The concept “no evidence of disease activity” (NEDA) has been formulated as a way of assessing treatment response in patients with multiple sclerosis.1 The recent inclusion of the rate of brain volume loss in the NEDA-4 criteria2 has opened a new front in the management of the disease, requiring assessment of brain atrophy as a means of monitoring treatment response.
Although an experienced radiologist will be able to detect changes in brain volume through a qualitative evaluation of the brain MRI studies, mainly as a function of the dilation of the ventricular spaces, we must also perform a quantitative analysis enabling precise identification of these modifications.
The method of choice for this assessment is the analysis of brain volumes (whole-brain and regional brain volume, white and grey matter volume) based on three-dimensional radiological sequences and the use of different automated or semi-automated software tools developed for this purpose. These methods are considered the gold standard due to their reproducibility and sensitivity.3 However, they frequently require a certain degree of manual supervision, since errors often occur in brain segmentation due to the particular anatomy of brain structures or the different imaging acquisition protocols used.4 Although these methods are valid and accurate, their implementation in daily clinical practice is far from a reality today for several reasons: correct use of these techniques requires specific training, and results may take several hours to be delivered and must be externally validated by a trained professional.5 Furthermore, there is a lack of standardised protocols for MRI studies, and precise normal ranges have not been defined.6
In contrast, we find manual linear measurements applicable to two-dimensional sequences, which are an indirect reflection of brain atrophy; in many cases, these are based on evaluation of the widening of the ventricular system due to the neurodegenerative process.7,8 The measurements mentioned in the literature include the width of the third ventricle,9 the bicaudate index,10 the bifrontal index,11 the Evans index,12 the corpus callosum index (CCI),13 and the area of the corpus callosum.14 These are simple measurements that require no specific training and can be rapidly obtained from conventional sequences. The main disadvantage is the lack of reproducibility due to the inherent uncertainty regarding the positioning of the linear markers between repeated examinations or between different observers.4 This is the main reason that their use does not extend beyond research works, with few studies in the literature specifically assessing its validity. We aimed to analyse the reproducibility of these measurements, taking special interest in CCI, as it is a recently described measurement that has been addressed by very few studies.
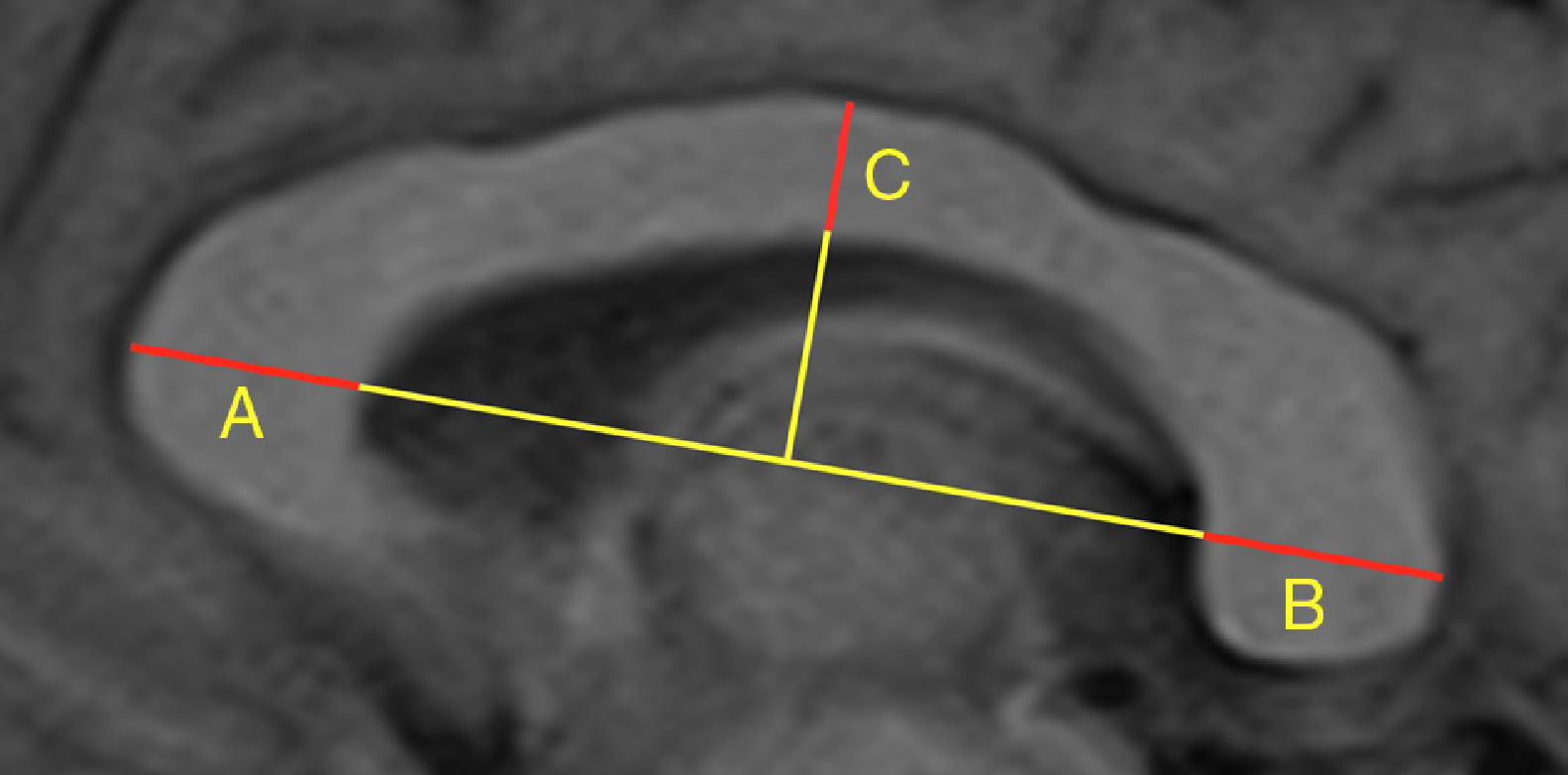
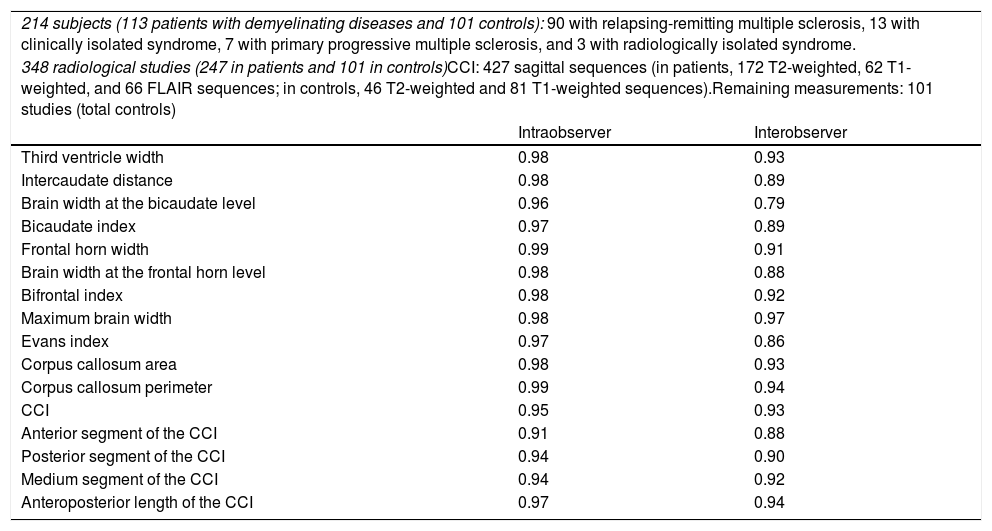
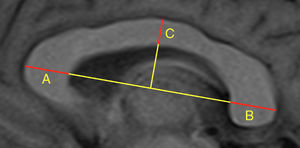
Our study included 113 patients with demyelinating diseases (mainly relapsing-remitting multiple sclerosis) and 101 healthy controls with normal results in brain MRI studies. In all controls, we calculated the width of the third ventricle, bicaudate index, bifrontal index, and Evans index, as well as the area and perimeter of the corpus callosum. In all subjects (patients and controls), we calculated the CCI, whose components are shown in Fig. 1. After an interval of several months, all measurements were calculated again by the same researcher, and a random sample of 100 subjects was analysed by a different researcher. The previous result was unknown in both cases. Concordance was assessed using the intraclass correlation coefficient; the results are shown in Table 1, which also shows the number of subjects and the radiological sequences analysed. We observed high intra- and interobserver reproducibility for all measurements studied.
Number of subjects and radiological sequences studied. Value of the intraclass correlation coefficient for the different linear measurements.
| 214 subjects (113 patients with demyelinating diseases and 101 controls): 90 with relapsing-remitting multiple sclerosis, 13 with clinically isolated syndrome, 7 with primary progressive multiple sclerosis, and 3 with radiologically isolated syndrome. | ||
| 348 radiological studies (247 in patients and 101 in controls)CCI: 427 sagittal sequences (in patients, 172 T2-weighted, 62 T1-weighted, and 66 FLAIR sequences; in controls, 46 T2-weighted and 81 T1-weighted sequences).Remaining measurements: 101 studies (total controls) | ||
| Intraobserver | Interobserver | |
| Third ventricle width | 0.98 | 0.93 |
| Intercaudate distance | 0.98 | 0.89 |
| Brain width at the bicaudate level | 0.96 | 0.79 |
| Bicaudate index | 0.97 | 0.89 |
| Frontal horn width | 0.99 | 0.91 |
| Brain width at the frontal horn level | 0.98 | 0.88 |
| Bifrontal index | 0.98 | 0.92 |
| Maximum brain width | 0.98 | 0.97 |
| Evans index | 0.97 | 0.86 |
| Corpus callosum area | 0.98 | 0.93 |
| Corpus callosum perimeter | 0.99 | 0.94 |
| CCI | 0.95 | 0.93 |
| Anterior segment of the CCI | 0.91 | 0.88 |
| Posterior segment of the CCI | 0.94 | 0.90 |
| Medium segment of the CCI | 0.94 | 0.92 |
| Anteroposterior length of the CCI | 0.97 | 0.94 |
CCI: corpus callosum index.
In the light of these results, we consider the two-dimensional linear measurements analysed to be a valid and reproducible tool that, either individually or in combination, may easily be applied to daily work flows in a demyelinating diseases clinic, enabling indirect assessment of brain atrophy and assisting in treatment decision-making. These results may lead the way to future research comparing two-dimensional and three-dimensional measurements.
This study was approved by the Research Ethics Committee of Asturias.
Please cite this article as: Pérez-Álvarez AI, Suárez-Santos P, González-Delgado M, Oliva-Nacarino P. Cuantificación de la atrofia cerebral en esclerosis múltiple mediante medidas bidimensionales. Neurología. 2020;35:433–435.