Ergot derivatives are drugs with vasoconstrictor effects that are used to abort migraine attacks. This study aims to determine how ergotamine derivatives are prescribed by physicians in Colombia, find variables associated with inappropriate prescribing, and review potential interactions in our patients.
MethodsWe reviewed 86411 formulas during April 2012, identifying the prescription by drug, dose, interval, duration of use, and indication. We interviewed 288 randomly selected patients in whom we also investigated concomitant use of (a) antihypertensive agents, (b) ischaemic heart disease treatments, (c) antiretrovirals, (d) other antimigraine drugs, and (e) macrolides, because of their potential for interactions.
ResultsWe identified 801 prescriptions from patients in 27 cities with a mean age of 35.1±14.1 years; 82.5% of the prescriptions were for women, 96.5% were written by primary care physicians, and 65.4% (n=524) corresponded to migraine treatments. There were 26 different prescription types and 797 prescriptions were incorrect with regard to usage recommendations (99.5%). Inappropriate prescribing was significantly associated with the health centre providing patient care (P=.005). Of the patients who were interviewed by telephone, 266 (92.4%) took the drug according to the erroneous indication. A total of 54 patients (6.7%) were treated with antihypertensive drugs, 24 (2.9%) with macrolides, and 5 (0.6%) with another concomitant antimigraine drug.
DiscussionMost patients take ergotamine improperly, apart from the fact that potential interactions may increase the risk of health problems such as ergotism and coronary events. Physicians will require assessment measures, updated information, and continuous training.
Los derivados ergotamínicos son medicamentos para abortar las crisis migrañosas, con un efecto vasoconstrictor, que poseen una forma específica de formulación. Se pretendió determinar la forma de prescripción de derivados ergotamínicos por médicos, las variables asociadas a inadecuadas prescripciones y las interacciones potenciales en pacientes de Colombia.
MétodosSe revisaron 86.411 fórmulas durante el mes de abril del 2012; se identificó la prescripción, con medicamento, dosis, intervalo, tiempo de uso e indicación. Se entrevistó a 288 pacientes seleccionados aleatoriamente, en los que además se buscó uso concomitante con: a) antihipertensivos; b) medicamentos para enfermedad cardíaca isquémica; c) antirretrovirales; d) otros antimigrañosos, y e) macrólidos a causa de sus interacciones.
ResultadosSe obtuvieron 801 prescripciones a pacientes en 27 ciudades del país con edad promedio de 35,1±14,1 años, el 82,5% en mujeres, el 96,5% de ellas realizadas por el médico de atención primaria, 524 (65,4%) de los casos para migraña; se hallaron 26 formas de prescripción distintas y 797 prescripciones incorrectas en cuanto a recomendaciones de uso (99,5%). La prescripción inadecuada se asoció significativamente a los centros de atención médica donde era atendido el paciente (p=0,005). De los pacientes entrevistados, 266 (92,4%) lo tomaron según la errónea indicación. En total, 54 (6,7%) pacientes tomaban antihipertensivos, 24 (2,9%) macrólidos y 5 (0,6%) más otro antimigrañoso concomitantemente.
DiscusiónLa mayoría de los pacientes están recibiendo ergotamina de manera inadecuada, sumado a las posibles interacciones que elevan el riesgo de problemas para la salud, como ergotismo y eventos coronarios. Deben implementarse medidas de evaluación, actualización y formación continua para médicos.
Migraine has a prevalence of 8.0% in men and 17.2% in women. It was estimated that 35 million patients in the USA presented migraines in 2005; in 2006, 12.6% of Spanish population experienced migraines and 1 in 4 female migraine sufferers had 4 or more severe attacks per month. During these attacks, more than 80% of patients are severely incapacitated and require bed rest.1–3
Pharmacological treatment for migraines is divided into 2 main categories: preventative and symptomatic. The aim of symptomatic treatment is to diminish pain using agents such as non-steroidal anti-inflammatory drugs, analgesics (alone or as combination therapy), and opioids if pain is very intense. It also includes migraine-specific drugs, such as triptans and ergot derivatives that are recommended for aborting migraine attacks.4–9
Ergotamine is a drug that can bind to serotonin, dopamine, and adrenaline receptors. It acts as an agonist and antagonist of multiple neurological circuits and elicits significant vasoconstriction of arteries, both in the brain and in general.10 It has been linked to different adverse drug reactions, especially vasomotor events, acute vascular insufficiency, and a disorder known as ergotism. Ergotism may be serious and it can develop independently from the drug dose and pharmacological interactions. It has also been associated with acute coronary events.11 In Colombia, use of ergotamine has become more widespread due to the low cost of the drug and the fact that it is offered by the Colombian compulsory health plan. This plan covers all basic drugs available to patients registered with the Colombian health and social security system (SGSSS). The sole indications for this drug are migraine and cluster headache. It is prescribed in doses proved to be effective and safe for aborting a migraine attack, and doses cannot exceed 6mg per day or 10mg per week.
The aim of this study is to determine how ergot derivatives are prescribed in Colombia, identify variables associated with how they are prescribed, and list potential drug interactions in patients registered with the SGSSS in 2012.
Patients and methodsWe conducted a cross-sectional study on the use of ergotamine in Colombian patients registered with the SGSSS. The study period was between 1 April and 30 April 2012. Audifarma S.A. is a logistic operator responsible for dispensing drugs to different institutional clients that provide health care services in Colombia. It currently supplies 1.5 million formulas per month to approximately 6 million users throughout the country. All the information on the supply of drugs is stored in a database. From this database, we can obtain statistics broken down by institutional client (for example, health insurance providers [HIP] or health care centres [HCC]), city, user, drug, disease, prescribing doctor's specialty, and dosage form.
The department of pharmacoepidemiology at Audifarma S.A. reviews statistics on a daily basis to find drugs that may be related to negative outcomes associated with medication (NOM) in specific cases. These negative outcomes are classified as necessity, effectiveness, and safety problems.
One of our doctors reviewed 86411 prescriptions randomly taken from the total prescribed during that month. The following variables were taken into account in data analysis:
- –
Sociodemographic variables: age, sex, city of residence.
- –
Pharmacological variables: antimigraine drug used (ergotamine+caffeine 1/100mg), dose (mg), dosage schedule (interval between doses) and treatment duration (days). Other pharmacological variables were the indication recorded by the doctor according to the International Classification of Diseases, the prescribing doctor's specialty, and whether drugs were prescribed as monotherapy or combination therapy. The following prescriptions were considered appropriate according to the manufacturer's instructions and clinical practice guidelines: taking a tablet with the first signs of migraine and adding one tablet every 30minutes if necessary (without exceeding 6mg in 24hours or 10mg per week). Researchers also checked whether the drug was prescribed for the approved indications.1,4,7
- –
Concomitant drugs: to establish drugs prescribed concomitantly, we listed all drugs in the following groups that are available in Colombia: (a) antihypertensive drugs (beta-adrenergic blockers, angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, alpha-adrenergic blockers, calcium channel blockers); (b) drugs for ischaemic heart disease (nitrovasodilators, ivabradine, trimetazidine); (c) antiretroviral drugs (protease inhibitors); (d) other antimigraine drugs (triptans); (e) macrolides, and (f) triazoles. In such cases, suitability of the drug was analysed according to the type of comorbidity. Lastly, we reviewed drug combinations that may provoke potentially dangerous interactions in order to recommend corrective actions.
To find how patients were taking antimigraine drugs, we randomly selected patients treated with ergot derivatives during the month of April and who gave their verbal informed consent. We contacted them over telephone and asked them about the following: diagnosis for which the drug was prescribed; how they were taking the drug (dose, schedule, treatment duration); presence of comorbidities such as hypertension, ischaemic heart disease, and human immunodeficiency virus; and use of other drugs able to cause interactions.
The protocol was approved by the Ethics Committee at the Faculty of Health Sciences at Universidad Tecnológica de Pereira. It was classified as “research without risk” in accordance with resolution No. 008430 of 1993 of the Colombian Ministry of Health, which establishes the scientific, technical and administrative standards for health research in compliance with the Declaration of Helsinki.12
The database was revised and validated by the department of pharmacoepidemiology at the company dispensing the drugs. Data analysis was performed using SPSS statistics software, version 20 for Windows (IBM-USA). For comparison of quantitative variables, we used the t-test or ANOVA method; for categorical variables, we used chi-square tests. Bivariate analyses were performed to identify the sociodemographic, pharmacological, and concomitant drug variables associated with inappropriate prescription. The level of statistical significance was set at P<.05.
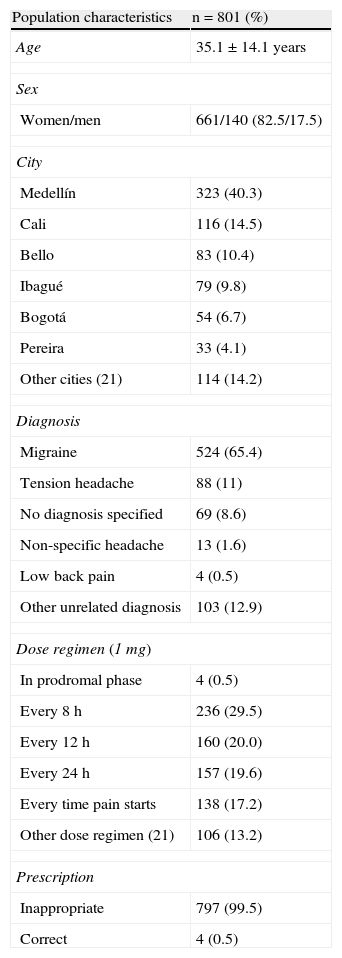
ResultsTable 1 shows sociodemographic and pharmacological characteristics associated with prescription of ergot derivatives. We obtained a total of 801 formulas containing ergotamine during the month of April 2012; they were predominantly prescribed to women and mean age was 35.1±14.1 years (range, 10-94 years). We identified patients from 15 health insurance providers and health care centres (HIP and HCC) in 27 different Colombian cities.
Sociodemographic, clinical, and pharmacological characteristics of 801 patients from 27 Colombian cities who were prescribed ergotamine in 2012
| Population characteristics | n=801 (%) |
| Age | 35.1±14.1 years |
| Sex | |
| Women/men | 661/140 (82.5/17.5) |
| City | |
| Medellín | 323 (40.3) |
| Cali | 116 (14.5) |
| Bello | 83 (10.4) |
| Ibagué | 79 (9.8) |
| Bogotá | 54 (6.7) |
| Pereira | 33 (4.1) |
| Other cities (21) | 114 (14.2) |
| Diagnosis | |
| Migraine | 524 (65.4) |
| Tension headache | 88 (11) |
| No diagnosis specified | 69 (8.6) |
| Non-specific headache | 13 (1.6) |
| Low back pain | 4 (0.5) |
| Other unrelated diagnosis | 103 (12.9) |
| Dose regimen (1mg) | |
| In prodromal phase | 4 (0.5) |
| Every 8h | 236 (29.5) |
| Every 12h | 160 (20.0) |
| Every 24h | 157 (19.6) |
| Every time pain starts | 138 (17.2) |
| Other dose regimen (21) | 106 (13.2) |
| Prescription | |
| Inappropriate | 797 (99.5) |
| Correct | 4 (0.5) |
General practitioners and primary care doctors provided most prescriptions (n=773, 96.5%); the remainder were written by paediatricians, family doctors, occupational medicine specialists, surgeons, and neurosurgeons (n=28, 3.4%). We found 26 different sets of prescription instructions, of which 797 (99.5%) were inappropriate with regard to usage recommendations and 277 (34.6%) were inappropriate because they were given for conditions for which the active ingredient had not been approved.
Relationship between inappropriate prescription and other variablesInappropriate prescription of the drug was significantly associated with the HIP and HCC providing patient care (P=.005). No significant differences were found regarding city, sex, prescribing doctor's specialty, diagnosis, or age group.
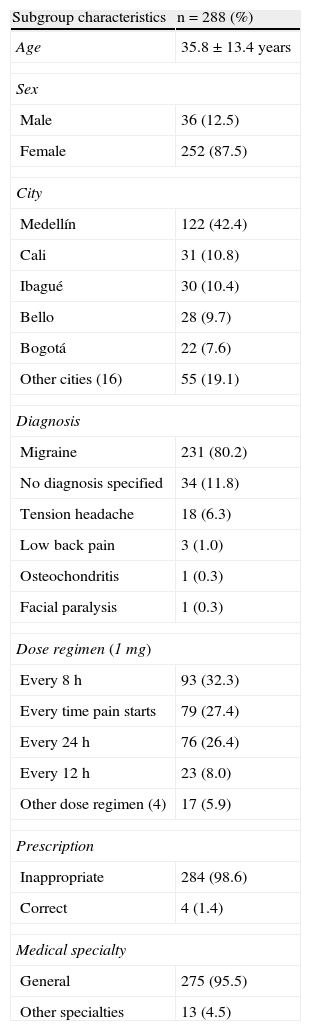
Analysis of the interviewed patient subgroupCharacteristics of the 288 patients selected for an interview are displayed in Table 2. We found a mean age of 35.8±13.4 years among these patients, who were predominantly women, from 21 cities and registered with 10 different HIPs and HCCs. Prescriptions were provided mainly by primary care doctors, with a small percentage written by paediatricians, occupational medicine specialists, family doctors, surgeons, and neurosurgeons.
Sociodemographic, clinical and pharmacological characteristics of 288 patients from 21 Colombian cities who were prescribed ergotamine in 2012
| Subgroup characteristics | n=288 (%) |
| Age | 35.8±13.4 years |
| Sex | |
| Male | 36 (12.5) |
| Female | 252 (87.5) |
| City | |
| Medellín | 122 (42.4) |
| Cali | 31 (10.8) |
| Ibagué | 30 (10.4) |
| Bello | 28 (9.7) |
| Bogotá | 22 (7.6) |
| Other cities (16) | 55 (19.1) |
| Diagnosis | |
| Migraine | 231 (80.2) |
| No diagnosis specified | 34 (11.8) |
| Tension headache | 18 (6.3) |
| Low back pain | 3 (1.0) |
| Osteochondritis | 1 (0.3) |
| Facial paralysis | 1 (0.3) |
| Dose regimen (1mg) | |
| Every 8h | 93 (32.3) |
| Every time pain starts | 79 (27.4) |
| Every 24h | 76 (26.4) |
| Every 12h | 23 (8.0) |
| Other dose regimen (4) | 17 (5.9) |
| Prescription | |
| Inappropriate | 284 (98.6) |
| Correct | 4 (1.4) |
| Medical specialty | |
| General | 275 (95.5) |
| Other specialties | 13 (4.5) |
Most patients were prescribed the drug for migraines, but it was also prescribed for other entities, such as tension headache, and more surprisingly, low back pain, osteochondritis, and facial paralysis.
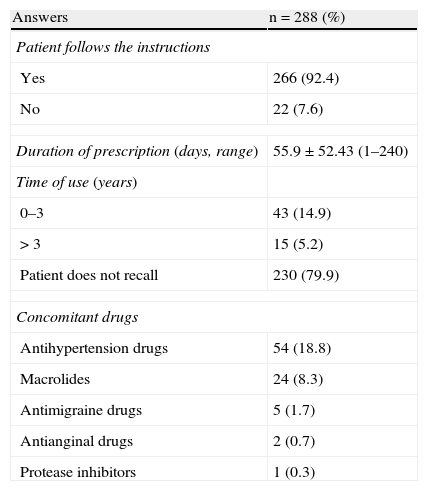
With regard to dose interval, the drug was correctly prescribed to only 4 patients (1.4%) and many cases of inappropriate use were found, including instructions to take it every 8, 12 or 24hours during several weeks or months. Most patients (92.4%) reported taking the drug as directed by the prescribing doctor while 22 patients (7.6%) reported not following instructions. Variables for patient compliance with administration instructions and timing, and those regarding concomitant drugs, especially hypertension drugs and macrolides, are shown in Table 3.
Results from the interview on the administration instructions and concomitant drugs in 288 patients from 21 cities of Colombia who were prescribed ergotamine, 2012
| Answers | n=288 (%) |
| Patient follows the instructions | |
| Yes | 266 (92.4) |
| No | 22 (7.6) |
| Duration of prescription (days, range) | 55.9±52.43 (1–240) |
| Time of use (years) | |
| 0–3 | 43 (14.9) |
| >3 | 15 (5.2) |
| Patient does not recall | 230 (79.9) |
| Concomitant drugs | |
| Antihypertension drugs | 54 (18.8) |
| Macrolides | 24 (8.3) |
| Antimigraine drugs | 5 (1.7) |
| Antianginal drugs | 2 (0.7) |
| Protease inhibitors | 1 (0.3) |
In a cohort of patients registered with the SGSSS, we determined that most ergotamine prescriptions were inappropriate. This information may be used by health care managers when making decisions to improve the quality of health care provided to patients who are prescribed ergotamine. Age and sex ratios in this patient group were similar to those reported in other studies; this can be expected because migraine is more likely to affect women.1–5,13–18
The vast majority of prescriptions were written by primary care doctors, which contrasts with observations in other studies (95.5% in our study vs 55% to 70% in other studies).19,20 This may be due to difficulties with patient access to specialists due to SGSSS regulations or even indicate patient under-registration.18 Prescribing ergotamine for other entities, such as tension headache, has been described in previous studies but this practice delivers poor results.20 This prescription cannot be justified given that the drug is only indicated in cases of migraine and cluster headache.21 It is also surprising to find ergotamine being prescribed for low back pain and osteochondritis, which has not been reported by any other study.
Even when we consider the variability of the medical care received by these patients, there is a surprising amount of variety in the prescription instructions, especially regarding doctors’ prescription habits. This finding is consistently confirmed by pharmacoepidemiological studies, as in this case, because ergotamine has only one drug formulation.22 It represents a medication error, or prescription error to be more specific, which in this case included using specifying doses, intervals, and treatment durations different from those recommended. Drug selection was also inappropriate given that ergotamine was used to treat problems other than migraine. Selecting this drug is also considered inappropriate for patients with hypertension or heart disease because of the risk of damage caused by the treatment itself.23
Inappropriate use of drugs has such significant and serious risks that between 44000 and 98000 patients are estimated to die because of medical errors in the USA, making this the seventh most common cause of death in that country.24,25 Medication errors represent 28% of all medical errors in the USA.26 Some descriptions have shown that prescription errors are mainly due to inability to identify them. This is especially true among inexperienced doctors who are responsible for between 4.2% and 82.0% of prescription errors.24
Given the magnitude of the problem, it is imperative to establish systems that consistently disclose potential errors, reduce the risks, and mitigate the consequences when mistakes arise. These risks have great clinical and financial repercussions, both for patients and for healthcare systems.26
Some of the actions carried out to reduce prescription errors involve establishing thresholds to reduce the possibility of error, using protocols that all professionals should follow, and implementing medical audits. At the same time, health care centres and universities should offer continuous training programmes to keep doctors’ knowledge up-to-date so that they can provide quality care to patients and reduce the risks inherent to inappropriate prescription.27,28
Treatment duration is another point to consider when prescribing ergotamine. This drug is associated with rebound headache a few hours after starting treatment, which may be confused with another migraine attack. This can lead the doctor to extend the treatment, thereby increasing the risk of ischaemia in different organs, especially if the treatment is not appropriate.27,28 Drug interactions can increase the number of adverse reactions. For example, triptans, especially sumatriptan, should not be administered in the 24hours after taking ergotamine, as the combination of these 2 agents has caused vasospastic episodes in patients.28 The literature also contains cases of ergotism arising as a complication of acute intoxication with or chronic abuse of ergot derivatives, especially in patients taking macrolides and protease inhibitors concomitantly. This occurs because these drugs inhibit the enzyme that metabolises ergotamine (CYP3A4), and concentrations of the drug therefore reach toxic levels.11,27,28 On the other hand, administration of ergotamine in patients with hypertension and ischaemic heart disease, as seen in our cohort, is contraindicated or requires extreme caution. The drug may increase the risk of adverse cardiovascular reactions, such as cerebral, cardiac, and peripheral ischaemic disorders, acute coronary syndromes, intermittent claudication, hypertension, tachycardia or bradycardia, headache, and kidney disorders.29
Based on the prescription instructions found in this study, we can state that bad habits predominate in the use of ergotamine, given that doctors cite doses and intervals that are not recommended by the literature for patients with comorbidities such as arterial hypertension or ischaemic heart disease. These comorbidities contraindicate use of ergot derivatives or other vasoconstrictors, or at the very least require doctors to proceed with extreme caution since vasoconstrictors can exacerbate these disorders.
In view of the results, it is clear that prescription errors and potential interactions between ergotamine and other drug groups are common and numerous patients are exposed to these risks. Therefore, risks should be previously assessed by the prescribing doctor and the patient must be duly informed. Regarding the limitations of the study, it should be noted that medical histories were not searched for undesirable effects recorded by doctors. We did not evaluate all prescription instructions in every city in the country and the study period was limited to one month. However, we should also highlight that our purpose was to evaluate how doctors prescribe the drug, rather than patient compliance or drug safety.
It could be argued that companies responsible for dispensing drugs to institutional clients should develop strategies permitting continuous assessment and updating for drug-related risks and periodically notify health care centres of such risks. Together with the doctors responsible for the patients, these companies should also follow up on every patient subjected to this risk and review the recommendations made by regulating entities in order to ensure safer use of the drug.28,30 Clinical practice guidelines for treating migraine must be developed. Novel drugs that have been safer and more effective than others should also be contemplated in these guidelines, which must highlight crisis prevention strategies.31–33 The continuous education needs of primary care doctors in the country must be revised. Doctors should receive training on the prescription of drugs for managing headaches and migraine, since so many patients seek medical advice for these conditions. This has already been evaluated in Spain.32
Although these strategies cannot solve all drug safety issues, they may offer ways of preventing possible risks to patients. We therefore recommend adopting them with a view to strengthening pharmacovigilance programmes in centres with databases to systematically record the drugs they dispense.
FundingThe study was financed by Universidad Tecnológica de Pereira and Audifarma S.A.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Machado-Alba JE, Morales-Plaza CD. Utilización de ergotamina: ¿saben los médicos en Colombia cómo prescribirla? Neurología. 2014;29:280–285.