Obstetric brachial plexus palsy (OBPP) usually has a favourable prognosis. However, nearly one third of all severe cases have permanent sequelae causing a high level of disability. In this study, we explore the effectiveness of ultrasound-guided injection of botulinum toxin A (BoNT-A) and describe the procedure.
Patients and methodsWe designed a prospective, descriptive study including patients with moderate to severe OBPP who were treated between January 2010 and December 2014. We gathered demographic data, type of OBPP, and progression. Treatment effectiveness was assessed with the Active Movement Scale (AMS), the Mallet classification, and video recordings.
ResultsWe gathered a total of 14133 newborns, 15 of whom had OBPP (1.6 per 1000 live births). Forty percent of the cases had severe OBPP (0.4/1000), a dystocic delivery, and APGAR scores <5; mean weight was 4038g. Mean age at treatment onset was 11.5 months. The muscles most frequently receiving BoNT-A injections were the pronator teres, subscapularis, teres major, latissimus dorsi, and pectoralis major. All the patients who completed the follow-up period (83%) experienced progressive improvements: up to 3 points on the AMS and a mean score of 19.5 points out of 25 on the Mallet classification at 2 years. Treatment improved muscle function and abnormal posture in all cases. Surgery was avoided in 3 patients and delayed in one. Adverse events were mild and self-limited.
ConclusionsDue to its safety and effectiveness, BoNT-A may be used off-label as an adjuvant to physical therapy and/or surgery in moderate to severe OBPP. Ultrasound may increase effectiveness and reduce adverse effects.
La parálisis braquial obstétrica (PBO) suele tener un pronóstico favorable; sin embargo, casi un tercio de los casos graves presentan secuelas permanentes que generan gran discapacidad. Valoramos la eficacia del tratamiento con infiltraciones ecoguiadas de toxina botulínica A (TB-A) y describimos el procedimiento.
Pacientes y métodosEstudio prospectivo, descriptivo, de los casos de PBO moderada-grave tratados entre enero del 2010 y diciembre del 2014. Recogimos datos demográficos, tipo de PBO y evolución. Valoramos eficacia con la escala de movimiento activo (EMA), Mallet Classification System y videofilmación.
ResultadosValoramos a 14.133 recién nacidos vivos, con 15 casos de PBO (1,06 por cada 1.000). El 40% casos graves (0,4/1000), nacidos de parto distócico, con APGAR <5 y peso medio de 4.038 g. Edad media de inicio de infiltraciones 11,5 meses. El pronator teres, subescapularis, teres major, latissimus dorsi y/o pectoralis major fueron los más frecuentemente infiltrados. Mejoría progresiva hasta 3 niveles en EMA y una media de 19,5 puntos sobre 25 en la escala de Mallet a los 2 años en todos los casos de PBO que completaron el seguimiento (83%). Todos mejoraron funcionalidad y posturas anormales. Evitamos la cirugía en 3 pacientes y se retrasó en uno. Los efectos adversos del tratamiento fueron leves y autolimitados.
ConclusionesPor su seguridad y eficacia, parece razonable la utilización «off label» de TB-A como tratamiento adyuvante a las terapias físicas y/o tratamiento quirúrgico en las PBO moderadas-graves. La ecografía podría aumentar la eficacia y disminuir efectos adversos.
Obstetric brachial plexus palsy (OBPP) is the most frequent neonatal peripheral neuropathy, with incidence ranging from 0.5 to 2 cases per 1000 live births in developed countries, depending on the series.1–4
Prognosis depends on the severity and extension of the lesions to the nerve roots. Fortunately, most cases spontaneously achieve complete recovery, or at least a near-normal range of motion and strength, in the first 6 to 8 weeks of life.5 However, in severe cases, permanent motor sequelae cause considerable disability; these cases require early identification and treatment.
Permanent functional limitation occurs in approximately one-third of severe lesions and mainly affects the muscles involved in elbow flexion, forearm supination, wrist extension, and the external rotation and abduction of the shoulder.5–7 Weakness of these muscle groups leads to an imbalance in strength with respect to the healthy antagonist muscles, resulting in significant functional limitation of the affected limb. Given its natural history, persistent muscle imbalance would limit the motion and strength of the affected limb, which frequently leads to the development of muscle contractures and occasionally bone deformities, mainly in the shoulder.8
It is therefore vitally important to identify the most severe cases early and to follow a systematic action protocol to limit permanent disability in children, whenever possible.
Botulinum toxin (BT) has been found to be effective and safe in the treatment of muscle imbalance, muscle co-contractions, and contractures; it may be used in these patients to weaken the healthy antagonist muscles with the aim of balancing strengths and enabling growth, strengthening, and functional improvement of the affected muscles through physical and occupational therapies, facilitating the development of a correct movement pattern during reinnervation, whenever possible.9–11
The aim of this study is to describe our experience with the treatment of OBPP with ultrasound-guided infiltrations of BT type A (BoNT-A), and to describe the procedure and action protocol.
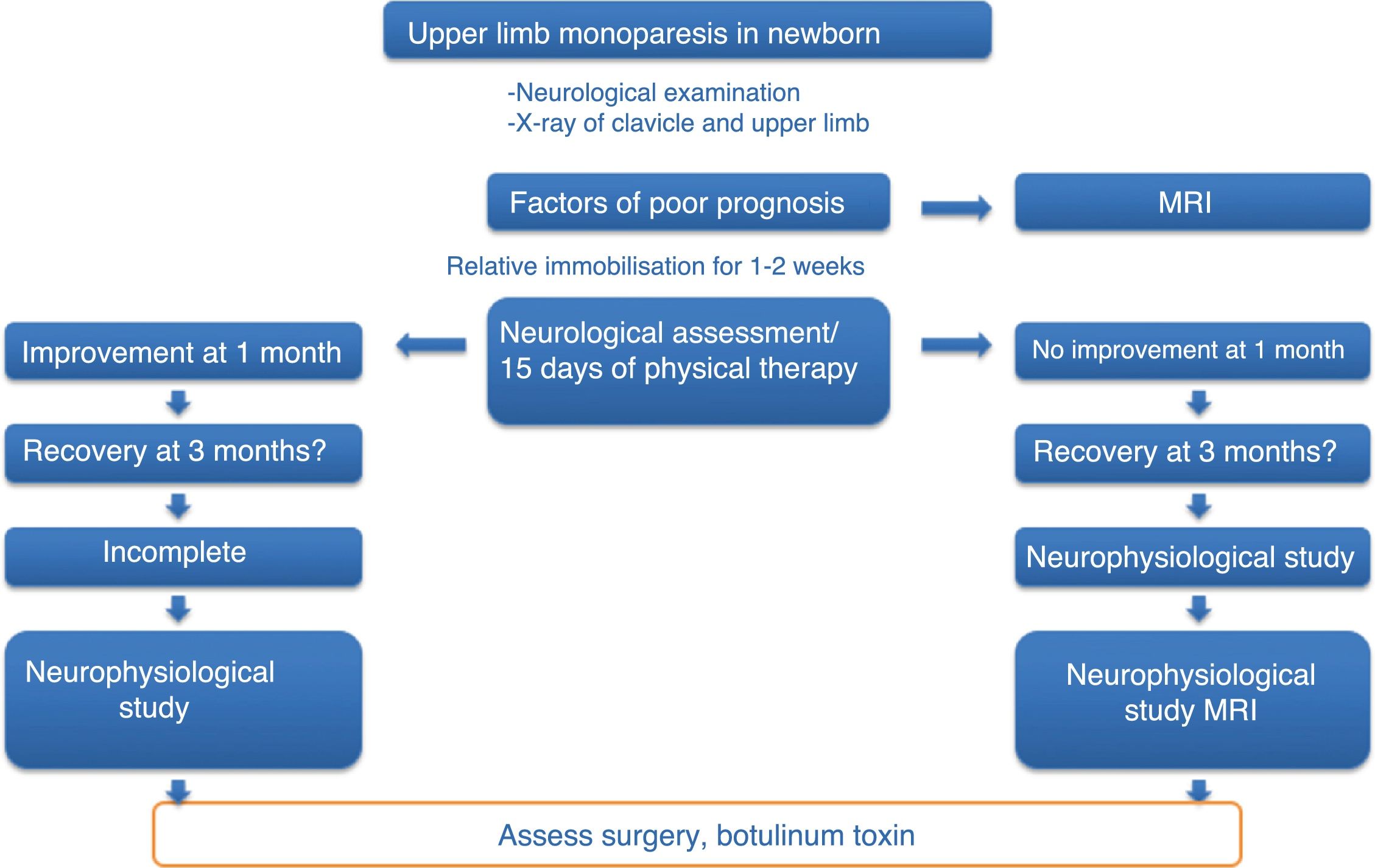
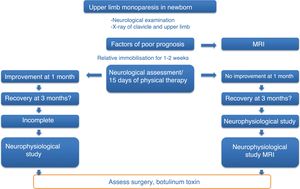
Patients and methodsThis is an open, non-randomised, prospective, descriptive study on the safety and effectiveness of treatment with ultrasound-guided infiltrations of BoNT-A, including all cases of moderate-to-severe OBPP diagnosed and treated at our centre between January 2010 and December 2014. Patients were included according to a previously established action protocol (Fig. 1), which is applied to all newborns with upper limb monoparesis. Once the diagnosis of OBPP was established, follow-up visits were scheduled to assess the clinical progression of newborns and include candidates for BoNT-A infiltrations; patients’ parents signed informed consent forms before treatment.
Inclusion criteria- -
Moderate-to-severe OBPP showing slight improvement with physical rehabilitation
- -
Recovery of biceps contraction at 6 to 9 months of life
- -
Muscle imbalances involving shoulder, elbow, forearm, or wrist muscles, leading to anomalous movement patterns and/or positions
- -
Biceps-triceps co-contraction
- -
Joint contractures (elbow flexion) in the affected arm
- -
Commitment to continue physical and occupational therapy before and after BoNT-A infiltrations
We excluded patients with mild OBPP with complete resolution in the first months of life, who therefore needed no treatment with BoNT-A.
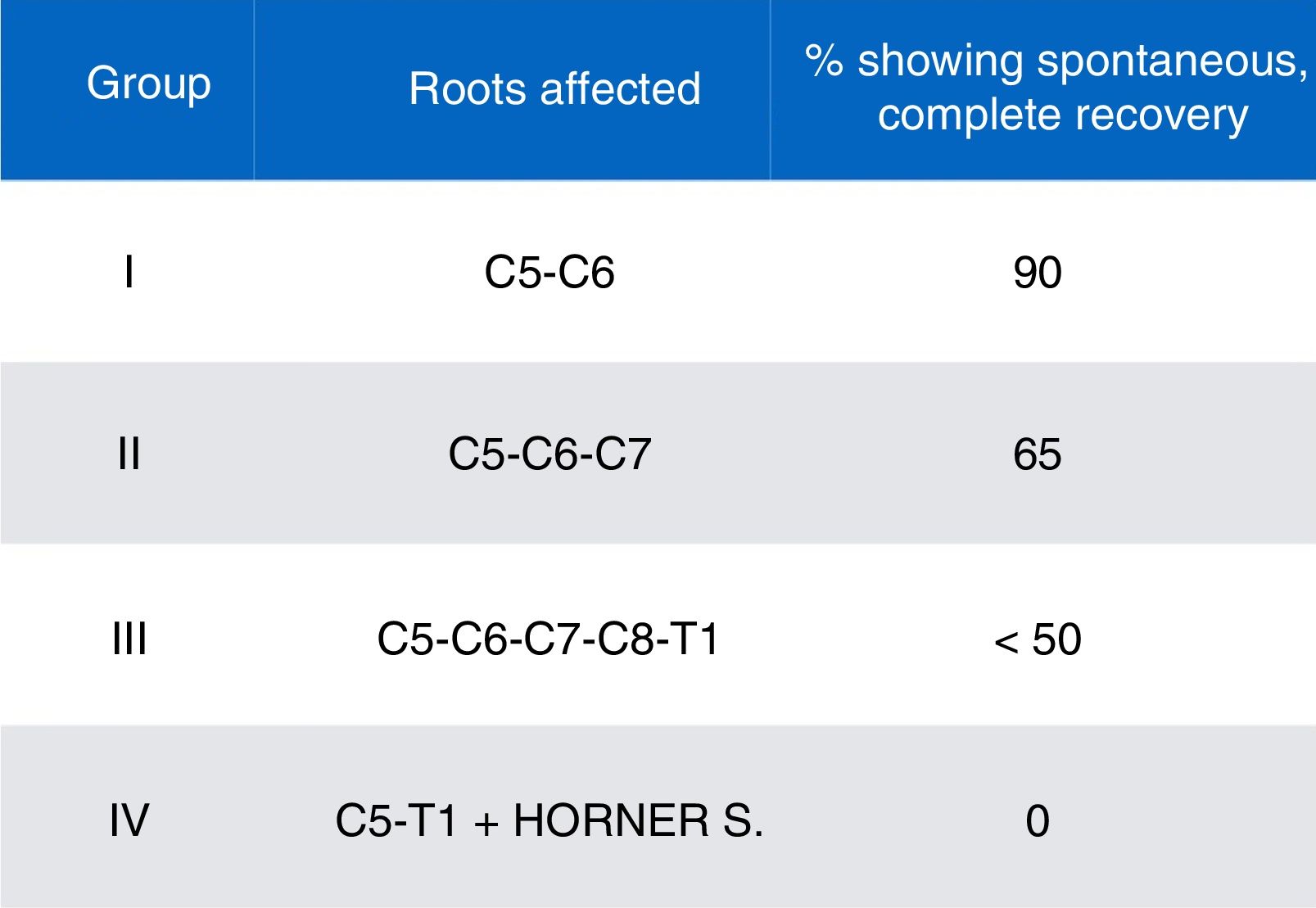
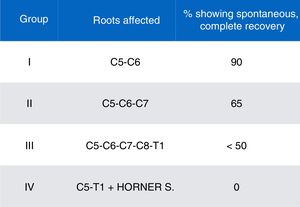
We gathered data on demographic variables (age, sex), obstetric history (gestational age at birth, type of delivery, birth weight), type of OBPP (affected side, affected nerve roots, and classification by severity and prognosis according to the schema proposed by Gilbert and Tassin/Narakas [Fig. 2]),12,13 and factors associated with poor prognosis (Horner syndrome, diaphragmatic paralysis, complete paralysis, lack of improvement with conservative treatment at 3 months of progression, lack of antigravity strength in the deltoids and triceps at the end of the third month of life).
ProcedureAll infiltrations were performed by the same physician, using ultrasound guidance, conscious analgesia/sedation with Kalinox® (50% protoxide of nitrogen and 50% medical oxygen; Air Liquide Healthcare, Spain), and topical analgesia with Emla® cream (lidocaine 25mg/g+prilocaine 25mg/g; AstraZeneca Farmacéutica Spain, SA) in collaborating patients; unconscious sedation with intravenous drugs (ketamine, midazolam, propofol, or fentanyl, depending on the patient) was also used in non-collaborating patients to minimise complications and adverse reactions and to improve efficacy.
- 1.
Muscle selection: performed on a case-by-case basis according to the previous examination of the affected limb, function, and/or deformity or the most frequent anomalous motion patterns.
- a.
Internal rotation of the shoulder: subscapularis muscles, teres major muscle, latissimus dorsi, and/or pectoralis major
- b.
Extension of the arm: triceps brachii
- c.
Pronation of the forearm: pronator teres and/or pronator quadratus
- d.
Wrist flexion: flexor carpi ulnaris or flexor carpi radialis muscles
- e.
Finger flexion: flexor digitorum superficialis or flexor digitorum profundus
- a.
- 2.
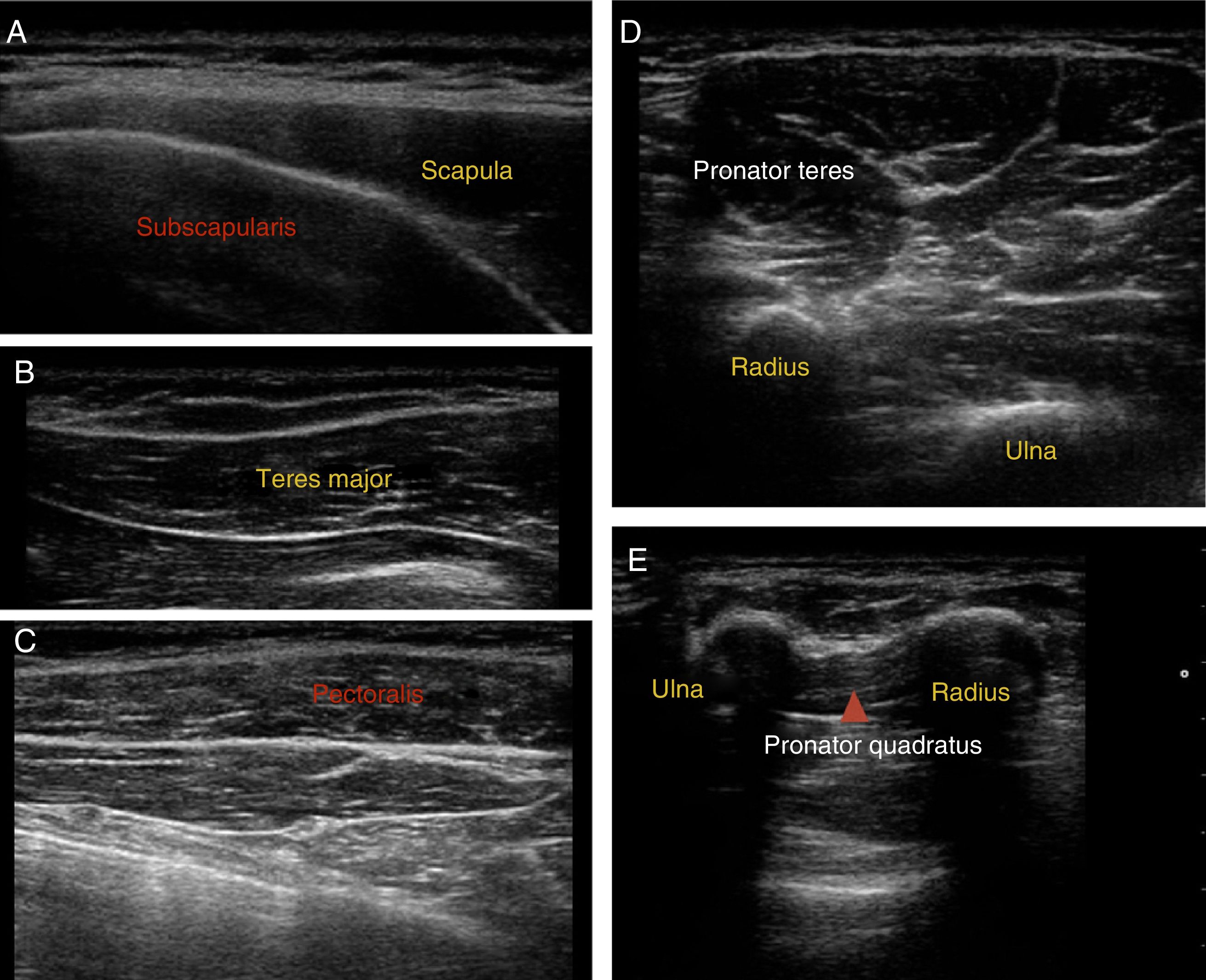
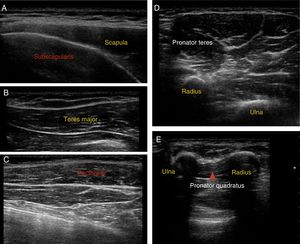
Muscle location: ultrasound guidance was used to locate target muscles and control the infiltration technique. Fig. 3 shows ultrasound images of the most frequently infiltrated muscles.
- 3.
Dosing: drugs were dosed according to the toxin used (Onabotulinum toxin A [Botox®, Allergan Pharmaceuticals Inc. TB-A, 100U vial] or Abobotulinum toxin A [Dysport®, Ipsen Pharmaceuticals, France, 500U vial]), the patient's body weight, the muscle to be infiltrated, and symptom severity. We referred to the recommendations established in expert guidelines for the treatment of spasticity, adjusted according to the personal experience of the physician performing the infiltrations.14,15
- 4.
For the subscapularis muscles, teres major, pectoralis major, triceps brachii, pronator teres, and flexor carpi ulnaris or flexor carpi radialis, we used 1 to 2U/kg Botox® or 2 to 4U/kg Dysport®. For the latissimus dorsi, we used 1 to 3U/kg Botox® or 2 to 6U/kg Dysport®, and 0.5 to 1U/kg Botox® or 1 to 2U/kg Dysport® for the remaining muscles (pronator quadratus, etc.).
- 5.
Dilution: we reconstituted both toxins with low volumes to prevent as far as possible their spread to other muscles: 1mL of 0.9% saline solution with no preservatives for the 100U vial of Onabotulinum toxin A (0.1mL=10U), and 2.5mL of 0.9% saline solution for the 500U vial of Abobotulinum toxin A (0.1mL=20U).
- 1.
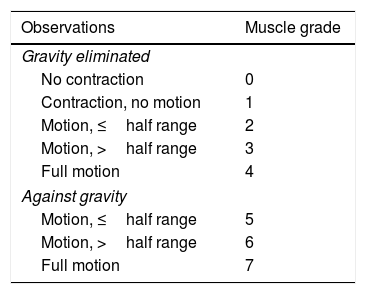
Active movement scale (AMS)16: based on the observation of spontaneous motion triggered by stimulation against and without gravity, before and after every infiltration. It assesses movement on an 8-point scale, with 0 indicating absence of muscle contraction and 7 indicating full motion (Table 1).
- 2.
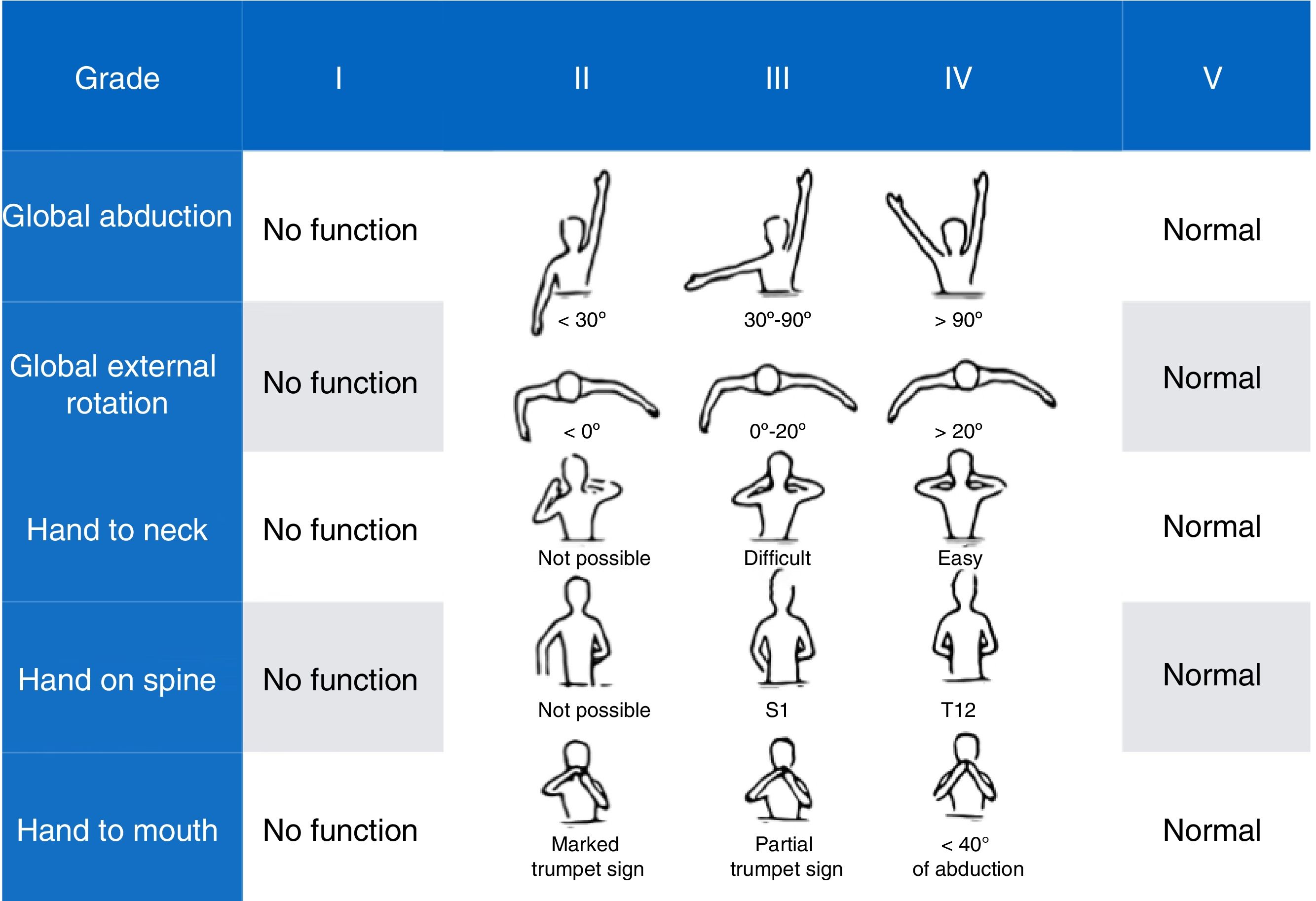
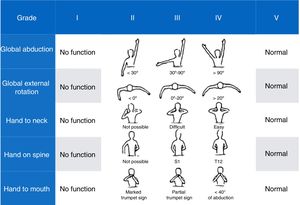
Mallet Classification System scale17: a scale assessing shoulder function. It includes 5 items: global abduction, global external rotation, and hand to neck, spine, and mouth; each is scored from 1 (no motion) to 5 (normal motion, symmetric to the unaffected side). Overall score ranges from a minimum of 5 to a maximum of 25 points (Fig. 4).
- 3.
Video recording before and after infiltration (with signed informed consent).
We conducted an interview during the follow-up consultations after infiltrations to detect possible adverse reactions to the toxins, which we classified as mild, moderate, or severe.
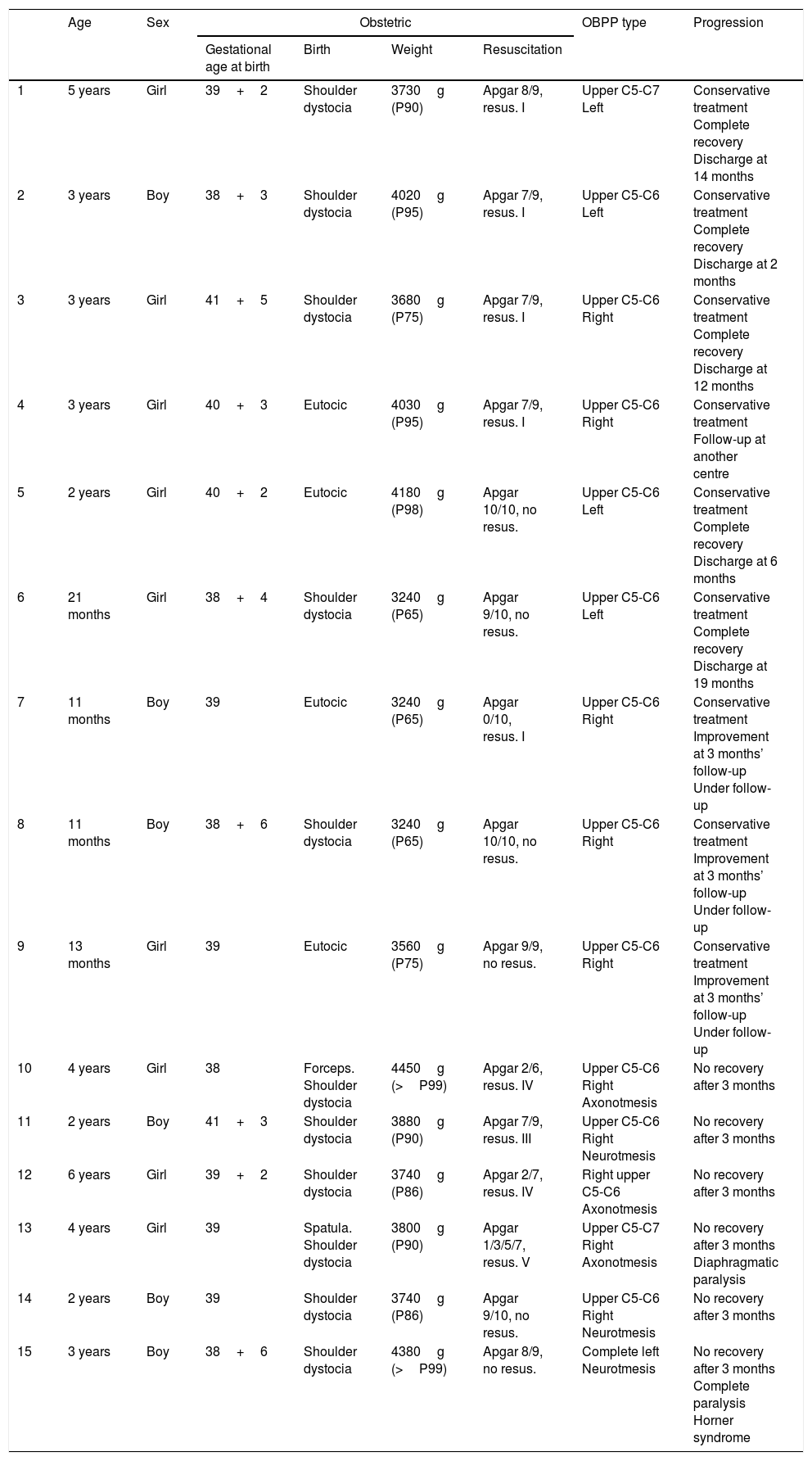
ResultsDuring the 4-year study period, we assessed 14133 live births; 15 were diagnosed with OBPP in the neonatal period, which represents an incidence of 1.06 cases per 1000 live births. All patients were delivered vaginally after full-term pregnancies, with a slight predominance of girls (60%). In terms of the overall analysis of the clinical characteristics of OBPP, upper nerve roots were affected in most cases, with 93% of the sample included in groups I and II; the right side was more frequently affected (2/3). Table 2 lists patients’ clinical and demographic characteristics.
Demographic and clinical characteristics of the sample.
| Age | Sex | Obstetric | OBPP type | Progression | ||||
|---|---|---|---|---|---|---|---|---|
| Gestational age at birth | Birth | Weight | Resuscitation | |||||
| 1 | 5 years | Girl | 39+2 | Shoulder dystocia | 3730g (P90) | Apgar 8/9, resus. I | Upper C5-C7 Left | Conservative treatment Complete recovery Discharge at 14 months |
| 2 | 3 years | Boy | 38+3 | Shoulder dystocia | 4020g (P95) | Apgar 7/9, resus. I | Upper C5-C6 Left | Conservative treatment Complete recovery Discharge at 2 months |
| 3 | 3 years | Girl | 41+5 | Shoulder dystocia | 3680g (P75) | Apgar 7/9, resus. I | Upper C5-C6 Right | Conservative treatment Complete recovery Discharge at 12 months |
| 4 | 3 years | Girl | 40+3 | Eutocic | 4030g (P95) | Apgar 7/9, resus. I | Upper C5-C6 Right | Conservative treatment Follow-up at another centre |
| 5 | 2 years | Girl | 40+2 | Eutocic | 4180g (P98) | Apgar 10/10, no resus. | Upper C5-C6 Left | Conservative treatment Complete recovery Discharge at 6 months |
| 6 | 21 months | Girl | 38+4 | Shoulder dystocia | 3240g (P65) | Apgar 9/10, no resus. | Upper C5-C6 Left | Conservative treatment Complete recovery Discharge at 19 months |
| 7 | 11 months | Boy | 39 | Eutocic | 3240g (P65) | Apgar 0/10, resus. I | Upper C5-C6 Right | Conservative treatment Improvement at 3 months’ follow-up Under follow-up |
| 8 | 11 months | Boy | 38+6 | Shoulder dystocia | 3240g (P65) | Apgar 10/10, no resus. | Upper C5-C6 Right | Conservative treatment Improvement at 3 months’ follow-up Under follow-up |
| 9 | 13 months | Girl | 39 | Eutocic | 3560g (P75) | Apgar 9/9, no resus. | Upper C5-C6 Right | Conservative treatment Improvement at 3 months’ follow-up Under follow-up |
| 10 | 4 years | Girl | 38 | Forceps. Shoulder dystocia | 4450g (>P99) | Apgar 2/6, resus. IV | Upper C5-C6 Right Axonotmesis | No recovery after 3 months |
| 11 | 2 years | Boy | 41+3 | Shoulder dystocia | 3880g (P90) | Apgar 7/9, resus. III | Upper C5-C6 Right Neurotmesis | No recovery after 3 months |
| 12 | 6 years | Girl | 39+2 | Shoulder dystocia | 3740g (P86) | Apgar 2/7, resus. IV | Right upper C5-C6 Axonotmesis | No recovery after 3 months |
| 13 | 4 years | Girl | 39 | Spatula. Shoulder dystocia | 3800g (P90) | Apgar 1/3/5/7, resus. V | Upper C5-C7 Right Axonotmesis | No recovery after 3 months Diaphragmatic paralysis |
| 14 | 2 years | Boy | 39 | Shoulder dystocia | 3740g (P86) | Apgar 9/10, no resus. | Upper C5-C6 Right Neurotmesis | No recovery after 3 months |
| 15 | 3 years | Boy | 38+6 | Shoulder dystocia | 4380g (>P99) | Apgar 8/9, no resus. | Complete left Neurotmesis | No recovery after 3 months Complete paralysis Horner syndrome |
Nine patients (60%) showed favourable progression with spontaneous, complete remission with conservative treatment, and were therefore excluded from the study. All patients were born after a full-term pregnancy and considered appropriate for gestational age; although they were born by non-instrumented vaginal delivery, shoulder dystocia occurred in 66% of births. No signs of foetal distress were observed, and no patient required resuscitation. In this subgroup with favourable progression, the most frequent type of OBPP was group I (8 cases), mainly affecting the sight side (5:4).
Our final sample included a total of 6 patients with moderate-to-severe OBPP (0.4/1000 live births). All were born after a full-term pregnancy by dystocic delivery (shoulder dystocia), with instrumentation (forceps) required for delivery in 2 cases. Weight ranged from 3740g to 4450g, with a mean weight of 4038g. Weight was considered high for gestational age in 2 cases, whereas the remaining patients were within percentiles 75 to 90. Three patients had an Apgar score <5, associated with foetal distress. By type of OBPP, the right upper nerve roots were most frequently affected (group II, 5 patients), followed by left total palsy (group IV). No patient presented bone lesions in neuroimaging studies, although we did observe a case of diaphragmatic paralysis among patients in the upper injury group.
All severe cases presented a lack of recovery (biceps contraction) with conservative treatment at the third month of life; from a neurophysiological perspective, a common negative prognostic factor was poor recovery in the electromyographical study due to severe nerve lesions (neuronotmesis and axonotmesis).
Infiltrations were started at ages ranging from the sixth to eighteenth month of life (mean 11.5 months), with a mean number of 3 infiltrations per patient. The most frequently infiltrated muscles were the subscapularis muscle, teres major, pectoralis major, latissimus dorsi, and pronator teres, which received infiltrations at least once in all treated cases, followed by the pronator quadratus and flexor carpi ulnaris.
Doses of BoNT-A were adjusted according to the patient's characteristics, response to previous infiltrations, and the type of toxin used. We used one adjusted dose per session and patient weight, which ranged from 8 to 10U/kg for onabotulinum toxin A, 100U vial, and 18 to 22U/kg for abobotulinum toxin A, 500U vial. We used an initial dose of 1U/kg of onabotulinum toxin A for the subscapularis muscle, teres major, pectoralis major, flexor carpi ulnaris or flexor carpi radialis muscle, flexor digitorum superficialis, and pronator quadratus, and 2U/kg for latissimus dorsi, biceps, triceps brachii, and pronator teres. Abobotulinum toxin A was initially administered at 4U/kg for the latissimus dorsi and pronator teres, 3U/kg for the subscapularis muscle, teres major, and pectoralis major, and 2U/kg for the remaining muscles.
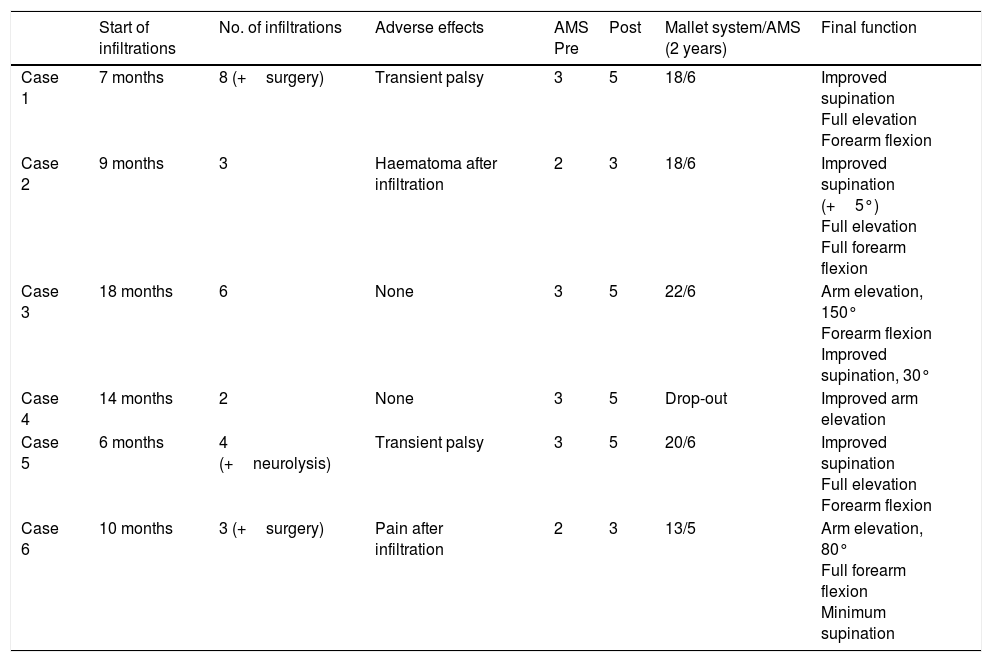
All patients received infiltrations at least twice, with 83.5% remaining under treatment and clinical follow-up. One patient was lost to follow-up due to a change of residence. From the first infiltration, we observed AMS score improvements of one level in the patient with total palsy and 2 levels in the remaining upper injury groups; symptoms improved 2 to 3 weeks after infiltration and mean duration of the relief was 4 months, with a drop of one level on the scale from that time. An additional infiltration before patients returned to the baseline situation, together with other treatments (occupational therapy, physical therapy, and/or surgery), enabled improvement of the limb to continue until patients reached level 6 on the AMS and a mean of 19.5 points out of 25 on the Mallet Classification System scale at 2 years’ progression in all cases of upper injury who completed follow-up.
Furthermore, regular infiltrations enabled us to avoid surgery in 3 patients and delay it on 2 occasions (case 1). All patients presented improvements in limb function and abnormal posture. Data on patients’ progression are listed in Table 3.
Progression of patients treated with botulinum toxin A.
| Start of infiltrations | No. of infiltrations | Adverse effects | AMS Pre | Post | Mallet system/AMS (2 years) | Final function | |
|---|---|---|---|---|---|---|---|
| Case 1 | 7 months | 8 (+surgery) | Transient palsy | 3 | 5 | 18/6 | Improved supination Full elevation Forearm flexion |
| Case 2 | 9 months | 3 | Haematoma after infiltration | 2 | 3 | 18/6 | Improved supination (+5°) Full elevation Full forearm flexion |
| Case 3 | 18 months | 6 | None | 3 | 5 | 22/6 | Arm elevation, 150° Forearm flexion Improved supination, 30° |
| Case 4 | 14 months | 2 | None | 3 | 5 | Drop-out | Improved arm elevation |
| Case 5 | 6 months | 4 (+neurolysis) | Transient palsy | 3 | 5 | 20/6 | Improved supination Full elevation Forearm flexion |
| Case 6 | 10 months | 3 (+surgery) | Pain after infiltration | 2 | 3 | 13/5 | Arm elevation, 80° Full forearm flexion Minimum supination |
Regarding adverse effects of the infiltration technique, 2 patients (cases 2 and 6) presented mild, self-limiting adverse effects (post-infiltration haematoma, muscle pain in the second infiltration); another 2 cases (1 and 5) presented weakness with almost complete, self-limited palsy of the limb, with functional recovery at about 3 weeks, also during the second infiltration and with an increased dose (Table 3). No patient presented adverse reactions related to analgesia/sedation.
DiscussionObstetric lesion of the brachial plexus is caused by a lateral traction injury to the nerve roots during delivery. Prognosis depends on the severity and extension of the lesion; although it is favourable in most cases, with complete recovery during the first months of life, almost one-third of patients display permanent sequelae limiting movement, strength, and even the size and thickness of the muscle affected.5–8
A conservative approach has been traditionally adopted, observing the degree of spontaneous recovery and later scheduling such palliative surgical treatment as tendon transfer, osteotomy, or arthrodesis. However, the current trend is towards early treatment during the first months of life.18
This approach is controversial due to the difficulty of properly selecting candidates and discriminating between those with a favourable or a poor prognosis. Biceps recovery is one of the basic pillars in natural progression, since recovery at 2 months is a predictor of spontaneous, complete recovery in most cases, whereas absence of contraction at 3 months is predictive of the need for surgical treatment.12
BT represents an intermediate approach between watchful waiting and invasive treatment. Its main indication in neurology has traditionally been the treatment of various syndromes progressing with muscle hyperactivity: spasticity of any aetiology, dystonias, hemifacial spasm, facial synkinesis, tics and tremors, etc.19 However, it may also be useful for numerous other indications, such as in muscle imbalances caused by nerve lesions, as in the case of OBPP.9,20
BoNT-A selectively, temporarily, and reversibly blocks cholinergic neurotransmission at the neuromuscular junction of the healthy antagonist muscles. It aims to compensate for the muscle imbalance generated by the plexopathy and prevent the development of pathological motor learning patterns and a possible aberrant reinnervation.9,10,20
Despite limited evidence on the efficacy of BoNT-A in this pathology, due to the scarcity of published studies and the lack of randomised controlled trials, the available data show benefits for the elbow joint, with improved flexion and supination from the first infiltrations and persisting for over a year when infiltrations are repeated every 3 to 5 months.9,10,21–23
In our series, all patients showed improvement on the scales used, both in the shoulder and the elbow joint from the first infiltration, with progressive improvement in both joints with periodic infiltrations every 3 to 6 months. This benefit was also subjectively reported by patients’ parents and observed when reviewing the audiovisusal material (video recordings) of the patients, with a significant improvement in movement patterns and shoulder function.
As in other published series, it was possible to use BoNT-A independently of other treatments, and even as coadjuvant treatment to brachial plexus surgery.23,24 The combination of both treatments in 2 of our patients resulted in better scores on the AMS and preserved shoulder elevation/abduction and elbow supination during progression. BoNT-A infusions enabled us to postpone surgery on 2 occasions in one patient; the level of improvement observed after surgery was higher than with BoNT-A alone.
Although our study presents some limitations, such as the limited sample size (N=15), we believe that our series is reasonably heterogeneous and representative of everyday clinical practice. We obtained an incidence rate similar to that published in other series, with a sample obtained from a population also similar in terms of culture and socioeconomic level; both the symptoms and the dysfunctions caused by the disease were those expected in this group of patients.
Our main contribution is the infiltration technique, since the precise localisation of BT in the target muscle is currently believed to be more important than dose and dilution in reducing adverse effects, avoiding spread to adjacent muscles, and obtaining the greatest possible therapeutic effects with the lowest possible dose.25 Unlike in other studies, our patients received ultrasound-guided infiltrations, increasing the accuracy of the technique, and with low dilution volumes to avoid toxin spread to other muscles. This may be the reason why we obtained better scores and limb function in all treated cases, with an acceptable rate of adverse effects. Due to this increased accuracy of infiltration, we were able to use somewhat lower doses than those of similar studies,10 with no negative impact on results.
Finally, we should highlight the importance of early identification of cases of OBPP requiring treatment (surgical or non-surgical) to prevent permanent motor sequelae. Given its safety and effectiveness, it seems reasonable to use this off-label indication of BoNT-A as an adjuvant treatment to physical therapies and/or surgical treatment for moderate-severe OBPP. It should be noted that our conclusions are limited by a lack of prospective and randomised studies with long-term follow-up and using functional assessment scales with standardised scoring systems, which may support our results.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García Ron A, Gallardo R, Huete Hernani B. Utilidad del tratamiento con infiltraciones ecoguiadas de toxina botulínica A en el desequilibrio muscular de niños con parálisis obstétrica del plexo braquial. Descripción del procedimiento y protocolo de actuación. Neurología. 2019;34:215–223.