Spinal cord infarction is a rare disease with a high rate of morbidity. Its diagnosis can be challenging and controversy remains regarding the best treatment. Few case series have been published.
MethodsWe conducted a retrospective review of cases of spinal cord infarction attended in a tertiary hospital from 1999 to 2020. Aetiology and clinical, imaging, and prognostic features were assessed.
ResultsForty-one patients (58.5% men, mean [standard deviation] age 61 [17] years) were included in the study. Thirty-one patients (75.6%) presented vascular risk factors. Motor deficits were recorded in 39 (95.1%), pain in 20 (48.8%), sensory deficits in 33 (80.4%), and autonomic dysfunction in 24 (58.5%). MRI was performed in 37 (90.2%) patients. Diffusion-weighted images were available for 12 patients, with 10 showing diffusion restriction. The thoracic region was the most frequently affected (68.2%). Vascular imaging studies were performed in 33 patients (80.4%). The most frequent aetiologies were aortic dissection (6 cases), atherosclerosis demonstrated by vascular imaging (6 cases), fibrocartilaginous embolism (6 cases), surgery (5 cases), and hypotension (4 cases). Aetiology was undetermined in 12 patients (29.3%), although 9 of these presented vascular risk factors. At the end of the follow-up period (median, 24 months; interquartile range, 3–70), 12 patients (29.2%) were able to walk without assistance. Vascular risk factors and paraparesis were significantly associated with poorer prognosis (P < .05).
DiscussionSpinal cord infarction may present diverse aetiologies, with the cause remaining undetermined in many patients. Long-term functional prognosis is poor, and depends on baseline characteristics and clinical presentation. MRI, and especially diffusion-weighted sequences, is useful for early diagnosis.
El infarto medular es una entidad infrecuente y con elevada morbilidad. El diagnóstico puede resultar difícil y el tratamiento óptimo sigue siendo controvertido. Existen pocas series de casos publicadas.
MétodosEstudio retrospectivo de infarto medular en un hospital terciario desde 1999 a 2020. Se evaluaron la etiología, las características clínicas, radiológicas, terapéuticas y pronósticas.
ResultadosSe incluyeron 41 pacientes (58,5% varones, edad media 61 ±17 años). Treinta y un pacientes (75,6%) presentaban factores de riesgo vascular (FRV). Presentaron déficit motor (39, 95,1%), dolor (20, 48,8%), déficit sensitivo (33, 80,4%) y alteración autonómica (24, 58,5%). Se realizó resonancia magnética (RM) en 37 pacientes (90,2%). En los 12 pacientes con secuencias de difusión, esta estaba alterada en 10. La localización más afectada fue la dorsal (68,2%). Se realizó estudio vascular en 33 pacientes (80,4%). Las etiologías más frecuentes fueron disección aórtica en 6, ateroesclerosis demostrada en estudio vascular en 6, embolia fibrocartilaginosa en 6, posquirúrgico en 5 e hipotensión en 4. El mecanismo etiológico quedó sin filiar en 12 pacientes (29,3%), 9 presentaban FRV. Al final del periodo de seguimiento (mediana 24 meses, rango intercuartílico 3-70), 12 pacientes (29,2%) presentaban deambulación autónoma. La presencia de FRV y la paraparesia se asociaron significativamente a peor pronóstico (p < 0,05).
DiscusiónEl infarto medular es una patología con una etiología variada, que en muchos de los pacientes queda sin resolver. El pronóstico funcional a largo plazo es malo y depende de las características basales del paciente y de la forma de presentación clínica. La RM, especialmente las secuencias de difusión, es útil en el diagnóstico precoz.
Spinal cord infarction is a rare entity accounting for 1% of all cases of ischaemic stroke.1 It represents a diagnostic challenge due to the poor specificity and great variability of the associated clinical signs.1–7 Furthermore, differential diagnosis includes a wide range of disorders, such as compressive myelopathy, infectious or autoimmune diseases, and other vascular disorders of the spinal cord, including dural arteriovenous fistulas.1 Magnetic resonance imaging (MRI) is the most useful tool for diagnosis, as it helps to differentiate spinal cord infarction from other causes of myelopathy; however, it may fail to detect alterations during the first hours after symptom onset.6 Ischaemia results in decreased cellular ATP levels, causing sodium channel malfunction and restricting the influx of water to the intracellular space.8 Over the past decades, diffusion-weighted imaging (DWI) has been used to establish an early diagnosis of brain ischaemia. Restricted diffusion, defined as high signal intensity on DWI sequences with low apparent diffusion coefficient (ADC) values, may help to establish an early diagnosis of spinal cord infarction.9 Vascular studies (CT angiography or arteriography of the supra-aortic trunks, aorta, or spinal cord blood vessels) are essential for aetiological diagnosis.1 However, aetiology is heterogeneous, and a definite aetiological diagnosis often cannot be established despite an extensive evaluation.1–7 Furthermore, treatment of spinal cord infarction continues to be controversial, and no clinical practice guidelines have been issued on the topic.1 Factors associated with poor prognosis should be identified in order to prevent futile treatment.
The literature includes few series of patients with spinal cord infarction.2–7 Zalewski et al.2 published a study of 133 patients and reviewed the diagnostic criteria for spontaneous spinal cord infarction. Robertson et al.3 focused on the long-term prognosis of spinal cord infarction in a series of 115 patients. Another study by Zalewski et al.4 included 75 patients and described the clinical and radiological characteristics of patients with spinal cord infarction following surgery. Other series have described the radiological characteristics of patients with spinal cord infarction in smaller patient samples.5,6 In our setting, Castro-Vilanova et al.7 described the clinical characteristics and complementary test results (MRI and CSF analysis) of 12 patients.
Given the current lack of data, we performed a descriptive study of the aetiological, clinical, radiological, treatment, and prognostic characteristics of patients diagnosed with spinal cord infarction.
Material and methodsWe conducted a retrospective, descriptive study of patients admitted to Hospital Ramón y Cajal between 1999 and 2020 and discharged with a diagnosis of spinal cord infarction. We included patients attended at the neurology department with a discharge diagnosis of spinal cord infarction, spinal cord ischaemia, or ischaemic myelopathy; patient data were gathered from our hospital’s clinical history database. All cases were reviewed independently by 2 reviewers (VRC and IC). All patients were evaluated by a neurologist at admission. We included all patients with spinal cord syndrome (anterior, posterior, central) of less than 72 hours’ progression and showing compatible neuroimaging findings and/or in whom other aetiologies had been ruled out (compressive myelopathy, myelitis, and vascular malformations). All patients were older than 18 years and were functionally independent before the event. We excluded patients with transient spinal cord symptoms, insufficient clinical data, and for whom other diagnoses could not be ruled out.
Data were collected on demographic and clinical variables at admission (age, sex, vascular risk factors, history of stroke, ischaemic heart disease, peripheral artery disease) and treatments received before the event. We also gathered data on clinical presentation: time from symptom onset to nadir, neurological examination results (motor function, pain and vibration sensitivity, deep tendon reflexes, sphincter function), and such other symptoms as pain or hypotension. Regarding MRI studies, we evaluated the presence of the classical pencil-like and owl-eyes patterns, as well as infarction of the adjacent vertebral bodies, oedema, or infarction with haemorrhagic transformation. In patients for whom diffusion-weighted or gadolinium-enhanced sequences were available, we evaluated the presence of diffusion restriction or contrast uptake, respectively. Other factors included the localisation and extension of spinal cord infarction (the spinal cord levels affected). During the study period, all MRI studies were performed with the Philips Ingenia 1.5 T or the Philips Achieva 1.5 T scanners.
The aetiology of spinal cord infarction was evaluated with a vascular study (CT angiography and arteriography of the supra-aortic trunks, aorta, and spinal cord blood vessels). The date of onset of antiplatelet or anticoagulation therapy was also recorded. The degree of disability after spinal cord infarction was evaluated with the modified Rankin Scale (mRS) at 3 months and at the end of follow-up. Patients who were able to walk unassisted but needed a cane or crutch were assigned an mRS score of 3. Patients were considered to walk independently if they did not require any type of walking aid.
Given the retrospective nature of our study, neither informed consent nor approval by a research ethics committee was necessary.
Categorical variables are presented as percentages, and continuous variables as means and standard deviation (SD) or medians and quartiles 1 and 3 (Q1-Q3), as appropriate. The Shapiro-Wilk test was used to test for normality. A univariate analysis was performed to identify variables showing significant differences between patients with good outcomes and those with poor outcomes (mRS > 2 at 3 months); the Fisher exact test was used for categorical variables. Statistical significance was set at P < .05. Statistical analysis was performed with the SPSS software (version 26).
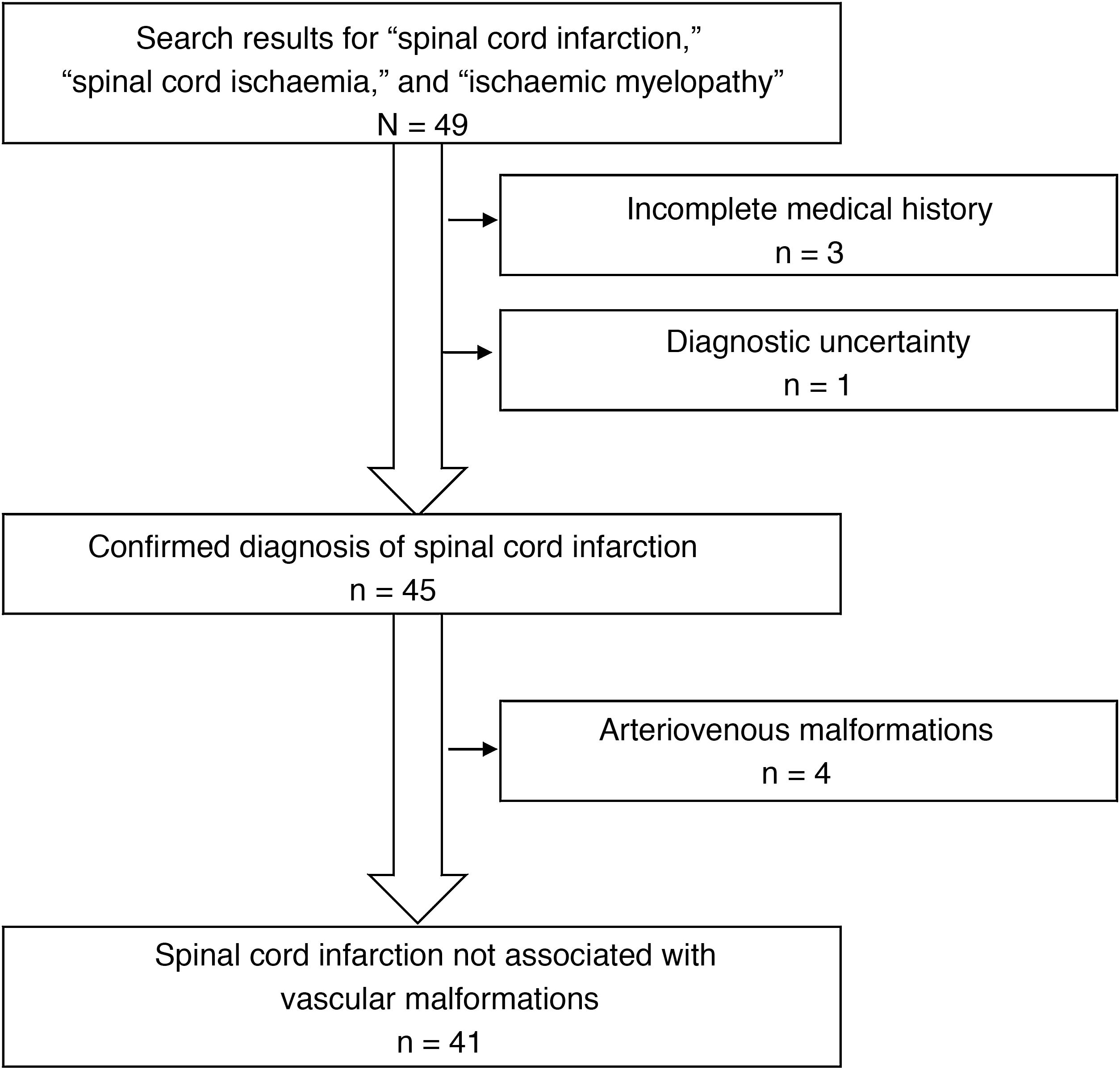
ResultsWe identified a total of 49 patients with a discharge diagnosis of spinal cord infarction, spinal cord ischaemia, or ischaemic myelopathy. Eight patients were excluded from our study, for the following reasons: arteriovenous malformations (4 patients), incomplete medical history (3), and diagnostic uncertainty (1) (Fig. 1). Of the 41 patients included, 24 were men (58.5%); mean age (SD) was 60.8 (16.5) years. Most patients presented vascular risk factors, with the most frequent being arterial hypertension. The patients’ clinical characteristics at admission are summarised in Table 1.
Clinical characteristics at admission.
| Baseline characteristics | |
|---|---|
| Mean age (SD), years | 60.8 (16.5) |
| Men | 24 (58.5%) |
| Vascular risk factors | 31 (75.6%) |
| Arterial hypertension | 22 (53.6%) |
| Smoking | 20 (48.7%) |
| Dyslipidaemia | 14 (34.1%) |
| Peripheral artery disease | 8 (19.5%) |
| Diabetes mellitus | 7 (17.1%) |
| Ischaemic heart disease | 6 (14.6%) |
| Overweight | 2 (4.8%) |
| Stroke | 1 (2.4%) |
Table 2 presents the characteristics of spinal cord infarction during the acute phase. Data on time from symptom onset to nadir were available in 17 cases; the median (Q1-Q3) was 6 hours (4-24). Most patients (95.1%) presented motor deficits, with paraparesis being the most frequent presentation. One patient presented isolated posterior spinal cord syndrome. Up to 48.8% of patients presented pain at onset. Tendon reflexes were diminished or absent in 20 patients (48.8%). The most frequent location of the infarction was the thoracic region (68.2%). Most patients (70.7%) were admitted to the neurology department, with 5 (12.1%) admitted to the intensive care unit, 3 (7.3%) to the vascular surgery department, and 4 (9.8%) to other wards.
Clinical characteristics of spinal cord infarction in our sample.
| Clinical presentation | |
|---|---|
| Time from symptom onset to nadir (hours), median (Q1-Q3) (n = 17) | 6 (4-24) |
| Motor deficits | 39 (95.1%) |
| Paraparesis | 28 (71.8%) |
| Tetraparesis | 8 (20.5%) |
| Hemiparesis | 3 (7.7%) |
| Sensory deficits | 33 (80.4%) |
| Proprioception | 1 (3%) |
| Sensory level | 24 (72.7%) |
| Pain | 20 (48.8%) |
| Sphincter dysfunction | 24 (58.5%) |
| Reflexes | |
| Hyporeflexia/areflexia | 20 (48.7%) |
| Extensor plantar reflex | 5 (12.1%) |
| Location | |
| Thoracic | 15 (36.6%) |
| Cervical | 8 (19.5%) |
| Thoracic–conus medullaris | 7 (17%) |
| Cervical-thoracic | 6 (14.6%) |
| Conus medullaris | 5 (12.2%) |
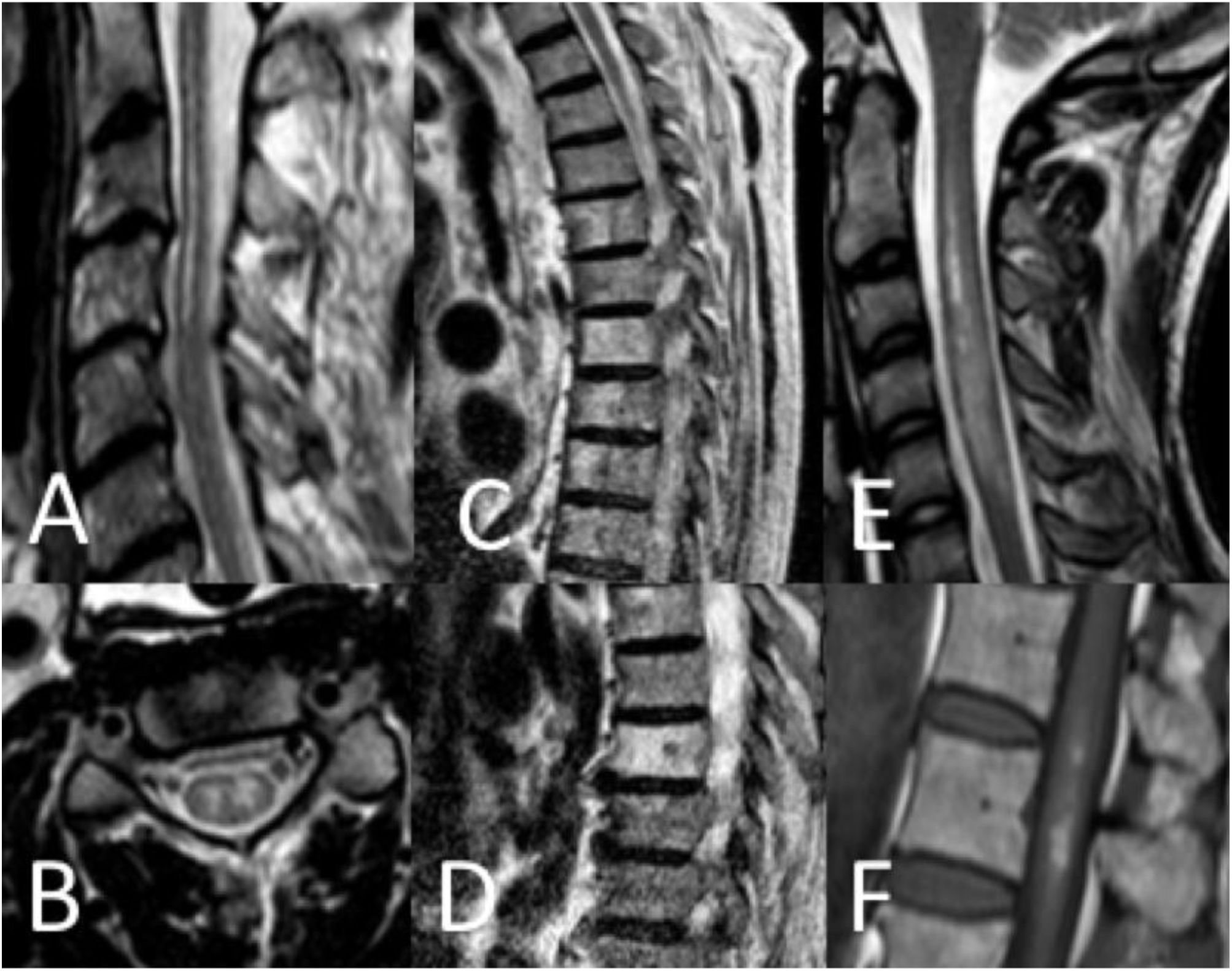
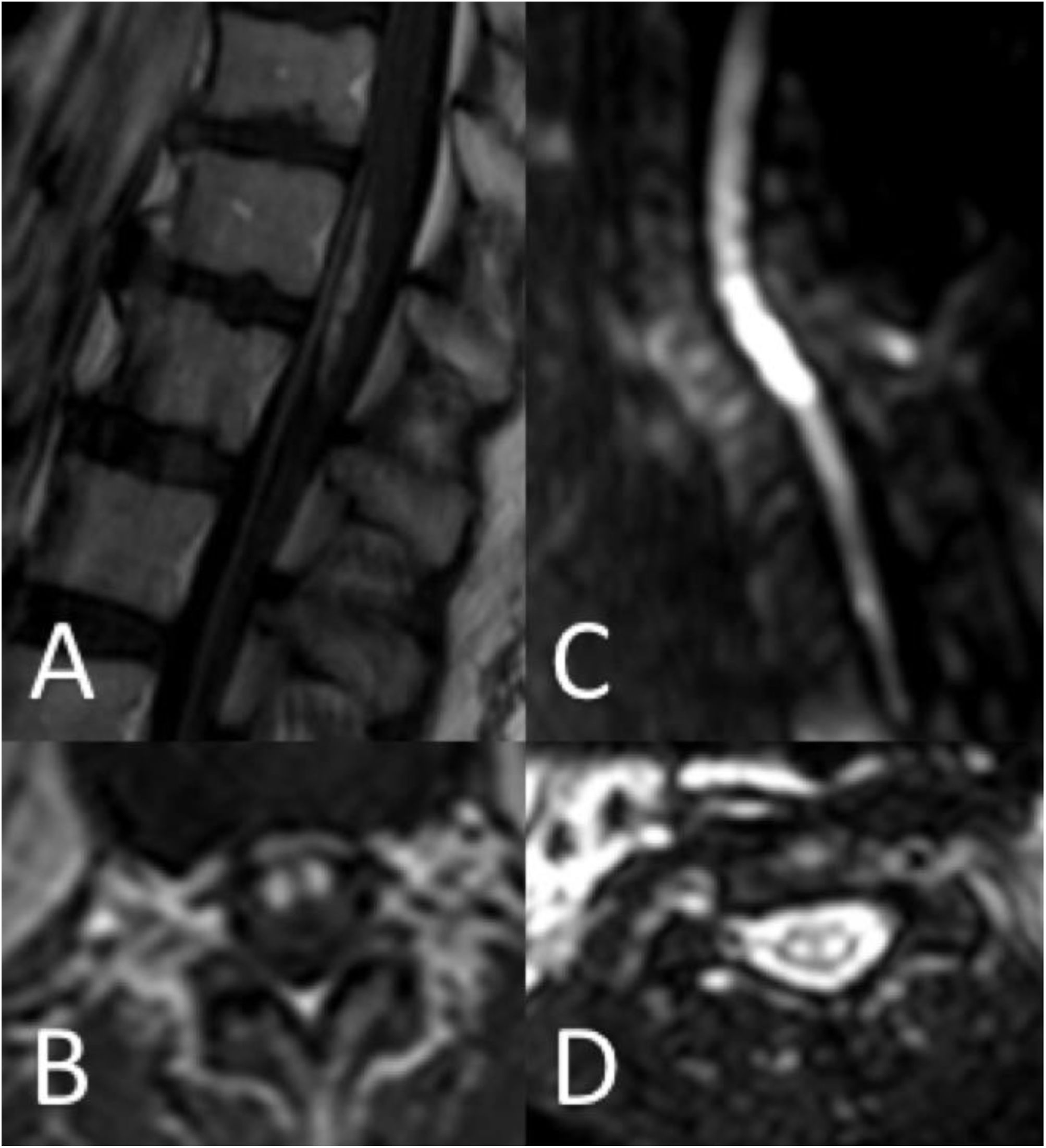
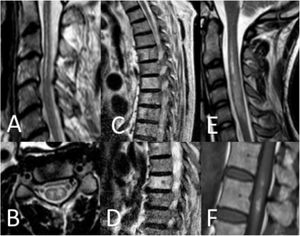
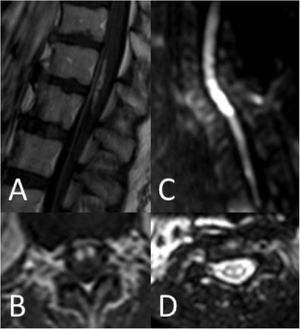
Thirty-seven patients (90.2%) underwent spinal cord MRI. In the remaining cases (9.8%), studies could not be performed due to the patients’ unstable clinical situation. In 5 cases (13.5%), the initial MRI scan revealed no alterations: in 3 patients, the study had been performed within 24 hours of symptom onset, and subsequent MRI studies revealed alterations compatible with spinal cord infarction. Radiological findings are presented in Table 3 and Figs. 2 and 3. Gadolinium-enhanced MRI studies were performed in 13 patients, and lesions displayed contrast uptake in 5 cases. DWI/ADC studies were performed in 12 patients, 10 of whom presented diffusion restriction (83.3%) (Fig. 3). In the patients showing diffusion restriction on DWI sequences, the median time (Q1-Q3) from symptom onset to DWI was 22 hours (14-34).
Radiological findings in our series.
| Radiological findings (n = 37) | n (%) |
|---|---|
| Pencil-like pattern | 17 (45.9) |
| Owl-eyes pattern | 15 (40.5) |
| Vertebral body infarction | 9 (24.3) |
| Oedema | 4 (10.8) |
| Haemorrhage | 1 (2.7) |
| Diffusion restriction (n = 12) | 10 (83.3) |
| Gadolinium uptake (n = 13) | 5 (38.5) |
| Spinal levels altered (n = 35) | |
| 1-3 | 18 (51.4) |
| 4-7 | 10 (28.6) |
| > 7 | 7 (20) |
Spinal cord MRI findings. A) Sagittal T2-weighted image: pencil-like pattern. B) Axial T2-weighted image: owl-eyes pattern. C and D) Sagittal T2-weighted images: vertebral body infarctions. E) Sagittal T2-weighted image: spinal cord oedema. F) Sagittal T1-weighted image: infarct with haemorrhagic transformation.
Vascular studies (CT angiography and/or arteriography) were performed in 33 patients (80.4%): the study focused on the supra-aortic trunks in 13 patients, the aorta in 22, and spinal cord blood vessels in 17. We observed significant atherosclerosis in 6 patients (18.1%), aortic dissection in 6 (18.1%), aortic aneurysms in 4 (12.1%), and anterior spinal artery thrombosis in 3 (9%). The vascular study revealed no abnormalities in 13 patients (39.4%).
Aetiology was undetermined in 12 patients (29.3%). Of these, 9 presented vascular risk factors. The most frequent aetiologies were aortic dissection (6 patients; 14.6%), atherosclerosis demonstrated in vascular studies (6; 14.6%), fibrocartilaginous embolism (6; 14.6%), surgery (5; 12.2%), and hypotension (4; 9.8%); in one case, hypotension was secondary to sildenafil use. Four of the patients presenting postsurgical spinal cord infarction had undergone aortic surgery, and another patient had undergone spinal surgery. Spinal cord infarction was attributed to atrial fibrillation in one patient, and in another case it was attributed to a state of hypercoagulability due to resistance to activated protein C and a prothrombin gene mutation.
Four of the 6 patients diagnosed with fibrocartilaginous embolism were women, and the mean age was 57 (15) years; all patients reported having performed a Valsalva manoeuvre, intense exercise, or a sudden movement of the trunk. Only 3 patients presented a single vascular risk factor (arterial hypertension, dyslipidaemia, or smoking). MRI revealed vertebral body infarction in 4 patients and disc protrusions in 5; the 4 patients undergoing vascular studies displayed no imaging alterations.
At discharge, 23 patients (56.1%) received antiplatelets and 3 (7.3%) received anticoagulation therapy. Prognostic variables are shown in Table 4. The 3-month mortality rate was 9.8%. Of these patients, 75% died due to multiple organ failure secondary to aortic dissection. At the end of follow-up (median [Q1-Q3], 24 [3-70] months), the mortality rate was 36.6%. The patients scoring 6 on the mRS were followed up for a median time of 43.5 months (21-108). The cause of death was unknown in 4 patients, cancer unrelated to the spinal cord infarction in 3, multiple organ failure in one, scleroderma in one, and basal ganglia haemorrhage in one. As these mortality data refer to the end of follow-up, and given the variability of follow-up times, we may conclude that the cause of death beyond 3 months of spinal cord infarction was unrelated to the event in most cases. However, it should be noted that 3-month mortality was greater in patients with aortic dissection.
After the follow-up period, only 12 patients (29.3%) walked independently (without the assistance of a walking aid). The variables significantly associated with poor functional prognosis (mRS > 2) at 3 months were presence of vascular risk factors (P = .01) and presentation with paraparesis (P = .02) (Table 5).
Differences between patients with and without functional dependence at 3 months after spinal cord infarction.
| mRS = 0-2 | mRS > 2 | P | |
|---|---|---|---|
| Age ≥ 60, mean (SD) | 18 (75) | 6 (25) | .99 |
| Men | 4 (16.7%) | 20 (83.3%) | .15 |
| Vascular risk factors | 5 (16.1%) | 26 (83.9%) | .01 |
| Arterial hypertension | 4 (18.2%) | 18 (81.8%) | .29 |
| Smoking | 4 (20%) | 16 (80%) | .48 |
| Dyslipidaemia | 2 (14.3%) | 12 (85.7%) | .28 |
| Diabetes mellitus | 2 (28.6%) | 5 (71.4%) | .99 |
| Ischaemic heart disease | 1 (16.7%) | 5 (83.3%) | .99 |
| Paraparesis | 4 (14.3%) | 24 (85.6%) | .02 |
| Tetraparesis | 3 (37.5%) | 5 (62.5%) | .66 |
| Sensory deficits | 10 (30.3%) | 23 (69.7%) | .41 |
| Sensory level | 8 (33.3%) | 16 (66.7%) | .31 |
| Pain | 6 (30%) | 14 (70%) | .73 |
| Sphincter dysfunction | 7 (29.2%) | 17 (70.8%) | .74 |
| Cervical involvement | 3 (37.5%) | 5 (62.5%) | .66 |
| Levels affected (≥ 4) | 4 (23.5%) | 13 (76.5%) | .47 |
| Aortic dissection | 0 | 6 (100%) | .17 |
| Atherosclerosis | 1 (16.7%) | 5 (71.4%) | .99 |
| Fibrocartilaginous embolism | 2 (33.3%) | 4 (66.7%) | .65 |
| Surgery | 0 | 5 (100%) | .30 |
| Hypotension | 0 | 4 (100%) | .56 |
Statistically significant differences are indicated in bold (P < .05).
mRS: modified Rankin Scale.
Spinal cord infarction is a rare entity whose diagnosis is challenging due to the great variability of clinical presentations and its broad differential diagnosis.1 Few series of patients with spinal cord infarction have been published.2–7 To our knowledge, ours is the study with the largest series of cases to be described in our setting. We should also highlight the high percentage of patients undergoing imaging studies and the long follow-up period.
As in previous studies,2–7 our patients were younger than those with cerebral infarction, with a mean age of approximately 60 years, and most (75.6%) presented vascular risk factors. Furthermore, unlike in stroke, in which neurological deficits present suddenly, our patients’ neurological symptoms developed more progressively. In fact, symptoms may take between 35-45 minutes to over 24 hours before they peak.2 Pain was a frequent manifestation of spinal cord infarction, presenting in 48.8% of cases, similarly to the rates described in the literature.2–7 Twenty-four patients (58.5%) presented sensory loss. In most patients, reflexes were absent or diminished, and only 5 patients showed extensor plantar reflexes. This is explained by the fact that assessment was performed during the acute phase of the event. The acute phase may last up to 6 weeks, and is associated with diminished or absent tendon reflexes and vegetative dysfunction, which may be severe in some cases.1 In fact, in our series, one patient with cervical spinal cord infarction developed severe hypotension, requiring orotracheal intubation.
MRI is the most useful tool for diagnosis of spinal cord infarction. Most infarctions appear as pencil-like hyperintensities on T2-weighted sequences. When lesions exclusively affect the grey matter, they present an owl-eyes pattern on axial T2-weighted sequences. Some cases may be associated with haemorrhagic transformation, with hyperintense lesions on T1-weighted sequences; other cases may present infarction of the adjacent vertebral body.5,6 Gadolinium uptake may also be observed.5,6 However, a considerable percentage of patients with spinal cord infarction show no MRI alterations, particularly when the study is performed in early stages of the event. Diffusion-weighted sequences are more sensitive in these cases.8 In our sample, 83.3% of patients undergoing DWI presented restriction on DWI/ADC maps. Alterations are observable several hours after symptom onset, and resolve within a week. The quality of DWI sequences at the level of the spinal cord may be suboptimal due to flow artefacts, proximity to bone, the longitudinal direction of white matter tracts, and the curvature of the spinal cord.9 However, although sensitivity results differ between series, they underscore the usefulness of DWI in early diagnosis; this tool should therefore be used whenever possible.
The aetiology of spinal cord infarction varies greatly, and a definitive diagnosis is not established in up to 68% of cases.2 The most frequent aetiologies are aortic surgery, aortic disease (aneurysm rupture or dissection), and vertebral artery dissection. Other less frequent causes are fibrocartilaginous embolism, hypercoagulability, cardioembolism, and hypotension.1 In exceptional cases, spinal cord infarction may be caused by treatment with sildenafil.10 In our series, we were unable to reach a definite aetiological diagnosis in a high percentage of patients (29.3%); however, many of these presented vascular risk factors, as in other series.2–7 In a considerable percentage of cases (14.6%), the event was attributed to fibrocartilaginous embolism. Diagnosis of this rare cause of spinal cord infarction can only be established in autopsy studies; it should be suspected in young patients with history of Valsalva manoeuvre, intense exercise, or trauma before the event, few or no vascular risk factors, and no other causes of spinal cord infarction.11
No treatment recommendations have been issued to date. In the acute phase, some isolated cases support the use of intravenous fibrinolysis.12–14 However, the only clinical trial designed to provide evidence on this topic had to be terminated due to recruitment problems. Some studies support the use of lumbar CSF drainage in patients with spinal cord infarction following aortic surgery; however, this treatment is not without complications.15 In the remaining patients, some authors recommend controlling vascular risk factors and starting antiplatelet or anticoagulation therapy when clinically appropriate1. Lastly, although antiplatelet therapy for fibrocartilaginous embolism is controversial, some authors recommend this treatment in patients presenting vascular risk factors.1 In our study, 56.1% of patients started antiplatelet therapy at discharge (including 2 patients diagnosed with fibrocartilaginous embolism) and 7.3% started anticoagulation therapy.
Spinal cord infarction presents elevated morbidity and mortality rates. Some studies suggest that spinal cord infarction has a better prognosis than stroke.16 However, other authors disagree.3,17,18 Severity of neurological symptoms at onset may be the most important prognostic factor.3 In our series, 29.3% of patients walked independently at the end of follow-up, and 2 factors were associated with poor prognosis: presence of vascular risk factors and presentation with paraparesis.
Our study has several limitations, including the relatively small size of our sample and its retrospective design.
ConclusionIn our experience, spinal cord infarction may have multiple aetiologies, which cannot be determined in many cases. MRI is an essential tool for diagnosis, with DWI sequences being particularly useful. Long-term functional prognosis is poor and depends on the patient’s baseline characteristics and the form of presentation. Further research is necessary to determine the optimal treatment in these patients.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
Conflicts of interestThe authors have no conflicts of interest to declare.