in order for this algorithm to be genuinely useful, it should be accompanied by patient education programmes to ensure adherence to protection measures (physical distancing, hygiene, use of face masks, etc) for the duration of the pandemic. Furthermore, we do not know the usefulness in clinical practice of testing populations at risk using this procedure.
Our algorithm may increase understanding of the prevalence and characteristics of SARS-CoV-2 infection in immunocompromised patients with MS. We aim to reassess its usefulness and make modifications based on the progression of the pandemic, and to report the results of its application.
ConclusionThis safety algorithm is intended to help to ensure the continued use of the most adequate therapeutic options on an individual basis. Taking into account the risk-benefit ratio, the application of our algorithm would be beneficial despite the fact that the actual number of contagious, asymptomatic/presymptomatic patients is unknown. Systematic performance of PCR tests, which provide the highest sensitivity for detecting presymptomatic individuals, combined with an early detection system would reduce infections and facilitate the identification of high-risk patients before they receive PIT.
FundingThe authors have received no funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Valero-López G, Carreón-Guarnizo E, Hernández-Clares R, Iniesta-Martínez F, Jiménez-Veiga J, Moreno-Docon A, et al. Tratamiento inmunosupresor en pulsos en esclerosis múltiple durante el desescalado de la epidemia por SARS-CoV-2. Algoritmo de seguridad. Neurología. 2020;35:357–362.