Despite use of currently available anti-epileptic drugs (AEDs), 30% of epilepsy patients are not seizure-free. The purpose of this study was to estimate the quality of life and economic impact in Spain of drug-resistant epilepsy (DRE), as defined by the International League Against Epilepsy (ILAE).
MethodsObservational retrospective 12-month study was conducted in Spain which included adults with focal epilepsy treated with at least two AEDs. Direct costs (€ 2010) were calculated based on health care resources used and their official unit costs. Costs were analysed from the perspectives of the Spanish National Health System (SNS) and society. The impact of DRE on patients’ quality of life was examined using the QOLIE 31-P, EQ-5D-3L, and NDDIE questionnaires.
ResultsWe analysed 263 patients out of the 304 recruited. According to ILAE criteria, 70.0% of the patients had drug-resistant epilepsy, while 20.3% achieved seizure freedom. From the viewpoint of the SNS, annual costs for resistant and seizure-free patients were € 4964 and € 2978 respectively (P<.01). Compared to resistant patients, seizure-free patients showed better scores on QOLIE-31P (70.8 vs 56.4, P<.0001) and EQ-5D-3L (75.6 vs 64.7, P<.001). Seizure-free patients showed a lower incidence of major depression compared to resistant patients according to the NDDIE scale (23% vs 8.3%, P<.05).
ConclusionsResults suggest that DRE is associated with increased use of healthcare resources and consequently with higher costs, poorer quality of life and higher incidence of major depression compared to seizure-free patients, thus representing a considerable burden on the SNS and society.
El 30% de los pacientes con epilepsia no permanece libre de crisis con los fármacos antiepilépticos (FAE) disponibles actualmente. El objetivo del estudio fue estimar el impacto económico y en calidad de vida de la epilepsia resistente a fármacos (ERF) en España, según la definición de la Liga Internacional contra la Epilepsia (ILAE).
MétodosEstudio observacional retrospectivo (12 meses) que incluyó pacientes adultos con epilepsia focal en tratamiento con, al menos, 2 FAE. Los costes sanitarios directos (€, 2010) se cuantificaron a partir de los recursos sanitarios y de sus costes unitarios. El análisis se realizó desde las perspectivas del Sistema Nacional de Salud (SNS) y la sociedad. El impacto de la ERF en la calidad de vida se valoró mediante los cuestionarios QOLIE 31-P, EQ-5D-3L y NDDIE.
ResultadosSe analizaron 263 pacientes (304 seleccionados). El 70,0% tenía ERF y el 20,3% estaban controlados. Bajo la perspectiva del SNS el coste anual por paciente con ERF y por paciente controlado fue de 4.964 y 2.978 €, respectivamente (p<0,01). En relación con los pacientes con ERF, los pacientes controlados presentaron mejores puntuaciones en el QOLIE-31P (70,8 vs 56,4, p<0,0001) y EQ-5D-3L (75,6 vs 64,7, p<0,001), y menor incidencia de depresión mayor según la escala NDDIE (23 vs 8,3%, p<0,05).
ConclusionesEn relación con la epilepsia controlada, la ERF se asocia a mayor consumo de recursos y costes, peor calidad de vida y mayor incidencia de depresión mayor, resultando, por tanto, en una considerable carga para el SNS y la sociedad.
Epilepsy is one of the most frequent chronic neurological diseases.1 In Europe, epilepsy affects 6 million people and 15 million people may experience an epileptic seizure at some point in their lives.2 In Spain, it is estimated that between 360000 and 400000 people are affected by this disease.3 Focal or partial epilepsy accounts for approximately 70% of all cases of epilepsy.4
Conventional treatment for epilepsy is based on long-term administration of antiepileptic drugs (AED). Although polytherapy with antiepileptic drugs results in long-term seizure remission in most patients, about 30% of the total – those with refractory or drug-resistant epilepsy (DRE) – do not achieve seizure freedom as a result of treatment with currently available AEDs.5
The International League Against Epilepsy (ILAE) has issued a standard definition of DRE, which had previously been described with a number of non-official definitions.6 According to the ILAE, DRE is defined as failure of adequate trials of two tolerated and appropriately chosen and used AED schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom. Failure is considered to occur if the patient does not remain seizure-free for a minimum of three times the longest pre-treatment interseizure interval, or 12 months, whichever is longer.7 The recent RATE-España consensus confirmed that Spanish neurologists found the ILAE's definition to be useful and applicable, in addition to confirming the need for a proactive attitude when managing DRE patients with a view to achieving an optimal level of seizure control.
Epilepsy is a neurological disease with a pronounced societal and healthcare impact in Spain.3,9 In addition to their economic impact, epileptic seizures significantly curtail daily life activities, decrease patients’ perceptions of quality of life, and augment morbidity and mortality rates.8 There have been prior attempts at measuring the cost of DRE in Spain.10 However, the real impact of drug-resistant epilepsy is not well-known in our country given that there was no standard definition of the entity prior to the ILAE's proposal.
The aim of ESPERA (European observational Study on ePileptic patiEnts Requiring at least two Antiepileptic drugs) was to measure the economic and quality of life impact of DRE, according to the ILAE's definition, in adults with focal epilepsy. The study was simultaneously carried out in France and Spain and this article shows results from the Spanish study.
Patients and methodsA multi-centre, cross-sectional, retrospective study was carried out in adults (age≥18 years) with focal epilepsy (focal seizures with or without secondary generalisation) and who had been receiving treatment with at least 2 AEDs during a minimum of 3 months at time of inclusion, whether or not they were seizure-free. We excluded patients who were participating in clinical trials of new antiepileptic drugs and those who were hospitalised at the time. Researchers were randomly selected from 2 lists comprising 349 general neurologists and epileptologists drawn from all of Spain's different geographical areas. Each researcher recruited all patients meeting the selection criteria in consecutive order during the inclusion period (October 2010–February 2011). We stratified patients according to their response to AEDs as defined by the ILAE. The study complies with the standards established by each participating institution's research ethics committee and the Declaration of Helsinki of 1975.
In addition, researchers consecutively recorded data from 20 adults with a firm diagnosis of focal epilepsy. The recorded data were then used to analyse the study sample, as we will explain further on.
Apart from sociodemographic data, records included the course of the disease from time of onset, current treatment and the patient's level of compliance, any prior treatments since onset of epilepsy, and health resources used during the 12 months prior to inclusion. Data were gathered from the patient interview when information about use of resources was not provided by the medical history.
We analysed costs from the perspective of the Spanish National Health System (SNS) based on outpatient and hospital resources, and from the perspective of society by also considering the patient's need for caregivers. The study contemplated the following resources: hospitalisations (emergency department admission, length of stay in the intensive care unit or neurosurgery/neurology departments), office visits (scheduled or emergency visits to the neurologist and consultations with other specialists or the primary care doctor), outpatient procedures (radiology, MRI, CT, EEG, etc.), AED treatment, and other non-pharmacological treatments including surgery.
The direct healthcare cost per patient was calculated based on the natural units of measurement for the healthcare resources consumed and their cost per unit. Unit costs for healthcare resources were obtained from each Spanish region's official fee schedule.11 The unit cost for a 1-day hospital stay in the intensive care unit or neurosurgery/neurology department was determined using the hospital discharge records from the SNS that are published by the Ministry of Health.12 The cost of hospitalisation was calculated as the mean stay in days according to the study results multiplied by the unit cost of each day in hospital. The costs of drug treatments (retail price+VAT) were calculated based on the data from Spain's General Council of Official Pharmacy Associations.13 The analysis from the perspective of the Spanish National Health System (SNS) took into account patients’ age and calculated drug costs payable by the SNS accordingly. The hourly fee paid to caregivers reflects the official rates for domestic employees.11 All prices were expressed in euros (2010); no discounted rates were applied since the follow-up period was one year long.
We measured health-related quality of life in epilepsy, presence of major depression, and general state of health using the QOLIE-31P,14 NDDIE,15 and EQ-5D-3L16 questionnaires, respectively. Patients completed the questionnaires themselves.
The Quality of Life in Epilepsy Inventory (QOLIE-31P)14 includes 31 items grouped in 7 domains: energy-fatigue, emotional well-being, daily activities, mental activity, medication effects, seizure worry, and overall quality of life. Scores for the 7 domains and overall quality of life ranged from 0 (worst score) to 100 (best score).
The NDDI-E questionnaire15 is a simple and reliable tool for screening for major depression in patients with epilepsy. It includes 6 items for assessing level of depression. We calculated each patient's overall score and the percentage of patients with major depression (defined by the questionnaire as having a score greater than or equal to 15).
The EQ-5D-3L16 is a general quality of life questionnaire that uses a visual analogue scale (VAS) and includes 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
Statistical analysis was performed using SAS software version 9.2. The first analysis examined the entire patient population and it was followed by a descriptive analysis comparing response to AEDs. Quantitative variables were described using means and standard deviations (SD). Qualitative variables were described according to number of cases and percentages. We compared results from the different groups using the t-test, analysis of variance or non-parametric tests for quantitative variables according to their distribution, and Pearson's chi-squared test, Yates's correction, or Fisher's exact test for qualitative variables. The differences between groups were considered statistically significant when probability of error was less than 5% (P<.05).
Patients’ results were weighted according to their share within the total study population using the following criteria: number of patients with focal epilepsy treated in the researcher's department, percentage of patients with DRE treated with polytherapy who consulted with researchers during the inclusion period, and yearly frequency of visits for each patient.
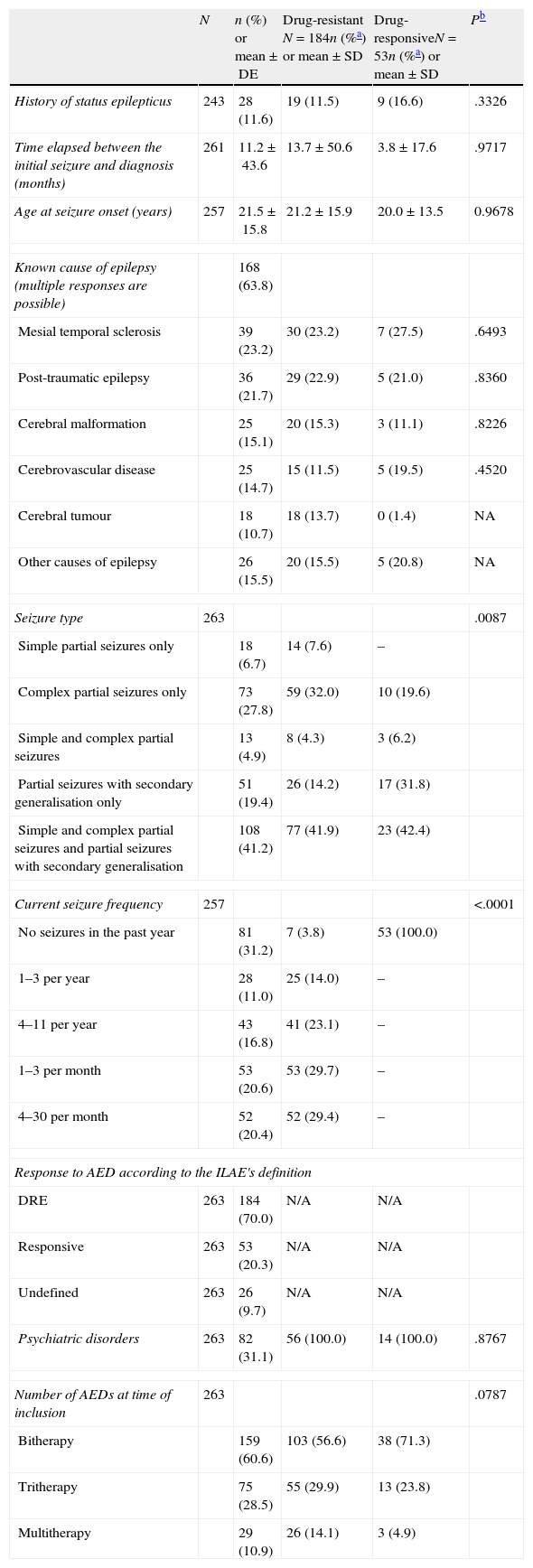
ResultsThe current study included 29 researchers from all over Spain, among which 34.5% may be considered epileptologists and the other 65.5%, general neurologists. Of the 304 participants selected, we analysed 263 patients with epilepsy who met the selection criteria: 39 patients were excluded since statistical weighting could not be performed with the recorded data, as were another 2 patients due to being treated in monotherapy. The sociodemographic and clinical characteristics are shown in Tables 1 and 2, respectively. According to the ILAE's definition, 70% of the included patients presented DRE, while 20.3% were drug-responsive and 9.7% were classified as undefined.
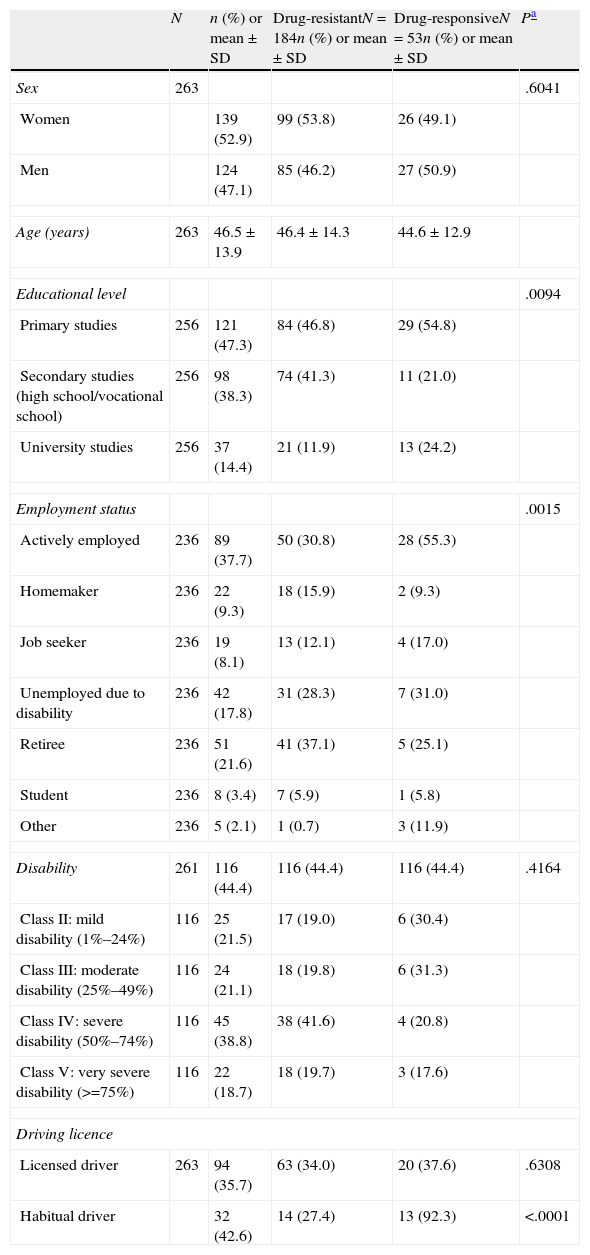
Sociodemographic characteristics of adults with focal epilepsy who are treated with at least 2 AEDs.
| N | n (%) or mean±SD | Drug-resistantN=184n (%) or mean±SD | Drug-responsiveN=53n (%) or mean±SD | Pa | |
| Sex | 263 | .6041 | |||
| Women | 139 (52.9) | 99 (53.8) | 26 (49.1) | ||
| Men | 124 (47.1) | 85 (46.2) | 27 (50.9) | ||
| Age (years) | 263 | 46.5±13.9 | 46.4±14.3 | 44.6±12.9 | |
| Educational level | .0094 | ||||
| Primary studies | 256 | 121 (47.3) | 84 (46.8) | 29 (54.8) | |
| Secondary studies (high school/vocational school) | 256 | 98 (38.3) | 74 (41.3) | 11 (21.0) | |
| University studies | 256 | 37 (14.4) | 21 (11.9) | 13 (24.2) | |
| Employment status | .0015 | ||||
| Actively employed | 236 | 89 (37.7) | 50 (30.8) | 28 (55.3) | |
| Homemaker | 236 | 22 (9.3) | 18 (15.9) | 2 (9.3) | |
| Job seeker | 236 | 19 (8.1) | 13 (12.1) | 4 (17.0) | |
| Unemployed due to disability | 236 | 42 (17.8) | 31 (28.3) | 7 (31.0) | |
| Retiree | 236 | 51 (21.6) | 41 (37.1) | 5 (25.1) | |
| Student | 236 | 8 (3.4) | 7 (5.9) | 1 (5.8) | |
| Other | 236 | 5 (2.1) | 1 (0.7) | 3 (11.9) | |
| Disability | 261 | 116 (44.4) | 116 (44.4) | 116 (44.4) | .4164 |
| Class II: mild disability (1%–24%) | 116 | 25 (21.5) | 17 (19.0) | 6 (30.4) | |
| Class III: moderate disability (25%–49%) | 116 | 24 (21.1) | 18 (19.8) | 6 (31.3) | |
| Class IV: severe disability (50%–74%) | 116 | 45 (38.8) | 38 (41.6) | 4 (20.8) | |
| Class V: very severe disability (>=75%) | 116 | 22 (18.7) | 18 (19.7) | 3 (17.6) | |
| Driving licence | |||||
| Licensed driver | 263 | 94 (35.7) | 63 (34.0) | 20 (37.6) | .6308 |
| Habitual driver | 32 (42.6) | 14 (27.4) | 13 (92.3) | <.0001 | |
SD: standard deviation; N: total number of patients; n: number of patients presenting the specified trait.
Clinical characteristics of adults with focal epilepsy and being treated with at least 2 AEDs.
| N | n (%) or mean±DE | Drug-resistant N=184n (%a) or mean±SD | Drug-responsiveN=53n (%a) or mean±SD | Pb | |
| History of status epilepticus | 243 | 28 (11.6) | 19 (11.5) | 9 (16.6) | .3326 |
| Time elapsed between the initial seizure and diagnosis (months) | 261 | 11.2±43.6 | 13.7±50.6 | 3.8±17.6 | .9717 |
| Age at seizure onset (years) | 257 | 21.5±15.8 | 21.2±15.9 | 20.0±13.5 | 0.9678 |
| Known cause of epilepsy (multiple responses are possible) | 168 (63.8) | ||||
| Mesial temporal sclerosis | 39 (23.2) | 30 (23.2) | 7 (27.5) | .6493 | |
| Post-traumatic epilepsy | 36 (21.7) | 29 (22.9) | 5 (21.0) | .8360 | |
| Cerebral malformation | 25 (15.1) | 20 (15.3) | 3 (11.1) | .8226 | |
| Cerebrovascular disease | 25 (14.7) | 15 (11.5) | 5 (19.5) | .4520 | |
| Cerebral tumour | 18 (10.7) | 18 (13.7) | 0 (1.4) | NA | |
| Other causes of epilepsy | 26 (15.5) | 20 (15.5) | 5 (20.8) | NA | |
| Seizure type | 263 | .0087 | |||
| Simple partial seizures only | 18 (6.7) | 14 (7.6) | – | ||
| Complex partial seizures only | 73 (27.8) | 59 (32.0) | 10 (19.6) | ||
| Simple and complex partial seizures | 13 (4.9) | 8 (4.3) | 3 (6.2) | ||
| Partial seizures with secondary generalisation only | 51 (19.4) | 26 (14.2) | 17 (31.8) | ||
| Simple and complex partial seizures and partial seizures with secondary generalisation | 108 (41.2) | 77 (41.9) | 23 (42.4) | ||
| Current seizure frequency | 257 | <.0001 | |||
| No seizures in the past year | 81 (31.2) | 7 (3.8) | 53 (100.0) | ||
| 1–3 per year | 28 (11.0) | 25 (14.0) | – | ||
| 4–11 per year | 43 (16.8) | 41 (23.1) | – | ||
| 1–3 per month | 53 (20.6) | 53 (29.7) | – | ||
| 4–30 per month | 52 (20.4) | 52 (29.4) | – | ||
| Response to AED according to the ILAE's definition | |||||
| DRE | 263 | 184 (70.0) | N/A | N/A | |
| Responsive | 263 | 53 (20.3) | N/A | N/A | |
| Undefined | 263 | 26 (9.7) | N/A | N/A | |
| Psychiatric disorders | 263 | 82 (31.1) | 56 (100.0) | 14 (100.0) | .8767 |
| Number of AEDs at time of inclusion | 263 | .0787 | |||
| Bitherapy | 159 (60.6) | 103 (56.6) | 38 (71.3) | ||
| Tritherapy | 75 (28.5) | 55 (29.9) | 13 (23.8) | ||
| Multitherapy | 29 (10.9) | 26 (14.1) | 3 (4.9) | ||
SD: standard deviation; DRE: drug-resistant epilepsy; AED: antiepileptic drug; N/A: not applicable/not available; N: total number of patients; n: number of patients presenting the specified trait.
The demographic characteristics were generally similar between patients with DRE and drug-responsive patients (Table 1). However, 3 disease-related characteristics differed significantly between the two groups. Concretely, 24.2% of the patients with drug-responsive epilepsy had a university education vs 11.9% of the DRE patients (P=.0095); 55.3% in the former category were actively employed vs 30.8% of the DRE patients (P=.0015); and 92.3% of those with drug-responsive epilepsy who had a driver licence drove habitually vs 27.4% of the DRE patients (P=.0001).
Patients’ clinical characteristics are listed in Table 2. The mean number of AEDs per patient was higher in those with drug-responsive epilepsy, at 2.6 (±0.7) vs 2.3 (±0.6) (P=.0037). Among all patients, 60.6% were treated with AED bitherapy, 28.5% with tritherapy, and 10.9% in polytherapy with more than 3 AEDs. The most frequent AEDs were levetiracetam (53.8%), carbamazepine (31.9%), lamotrigine (28.6%), valproic acid (21.9%), oxcarbazepine (19.2%), and zonisamide (18.2%).
The health resources used and their associated unit costs are listed in Table 3. During the preceding 12 months, 38 patients (14.6%) were admitted to hospital due to epilepsy at least once. The mean number of hospitalisations per patient per year was 1.3 (±0.7), and mean length of hospital stay was 5.6 days (±6.4). The main reasons for admitting patients were epileptic seizures (37.6%), evaluating status epilepticus (20.0%), and video-EEG monitoring (24.8%). Eleven patients were hospitalised in the neurosurgery unit for a mean stay of 8.9 days (±5.7), and 16 patients were hospitalised in the intensive care unit for a mean stay of 4.2 days (±3.6). The mean cost per hospitalisation was €5234 (±€6697).
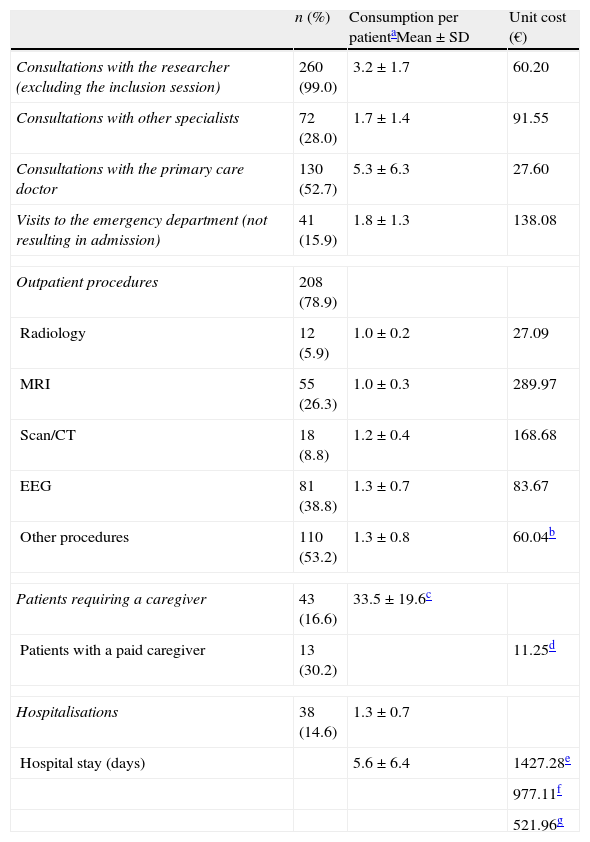
Resource consumption associated with epilepsy in the past 12 months.
| n (%) | Consumption per patientaMean±SD | Unit cost (€) | |
| Consultations with the researcher (excluding the inclusion session) | 260 (99.0) | 3.2±1.7 | 60.20 |
| Consultations with other specialists | 72 (28.0) | 1.7±1.4 | 91.55 |
| Consultations with the primary care doctor | 130 (52.7) | 5.3±6.3 | 27.60 |
| Visits to the emergency department (not resulting in admission) | 41 (15.9) | 1.8±1.3 | 138.08 |
| Outpatient procedures | 208 (78.9) | ||
| Radiology | 12 (5.9) | 1.0±0.2 | 27.09 |
| MRI | 55 (26.3) | 1.0±0.3 | 289.97 |
| Scan/CT | 18 (8.8) | 1.2±0.4 | 168.68 |
| EEG | 81 (38.8) | 1.3±0.7 | 83.67 |
| Other procedures | 110 (53.2) | 1.3±0.8 | 60.04b |
| Patients requiring a caregiver | 43 (16.6) | 33.5±19.6c | |
| Patients with a paid caregiver | 13 (30.2) | 11.25d | |
| Hospitalisations | 38 (14.6) | 1.3±0.7 | |
| Hospital stay (days) | 5.6±6.4 | 1427.28e | |
| 977.11f | |||
| 521.96g | |||
Each patient attended a mean of 3.2 (±1.7) neurology consultations per year. Approximately half of the patients (52.7%) visited their primary care doctor (mean of 5.3 (±6.3) visits per year) and about a third (28.0%) consulted with other specialists (mean of 1.7 (±1.4) visits per year). Of the patient total, 15.9% came to the emergency department at least once. In the preceding 12 months, a total of 208 patients (78.9%) had required at least one outpatient diagnostic procedure due to epilepsy. EEG was the most commonly used test (38.8%). Lastly, 16.6% of patients needed a caregiver, and that an individual was paid in 30.2% of all cases. Caregivers worked a mean of 33.5 (±19.6) weekly hours.
From the perspective of the SNS, the mean annual direct cost per adult with focal epilepsy treated with at least 2 AEDs was €4505 (±€5920). This figure represents the sum of the cost of AEDs (€3027±€2252), the cost of hospitalisation (€968±€5060), the cost of consultations (€351±€285), and the cost of outpatient tests (€158± €254). From the perspective of society, costs reached €5744 (±€7551). This figure includes the cost shown above plus that of the paid caregivers (€939 [±€4395]); caregivers represented the third-largest fraction of the total cost.
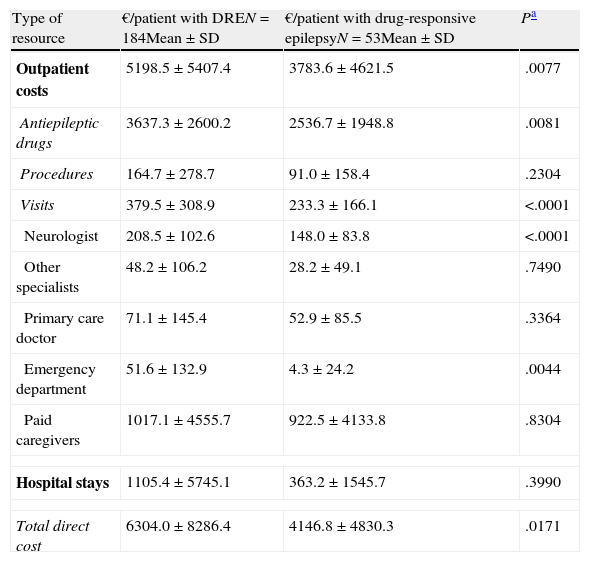
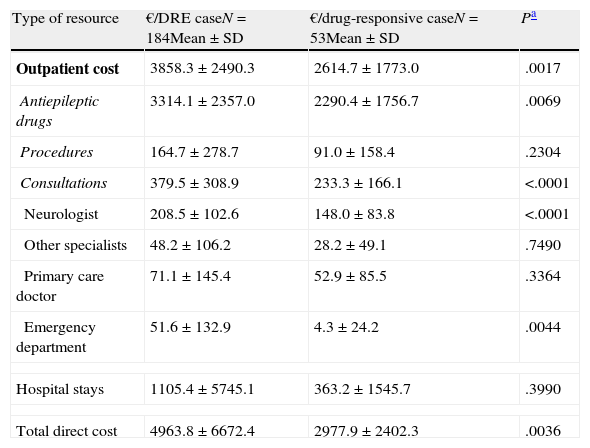
The mean annual cost per patient was higher in DRE patients than in those with drug-responsive epilepsy, from both a societal perspective (€6304 [±€8286] vs €4147 [±€4830], P=.0171) and according to the SNS (€4964 [±€6672] vs €2978 [±€2402]; P=.0036) (Tables 4 and 5). From both perspectives, DRE patients generated more costs associated with AED therapy, hospitalisations, visits to the neurologist, and visits to the emergency department (Tables 4 and 5). From a quantitative point of view, the difference between DRE patients and patients with drug-responsive epilepsy was mainly due to the cost of AEDs (52%) and hospital stays (37%), which both represented nearly 90% of the added costs associated with DRE patients.
Mean epilepsy-related cost per patient and year according to response to drugs (social perspective).
| Type of resource | €/patient with DREN=184Mean±SD | €/patient with drug-responsive epilepsyN=53Mean±SD | Pa |
| Outpatient costs | 5198.5±5407.4 | 3783.6±4621.5 | .0077 |
| Antiepileptic drugs | 3637.3±2600.2 | 2536.7±1948.8 | .0081 |
| Procedures | 164.7±278.7 | 91.0±158.4 | .2304 |
| Visits | 379.5±308.9 | 233.3±166.1 | <.0001 |
| Neurologist | 208.5±102.6 | 148.0±83.8 | <.0001 |
| Other specialists | 48.2±106.2 | 28.2±49.1 | .7490 |
| Primary care doctor | 71.1±145.4 | 52.9±85.5 | .3364 |
| Emergency department | 51.6±132.9 | 4.3±24.2 | .0044 |
| Paid caregivers | 1017.1±4555.7 | 922.5±4133.8 | .8304 |
| Hospital stays | 1105.4±5745.1 | 363.2±1545.7 | .3990 |
| Total direct cost | 6304.0±8286.4 | 4146.8±4830.3 | .0171 |
Mean epilepsy-related cost per patient per year according to response to drugs (costs borne by the Spanish National Health System).
| Type of resource | €/DRE caseN=184Mean±SD | €/drug-responsive caseN=53Mean±SD | Pa |
| Outpatient cost | 3858.3±2490.3 | 2614.7±1773.0 | .0017 |
| Antiepileptic drugs | 3314.1±2357.0 | 2290.4±1756.7 | .0069 |
| Procedures | 164.7±278.7 | 91.0±158.4 | .2304 |
| Consultations | 379.5±308.9 | 233.3±166.1 | <.0001 |
| Neurologist | 208.5±102.6 | 148.0±83.8 | <.0001 |
| Other specialists | 48.2±106.2 | 28.2±49.1 | .7490 |
| Primary care doctor | 71.1±145.4 | 52.9±85.5 | .3364 |
| Emergency department | 51.6±132.9 | 4.3±24.2 | .0044 |
| Hospital stays | 1105.4±5745.1 | 363.2±1545.7 | .3990 |
| Total direct cost | 4963.8±6672.4 | 2977.9±2402.3 | .0036 |
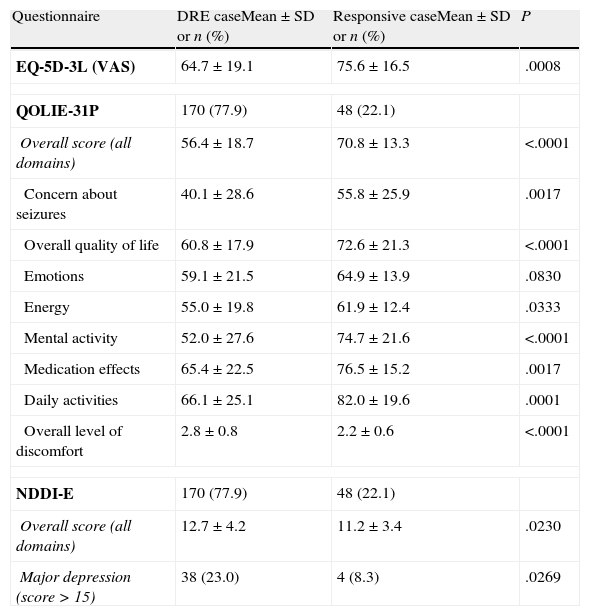
Two hundred three patients (92.4%) answered the questionnaires. The overall quality of life score on the QOLIE-31P questionnaire was 60.4 (±18.5). Response to AEDs was observed to be closely related to self-reported quality of life (Table 6). In addition, QOLIE-31P overall scores decreased as seizure frequency of increased. The mean score was 69.2 (±15.0) in seizure-free patients vs 56.7 (±18.1) in patients with at least 1 seizure per month (P<.0001).
Mean scores for response to drugs taken from self-administered questionnaires.
| Questionnaire | DRE caseMean±SD or n (%) | Responsive caseMean±SD or n (%) | P |
| EQ-5D-3L (VAS) | 64.7±19.1 | 75.6±16.5 | .0008 |
| QOLIE-31P | 170 (77.9) | 48 (22.1) | |
| Overall score (all domains) | 56.4±18.7 | 70.8±13.3 | <.0001 |
| Concern about seizures | 40.1±28.6 | 55.8±25.9 | .0017 |
| Overall quality of life | 60.8±17.9 | 72.6±21.3 | <.0001 |
| Emotions | 59.1±21.5 | 64.9±13.9 | .0830 |
| Energy | 55.0±19.8 | 61.9±12.4 | .0333 |
| Mental activity | 52.0±27.6 | 74.7±21.6 | <.0001 |
| Medication effects | 65.4±22.5 | 76.5±15.2 | .0017 |
| Daily activities | 66.1±25.1 | 82.0±19.6 | .0001 |
| Overall level of discomfort | 2.8±0.8 | 2.2±0.6 | <.0001 |
| NDDI-E | 170 (77.9) | 48 (22.1) | |
| Overall score (all domains) | 12.7±4.2 | 11.2±3.4 | .0230 |
| Major depression (score>15) | 38 (23.0) | 4 (8.3) | .0269 |
The overall mean score on the NDDI-E questionnaire for all patients was 12.3 (±4.1). The overall NDDI-E score for DRE patients was higher (reflecting a poorer state of health) compared to that in the drug-responsive group (12.7 vs 11.2; P=.0230). More DRE patients presented major depression compared to those with drug-responsive epilepsy (23.0% vs 8.3%; P=.0253). Patients with drug-responsive epilepsy also delivered better results than DRE patients on the EQ-5D-3L test measuring perception of general quality of life. The former group had a mean score of 75.6 (±16.5), while DRE patients had a mean score of 64.7 (±19.1) (P=.0008).
DiscussionOn an individual level, epilepsy places considerable social and psychological strain on patients who may be unable to obtain a driving license and who may suffer employment or educational difficulties, social stigma, medication dependence, or emotional disorders and depression. Seizure frequency is one of the most important factors affecting the patient's perceived quality of life. In fact, quality of life in patients with controlled epilepsy is similar to that in healthy subjects.17 Epilepsy also places a significant economic burden on society as whole, and this has been studied in Spain, across Europe, and in other parts of the world.9,18–21
The International League Against Epilepsy (ILAE) has standardised the definition of medically refractory or drug-resistant epilepsy. This concept was open to interpretation prior to until the ILAE's proposed definition.6 To our knowledge, the present study is the first to analyse costs generated by patients with DRE (as defined by the ILAE) and the impact of DRE on patients’ quality of life.
Our study reveals significant differences between the demographic characteristics of patients with DRE (as defined by the ILAE) and those with drug-responsive epilepsy in several relevant aspects related to the disease. In this sense, the educational level of patients with drug-responsive epilepsy was higher than that of DRE patients. Additionally, more seizure-free patients were actively employed and held driver licences. This testifies to the impact of DRE on the level of the individual. The study by Marinas et al.22 carried out in Spain 4 years ago also demonstrates that patients with drug-responsive epilepsy are more likely to be actively employed (64.4%) than patients with drug-resistant epilepsy (44.7%).22 The ESPERA study reflects a lower rate of employment among DRE patients than that found by Marinas et al.22 This result may have been influenced by the socio-economic decline in Spain. We should point out that the definition of DRE employed by Marinas et al.22 specified the failure of 3 AEDs, and this fact highlights the current decline in DRE patients’ employment status.
Our study shows that DRE patients use more healthcare resources and therefore incur greater costs than patients who are seizure-free. According to our study, DRE patients generate an additional cost to society of €2157 per patient per year, and an additional cost of €1986 to the SNS, compared to seizure-free patients. Considering that there are between 360000 and 400000 cases of epilepsy in Spain, of which 30%5,23 respond poorly to treatment, the additional cost associated with DRE would range from 216 to 240 million euros per year in our country.
The difference between mean annual costs generated by patients with DRE and patients with drug-responsive epilepsy is mainly due to the costs of AEDs and hospital stays. Outpatient diagnostic procedures and consultations with doctors also contribute to the cost increase to a lesser extent. From the viewpoint of society, the added cost of caregivers accounts for 4% of the difference between patients with DRE and those with drug-responsive epilepsy.
Although prior studies have estimated the costs of epilepsy in adults,9,10 the ESPERA study is the first to adopt the ILAE's definition of DRE in a comparison of resource use and costs between patients with DRE and those with drug-responsive epilepsy. According to the LINCE study,10 the mean annual cost generated by a patient with DRE (according to the definition used by that study) was €6838, of which 72% corresponds to direct healthcare costs, 24% to indirect costs, and 4% to direct non-healthcare costs. As our study does not evaluate indirect costs associated with patients’ workplace productivity, it cannot be directly compared to the LINCE study. In spite of methodological differences, results from the ESPERA study are congruent with the earlier estimates. Specifically, direct non-healthcare costs resulting from the need for paid caregivers accounted for 16% of the total for patients with DRE in our study, while the mean annual direct cost of healthcare resources per patient was €5287, representing 84% of the total. Since the study by Pato et al.9 included more seizure-free patients (40%), its results cannot be compared to those from the present study.
The ESPERA study observed that, in addition to generating higher costs, DRE patients present a poorer quality of life (according to the QOLIE-31P and EQ-5D questionnaires) and a higher incidence of major depression (according to the NDDI-E questionnaire) compared to seizure-free patients. Other studies have found similar results, highlighting that the patient's perceived quality of life is affected by both seizure frequency and severity.24 Likewise, the association between major depression and seizure frequency, as measured by the NDDI-E questionnaire, has also been observed in prior studies.25 Both healthcare professionals26 and patients27 have highlighted the importance of improving quality of life in patients with epilepsy. The ESPERA study, which included a large sample of Spanish patients, confirmed that achieving seizure control is very important to a patient's perceived quality of life and state of mind.
With regard to our study's limitations, resources associated with DRE may have been underestimated as a result of using retrospective methodology, which is subject to recall bias. Likewise, the current study may limit the difference between costs generated by DRE patients and seizure-free patients due to the selection bias resulting from including patients treated with 2 or more AEDs. Given that patients who had achieved seizure freedom with monotherapy were excluded, the difference may be greater than that expressed by our results. Another limitation is that our study focuses solely on direct costs and excludes indirect costs resulting from patients’ loss of productivity or premature death.
ConclusionsThe cost and quality of life of patients with epilepsy vary considerably depending on the level of seizure control achieved. Inadequate seizure control entails a cost increase of more than 50%, which produces a heavy economic burden that may be avoidable. Furthermore, inadequate seizure control is associated with a higher incidence of major depression and poorer quality of life as perceived by the patient. Inadequate control over seizures is also related to lower educational level and poorer employment prospects, both of which are important considerations for the patient and society. Achieving seizure freedom would therefore improve the patient's quality of life and decrease the costs borne by the SNS and society.
Conflicts of interestThis study was funded by GlaxoSmithKline.
L. Lévy-Bachelot and L. Hernández are employed by GlaxoSmithKline; J. Lahuerta was employed by GlaxoSmithKline at the time the study was completed; M. Doz is employed by CEMKA-EVAL, an independent consulting firm coordinating the study in Spain and France; M. Cuesta is employed by Oblikue Consulting S.L., an independent company which coordinates the study in Spain.
Manuel Mazabel Flores, Hospital. Nuestra Señora de Regla; Dulce Campos Blanco, Hospital Clínico de Valladolid; Jaume Burcet Darde, Hospital Comarcal El Vendrell; Francisco Barriga Herández, Hospital Alcorcón; Apolinar Gómez Díaz-Castroverde, Hospital de Cabueñes; Joaquin Ojeda Ruiz, Hospital Infanta Sofia; Javier Abella Corral, Hospital Arquitecto Marcide; Anne Gómez Caicoya, Clínica Quirón; Meritxell Martinez Ferri, Mútua de Terrassa; Ana Clara Ricciardi Ciocchini, Hospital Granollers; Mercedes Falip Centelles, Hospital de Bellvitge; Francisco Javier López González, Complexo Hospitalario Universitario de Santiago; Virgilio Hernando Requejo, Hospital Severo Ochoa; Albert Molins Albanell, Hospital Josep Trueta; Xiana Rodriguez Osorio, Complexo Hospitalario Universitario Santiago; Carmina Díaz Martí, Hospital Villajoyosa; Maria Teresa Adeva Bartolomé, Hospital Recoletas; Ruth Marrero Abrante, Hospital Universitario de Canarias; Jose Manuel Fernández Carril, Hospital Guadalajara; José Marin Marin, Hospital Reina Sofía, Murcia; Nuria García Barragán, Hospital Universitario Ramón y Cajal; Javier Salas Puig, Hospital Vall d’Hebron; Maria Aguirregomozcorta Gil, Hospital de Figueres; Juan Carlos García-Moncó, Hospital Galdákano; Vicente Ivañez Mora, Hospital La Paz; Diego Tortosa Conesa, Hospital Virgen de la Arrixaca; Imanol Iriondo Etxenagusia, Hospital Basurto; Olga Carmona Codina, Hospital de Figueres; Antonio José Moreno Rojas, Hospital Son Dureta; Jorge Ballabriga Planas, Hospital Son Llatzer; Eloy Elices Palomar, Clínica Rotger.
Please cite this article as: Villanueva V, et al. Impacto económico y en calidad de vida de la epilepsia resistente en España: estudio ESPERA. Neurología. 2013;28:195–204.