Post-traumatic stress disorder (PTSD) has been proposed as a risk factor for chronification of migraine. The aim of this study was to investigate the frequency of PTSD and traumatic life events (TE) in patients with episodic (EM) and chronic migraine (CM) and their impact on clinical parameters, other comorbidities, and migraine biomarkers.
Material and methodsPatients with EM and CM according to the International Classification of Headache Disorders (third edition; beta version) were recruited at a headache unit and a primary care centre. We used questionnaires validated for research on PTSD, TEs, cranial autonomic symptoms, comorbidities (depression, anxiety, and fatigue), disability, migraine impact, and quality of life. Baseline serum levels of CGRP, VIP, and PACAP were determined by ELISA.
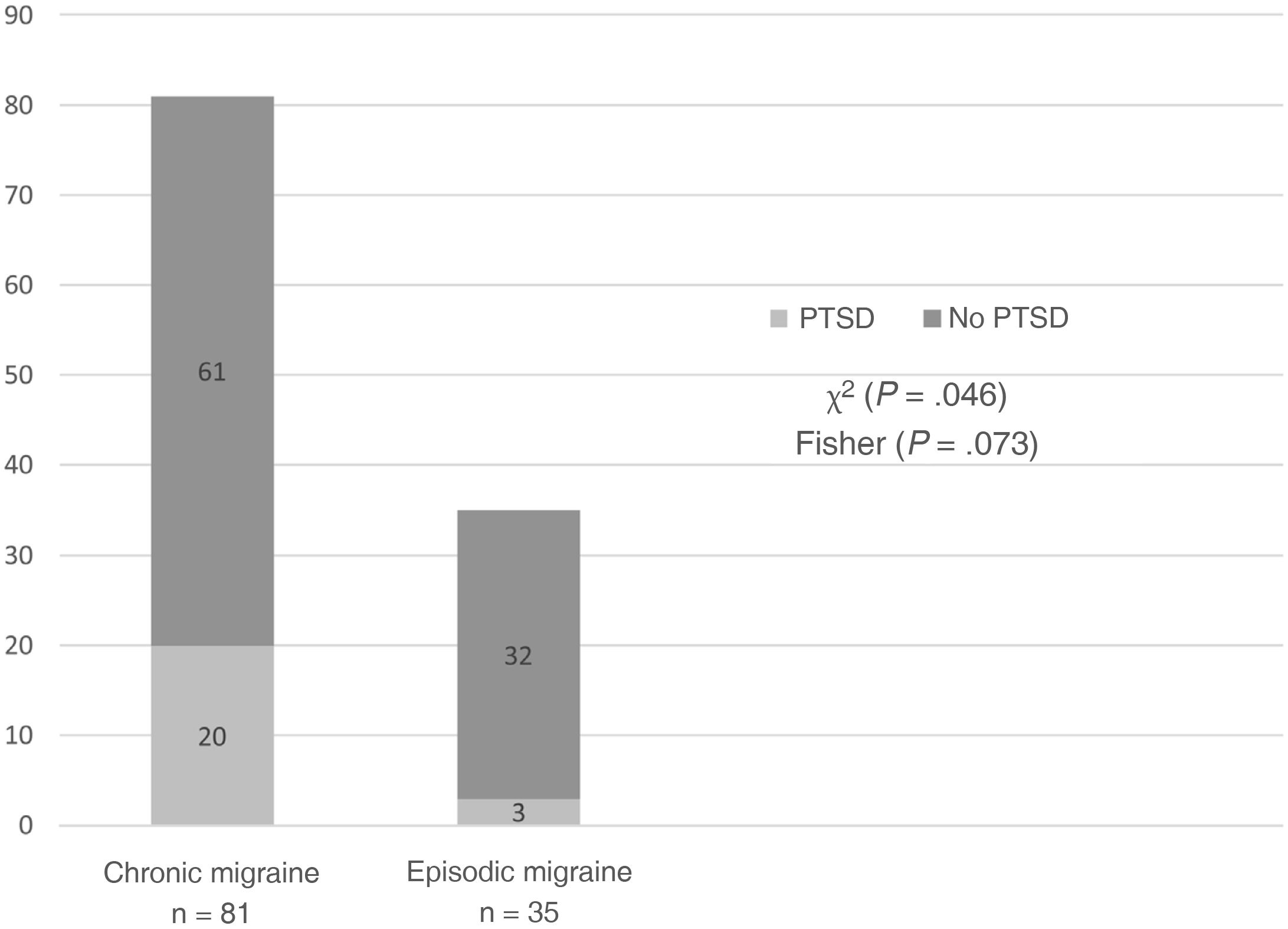
ResultsThe study included 116 patients: 35 with EM and 81 with CM. Nineteen presented refractory migraine. PTSD was detected in 23 patients (19.8%): 20 with CM and 3 with EM (chi-square: P = .046; Fisher T: P = .073). No significant differences were identified between the EM and CM groups for frequency of any TE nor the number of TEs per patient. A total of 5/19 patients with refractory migraine had experienced sexual violence (vs 2/97 with non-refractory migraine; P = .002). PTSD was associated with more autonomic symptoms; higher scores on anxiety, depression, and fatigue scales; and poorer quality of life; it did not change neuropeptide levels.
ConclusionsOur results suggest that PTSD is frequent in patients with migraine, and especially CM, in our setting; history of sexual violence is particularly frequent in patients with refractory migraine. PTSD has a negative impact on migraine, with higher numbers of comorbidities and poorer quality of life; therefore, further research is needed in this patient group.
El trastorno por estrés postraumático (TEPT) se ha postulado como factor de cronificación de la migraña. Nuestro objetivo fue investigar la frecuencia del TEPT y eventos vitales traumáticos (ETs) en pacientes con migraña episódica (ME) y crónica (MC) y su impacto sobre parámetros clínicos, otras comorbilidades y biomarcadores de migraña.
Material y métodosSe reclutaron pacientes con ME y MC según CIC-3β en una Unidad de Cefaleas y un centro de Atención Primaria. Se utilizaron cuestionarios validados para investigar TEPT, ETs, síntomas autonómicos craneales, comorbilidades (depresión, ansiedad, fatiga), discapacidad, impacto de la migraña y calidad de vida. Se determinaron los niveles séricos basales de CGRP, VIP y PACAP por ELISA.
Resultados116 pacientes fueron incluidos: 35 ME y 81 MC. 19 sufrían migraña refractaria (MR). Se detectó TEPT en 23 casos (19,8%): 20 MC y 3 ME (Chi2p = 0,046; T de Fisher p = 0,073). La frecuencia de ningún ET ni el número de ETs por paciente fue diferente entre MC y ME. 5/19 MR habían sufrido violación (vs 2/97 no MR; p = 0,002). El TEPT se asoció con más síntomas autonómicos, mayor puntuación en escalas de ansiedad, depresión y fatiga, y menor calidad de vida, y no modificó los niveles de neuropéptidos.
ConclusionesEste estudio sugiere que el TEPT es frecuente en pacientes con migraña, especialmente MC, también en nuestro medio, y que particularmente el antecedente de violencia sexual es frecuente en MR. El TEPT impacta negativamente sobre la migraña, asociando más comorbilidades y peor calidad de vida, por lo que es preciso investigarlo en estos pacientes.
Migraine, a neurological condition presenting with episodes of disabling headache, is one of the main causes of years lived with disability.1 According to the frequency of attacks, it may be classified as either episodic (EM) or chronic migraine (CM).2 Patients with CM present more severe disability,3,4 not only due to the high frequency of migraine attacks but also to the greater frequency of comorbidities associated with poorer prognosis, such as vascular risk factors and certain respiratory diseases and psychiatric disorders.4,5 The latter include major depressive disorder, bipolar disorder, and anxiety disorders.5 A comprehensive study conducted in the United States found that patients with CM were twice as likely to present anxiety and depression at some point in their lives as patients with EM.4 Although the association with anxiety and depression has been extensively studied, other associations have also been proposed, although with a lower level of evidence, such as that between CM and post-traumatic stress disorder (PTSD). Some researchers have reported a greater frequency of PTSD among patients with migraine than in the general population, suggesting a possible pathogenic role of this condition in migraine transformation.6–8 Other studies have shown that presence of PTSD considerably increases the burden of migraine.6,9
It is unclear whether mere exposure to a traumatic event is also associated with the risk of presenting migraine. Experiencing a traumatic event is a necessary condition for the development of PTSD. This condition is characterised by intrusive thoughts, avoidance behaviour, cognitive alterations, and mood disorders. Although these symptoms usually appear within 3 months of the event, they may also present months or years later.10 However, exposure to a traumatic event is not sufficient to develop PTSD: according to several epidemiological studies, the prevalence of history of traumatic experiences in the general population ranges from 39.1% to 89.6%,11 whereas the prevalence of PTSD ranges from 1% to 12.3%, depending on the setting.12 Some retrospective studies report an association between traumatic events during childhood and migraine in adulthood; an association between these traumatic events and migraine severity has also been described.13,14 Other authors, in contrast, suggest that this association is only observed in the case of repetitive traumatic events,15 while others argue that only PTSD, but not exposure to traumatic events or number of traumatic events, is associated with diagnosis of migraine.8
Although migraine is diagnosed clinically,2 significant advances have been made in recent years in the field of plasma biomarkers for the diagnosis, treatment, and follow-up of this condition. At present, the calcitonin gene-related peptide (CGRP) is the most promising biomarker for CM.16 Such other biomarkers as vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase–activating polypeptide (PACAP) have shown less satisfactory results.16
The aim of this study was to analyse the frequency of PTSD and traumatic events in patients with migraine, as well as the differences between CM and EM. As a secondary objective, we evaluated the impact of PTSD on clinical variables, comorbidities, and biomarkers of migraine.
Material and methodsWe conducted a cross-sectional study of patients diagnosed with CM and EM according to the third edition of the International Classification of Headache Disorders.2 Patients were consecutively recruited from a headache unit (Hospital Universitario Marqués de Valdecilla, Santander, Spain) and a primary care centre (Centro de Salud Camargo Costa, Camargo, Spain) between March 2016 and May 2019. The study was approved by the ethics committee for Cantabria, and all patients gave written informed consent to participate. We included patients aged 18 to 65 years with a diagnosis of EM or CM of at least one year’s progression. We excluded patients with secondary headache disorders and those whose headache diaries provided insufficient clinical information from the previous 3 months. The European Headache Federation defines refractory migraine (RM) as migraine in which all of the available preventive treatments have failed, and debilitating headache is present at least 8 days per month for at least 6 consecutive months.17 Data were gathered on the frequency of PTSD and traumatic events; cranial autonomic symptoms linked to headache; such comorbidities as anxiety, depression, and fatigue; perceived quality of life; disability; and migraine impact, using validated questionnaires (Table 1), which were administered at an appointment scheduled for that purpose. Our multidisciplinary team included 2 neurologists specialising in headache and 2 clinical psychologists.
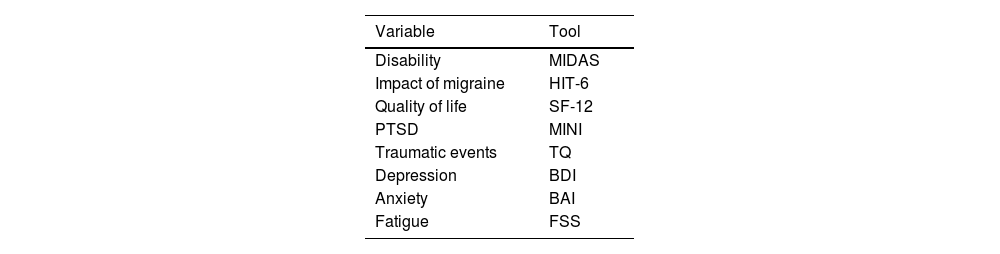
Tools used for the assessment of disability, migraine impact, quality of life, post-traumatic stress disorder, traumatic events, and comorbidities of migraine.
| Variable | Tool |
|---|---|
| Disability | MIDAS |
| Impact of migraine | HIT-6 |
| Quality of life | SF-12 |
| PTSD | MINI |
| Traumatic events | TQ |
| Depression | BDI |
| Anxiety | BAI |
| Fatigue | FSS |
BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; FSS: Fatigue Severity Scale; HIT-6: Headache Impact Test; MIDAS: Migraine Disability Assessment; MINI: Mini–International Psychiatric Interview; PTSD: post-traumatic stress disorder; SF-12: Short-Form Health Survey; TQ: Trauma Questionnaire.
This structured interview is divided into modules identified by letters and exploring the main psychiatric disorders included in axis I of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).18 The first module of the MINI explores the patient’s PTSD symptoms in the past month. The tool presents good psychometric properties for use in the clinical and research settings.18
Trauma Questionnaire (TQ)This questionnaire comprises 3 sections. The first (18 items) explores the experience of traumatic events over the patient’s life, their age at occurrence of the events, and their duration. The second (9 items) focuses on the characteristics of the event indicated by the patient as being the most disturbing. The third evaluates the consequences of said event at any time since its occurrence, with 18 items about the DSM-IV criteria for PTSD.19 The Spanish-language version of the questionnaire has shown adequate psychometric properties.20
Beck Anxiety Inventory (BAI)The BAI comprises 21 items exploring somatic symptoms of anxiety.21 Patients are asked to report to what extent they have experienced a series of symptoms over the past week. Responses are scored from 0 (not at all) to 3 (severely). The sum of all item scores is calculated, giving a maximum possible score of 63.
Beck Depression Inventory (BDI)This 21-item scale rates the severity of depressive symptoms and behaviours from 0 to 3. These categories reflect the DSM-IV diagnostic criteria for depression, with the total score ranging from 0 to 63 points. The BDI is one of the most widely used tools to evaluate depression.22
Cranial autonomic symptoms linked to headacheWe used a brief questionnaire about cranial autonomic symptoms (sensation of fullness of the face or eyes, tearing, reddening of the eyes or face, ptosis, gritty sensation in the eyes, nasal congestion, or rhinorrhoea) before or during headache; patients are asked to report whether these symptoms occur frequently, occasionally, or never.
Fatigue Severity Scale (FSS)The FSS includes 9 items on the defining features of fatigue; patients must express their level of agreement from 1 (strongly disagree) to 7 (strongly agree).23
Short-Form Health Survey (SF-12)With only 12 items, this abbreviated version of the 36-item Short-Form Health Survey explores the patient’s physical and mental health over the past 4 weeks.24
Migraine Disability Assessment (MIDAS)The MIDAS is one of the most widely used tools for the assessment of migraine-associated disability.25 It includes 5 questions about limitations in different domains of daily life (work; household chores; and social, family, and leisure activities) over 3 months.
Headache Impact Test (HIT-6)This 6-item questionnaire evaluates pain, social functioning, cognitive function, psychological distress, and vitality, and has been shown to be reliable for screening and monitoring the impact of headache.26
Determination of serum neuropeptidesWe determined levels of the neuropeptides CGRP, VIP, and PACAP in peripheral blood. At the headache unit, 5-mL blood samples were collected from the ulnar vein, with patients in fasting conditions. Blood samples were collected after a migraine-free period of at least 72 hours. Samples were collected in BD Vacutainer® tubes without EDTA (Becton, Dickinson and Company; Franklin Lakes, NJ, USA), centrifuged at 3500 rpm, at room temperature, within 10 minutes of collection, and subsequently aliquoted and stored at –80°C. The samples were analysed with commercially available ELISA kits from BlueGene Biotech (Pudong New District, Shanghai, China) for PACAP and from Cloud-Clone Corp. (Wuhan, China) for CGRP and VIP, according to the manufacturers’ instructions. The detection ranges of the kits were 12.35–1000 pg/mL for CGRP, 6.17–500 pg/mL for VIP, and 0–1000 pg/mL for PACAP. The procedure was established in a previous study designed to evaluate the diagnostic value of these neuropeptides in migraine.27
Statistical analysisIn the descriptive statistical analysis, we calculated means and standard deviations for normally distributed variables and medians and quartiles 1 and 3 for non–normally distributed variables. Normally distributed quantitative variables were compared with the t-test, whereas non–normally distributed quantitative variables were compared with the Mann–Whitney U test Qualitative variables were compared with the χ2 test or the Fisher exact test when n < 5. Correlations between 2 variables were studied using the Spearman rho test for non-normally distributed variables. In all comparisons, we assumed an α value of 0.05.
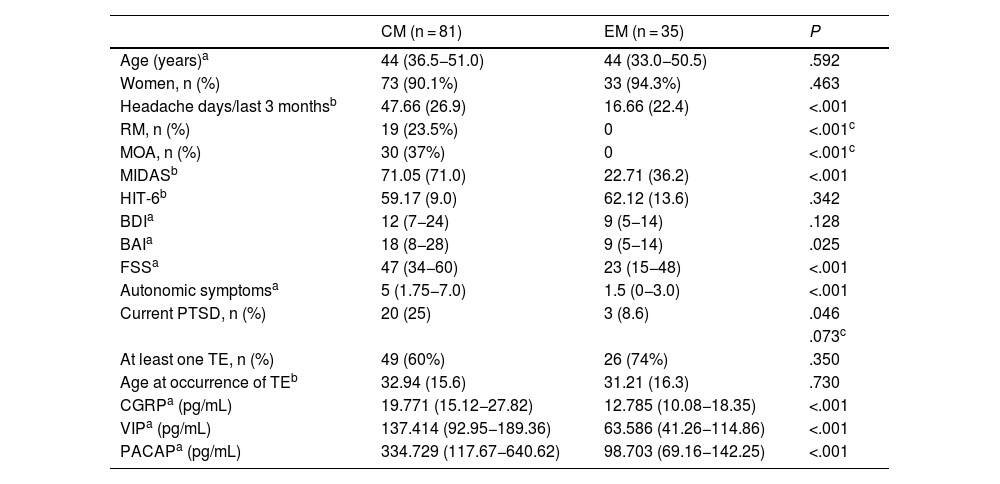
ResultsDescription of the sampleOur sample included 116 patients: 81 with CM and 35 with EM. Women accounted for 91% of the sample (n = 106). No statistically significant differences were observed in sex or age distribution. In the CM group, 19 patients met criteria for RM and 30 presented medication-overuse headache (Table 2).
Demographic and clinical characteristics, comorbidities, and serum neuropeptide levels of our patients with chronic and episodic migraine.
| CM (n = 81) | EM (n = 35) | P | |
|---|---|---|---|
| Age (years)a | 44 (36.5−51.0) | 44 (33.0−50.5) | .592 |
| Women, n (%) | 73 (90.1%) | 33 (94.3%) | .463 |
| Headache days/last 3 monthsb | 47.66 (26.9) | 16.66 (22.4) | <.001 |
| RM, n (%) | 19 (23.5%) | 0 | <.001c |
| MOA, n (%) | 30 (37%) | 0 | <.001c |
| MIDASb | 71.05 (71.0) | 22.71 (36.2) | <.001 |
| HIT-6b | 59.17 (9.0) | 62.12 (13.6) | .342 |
| BDIa | 12 (7−24) | 9 (5−14) | .128 |
| BAIa | 18 (8−28) | 9 (5−14) | .025 |
| FSSa | 47 (34−60) | 23 (15−48) | <.001 |
| Autonomic symptomsa | 5 (1.75−7.0) | 1.5 (0−3.0) | <.001 |
| Current PTSD, n (%) | 20 (25) | 3 (8.6) | .046 |
| .073c | |||
| At least one TE, n (%) | 49 (60%) | 26 (74%) | .350 |
| Age at occurrence of TEb | 32.94 (15.6) | 31.21 (16.3) | .730 |
| CGRPa (pg/mL) | 19.771 (15.12−27.82) | 12.785 (10.08−18.35) | <.001 |
| VIPa (pg/mL) | 137.414 (92.95−189.36) | 63.586 (41.26−114.86) | <.001 |
| PACAPa (pg/mL) | 334.729 (117.67−640.62) | 98.703 (69.16−142.25) | <.001 |
BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; CGRP: calcitonin gene-related peptide; CM: chronic migraine; EM: episodic migraine; FSS: Fatigue Severity Scale; HIT-6: Headache Impact Test; MIDAS: Migraine Disability Assessment; MOA: medication-overuse headache; PACAP: pituitary adenylate cyclase–activating polypeptide; PTSD: post-traumatic stress disorder; RM: refractory migraine; TE: traumatic event; VIP: vasoactive intestinal peptide.
The mean (SD) MIDAS scale score was higher in the CM group than in the EM group (71.05 [71] vs 22.71 [36.2]; P < .001); however, no significant differences were observed in HIT-6 scores. Patients with CM presented higher median (Q1–Q3) scores on the BAI (18 [8–28] vs 9 [5–14]; P = .025) and FSS (47 [34−60] vs 23 [15−48]; P < .001), but no significant intergroup differences were observed in BDI scores. Patients with CM reported a higher median number of cranial autonomic symptoms linked to headache than patients with EM (5 [1.7–7.0] vs 1.5 [0–3]; P < .001). Median interictal serum levels of CGRP, VIP, and PACAP were significantly higher in patients with CM than in those with EM (CGRP: 19.771 [15.12−27.82] vs 12.785 [10.08−18.35]; VIP: 137.414 [92.95−189.36] vs 63.586 [41.26−114.86]; PACAP: 334.729 [117.67−640.62] vs 98.703 [69.16−142.25]; P < .001 for all comparisons) (Table 2).
Frequency of post-traumatic stress disorder and traumatic eventsA total of 23 patients (19.8%) met criteria for PTSD in the previous month. Of these, 20 patients (87%) presented CM and 3 (13%) had EM; a trend was observed toward greater frequency of PTSD in patients with CM (χ2 = 3.995, P = .046; Fisher exact test P = .073) (Table 2, Fig. 1).
A total of 75 patients (64.7%) reported having experienced a traumatic event at some point in their lives, with no significant differences between groups (49 patients with CM and 26 with EM; χ2 = 2.034, P = .350) (Table 2). The most frequent traumatic event was the unexpected death of a loved one, in 53 patients (34 with CM and 19 with EM; P = .222).
No significant differences were observed in the type or total number of traumatic events between groups (χ2 = 7.806, df = 7; P = .350). We did observe significant differences between patients with and without PTSD. Patients with PTSD in the previous month had more frequently experienced a serious threat or harm to a relative or close friend (6/23 vs 5/93 patients without PTSD; P = .008), physical abuse (9/23 vs 6/93; P < .001), and rape (4/23 vs 3/93; P = .028). Patients without PTSD in the previous month more frequently reported a car, train, or aviation accident (20/93 vs 11/23; P = .011), the unexpected death of a loved one (35/93 vs 18/23; P = .001), and nearly drowning (10/93 vs 7/23; P = .025). No significant differences were observed in the frequencies of the remaining traumatic events.
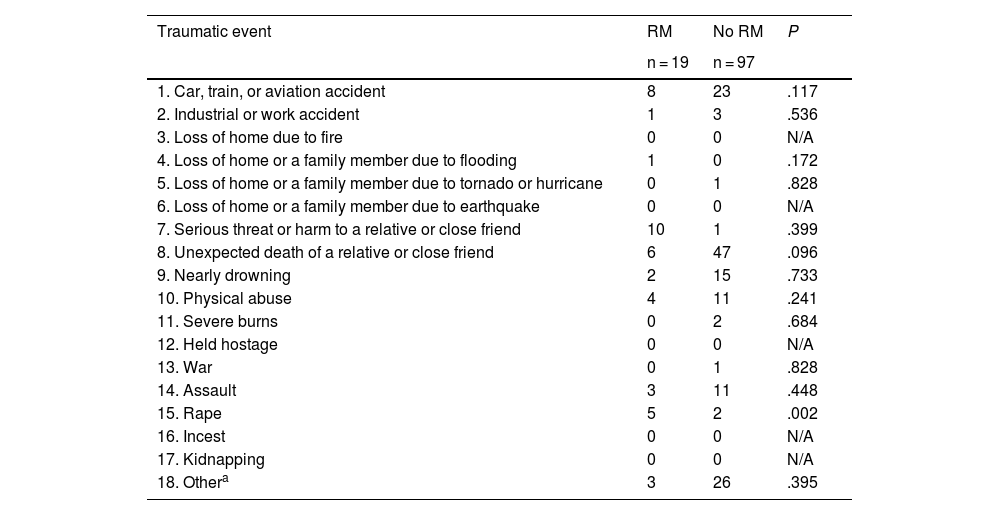
Table 3 presents the distribution of traumatic events in patients with and without RM. The total number of traumatic events per patient was similar in patients with and without RM (χ2 = 9.670, df = 7; P = .208). However, by type of traumatic event, rape was more frequently reported by patients with RM than those without RM: 5 of the 19 patients with RM (26%) had been raped, compared to 2/97 (2%) of patients without RM (P = .002). This event occurred at a mean age of 15.9 (9.0) years, a significantly younger age than in the remaining traumatic events, which occurred at a mean age of 33.26 (15.58) years (P = .050). Rape was the only type of traumatic event that was found to be more frequent in patients with RM.
Distribution of traumatic events (TQ) between patients with and without refractory migraine.
| Traumatic event | RM | No RM | P |
|---|---|---|---|
| n = 19 | n = 97 | ||
| 1. Car, train, or aviation accident | 8 | 23 | .117 |
| 2. Industrial or work accident | 1 | 3 | .536 |
| 3. Loss of home due to fire | 0 | 0 | N/A |
| 4. Loss of home or a family member due to flooding | 1 | 0 | .172 |
| 5. Loss of home or a family member due to tornado or hurricane | 0 | 1 | .828 |
| 6. Loss of home or a family member due to earthquake | 0 | 0 | N/A |
| 7. Serious threat or harm to a relative or close friend | 10 | 1 | .399 |
| 8. Unexpected death of a relative or close friend | 6 | 47 | .096 |
| 9. Nearly drowning | 2 | 15 | .733 |
| 10. Physical abuse | 4 | 11 | .241 |
| 11. Severe burns | 0 | 2 | .684 |
| 12. Held hostage | 0 | 0 | N/A |
| 13. War | 0 | 1 | .828 |
| 14. Assault | 3 | 11 | .448 |
| 15. Rape | 5 | 2 | .002 |
| 16. Incest | 0 | 0 | N/A |
| 17. Kidnapping | 0 | 0 | N/A |
| 18. Othera | 3 | 26 | .395 |
N/A: not applicable; RM: refractory migraine; TQ: Trauma Questionnaire.
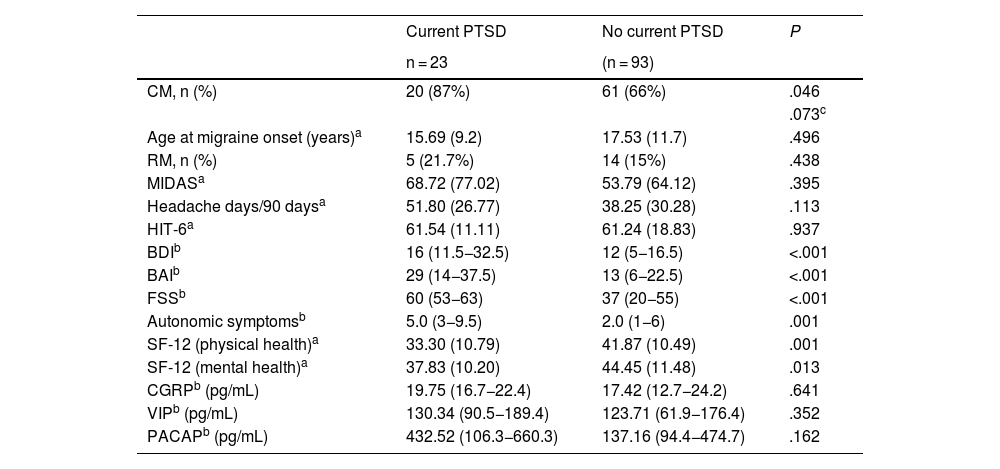
We compared clinical variables, other comorbidities, and baseline serum levels of neuropeptides involved in the pathophysiology of migraine between patients with and without PTSD in the previous month (Table 4).
Clinical differences, comorbidities, and baseline serum neuropeptide levels of our patients with and without post-traumatic stress disorder.
| Current PTSD | No current PTSD | P | |
|---|---|---|---|
| n = 23 | (n = 93) | ||
| CM, n (%) | 20 (87%) | 61 (66%) | .046 |
| .073c | |||
| Age at migraine onset (years)a | 15.69 (9.2) | 17.53 (11.7) | .496 |
| RM, n (%) | 5 (21.7%) | 14 (15%) | .438 |
| MIDASa | 68.72 (77.02) | 53.79 (64.12) | .395 |
| Headache days/90 daysa | 51.80 (26.77) | 38.25 (30.28) | .113 |
| HIT-6a | 61.54 (11.11) | 61.24 (18.83) | .937 |
| BDIb | 16 (11.5−32.5) | 12 (5−16.5) | <.001 |
| BAIb | 29 (14−37.5) | 13 (6−22.5) | <.001 |
| FSSb | 60 (53−63) | 37 (20−55) | <.001 |
| Autonomic symptomsb | 5.0 (3−9.5) | 2.0 (1−6) | .001 |
| SF-12 (physical health)a | 33.30 (10.79) | 41.87 (10.49) | .001 |
| SF-12 (mental health)a | 37.83 (10.20) | 44.45 (11.48) | .013 |
| CGRPb (pg/mL) | 19.75 (16.7−22.4) | 17.42 (12.7−24.2) | .641 |
| VIPb (pg/mL) | 130.34 (90.5−189.4) | 123.71 (61.9−176.4) | .352 |
| PACAPb (pg/mL) | 432.52 (106.3−660.3) | 137.16 (94.4−474.7) | .162 |
BAI: Beck Anxiety Inventory; BDI: Beck Depression Inventory; CGRP: calcitonin gene-related peptide; CM: chronic migraine; EM: episodic migraine; FSS: Fatigue Severity Scale; HIT-6: Headache Impact Test; MIDAS: Migraine Disability Assessment; PACAP: pituitary adenylate cyclase–activating polypeptide; PTSD: post-traumatic stress disorder; RM: refractory migraine; SF-12: Short-Form Health Survey; VIP: vasoactive intestinal peptide.
Patients with PTSD scored higher on the BDI (16 [11.5−32.5] vs 12 [5−16.5]; P < .001), BAI (29 [14−37.5] vs 12 [5−16.5]; P < .001), and FSS (60 [53−63] vs 37 [20−55]; P < .001), and presented poorer quality of life according to the physical (33.30 [10.79] vs 41.87 [10.49]; P = .001) and mental domains of the SF-12 (37.83 [10.20] vs 44.45 [11.48]; P = .013). No significant differences were observed in the mean number of headache days in the last 3 months, or in MIDAS or HIT-6 scores.
Patients with PTSD reported a higher median number of cranial autonomic symptoms linked to headache than patients without PTSD (5.0 [3–95] vs 2.0 [1–6]; P = .001) (Table 4).
No significant differences in interictal serum levels of CGRP, VIP, or PACAP were found between patients with and without PTSD (Table 4). Serum levels of VIP (rho = 0.247; P = .011) and PACAP (rho = .242; P = .013) showed a positive correlation with the results of the questionnaire on autonomic symptoms.
DiscussionThe relevance of migraine comorbidities lies in the potential existence of common pathophysiological mechanisms.28 In this line of reasoning, it has been suggested that PTSD may affect the neural circuits participating in stress response and migraine-related brain hyperexcitability.29 Furthermore, migraine prognosis may improve with the identification and management of PTSD, as has been reported in other conditions associated with chronic pain.30
We used the MINI, which evaluates the presence of symptoms of PTSD in the previous month, to determine the frequency of PTSD in a sample of patients with migraine recruited from a headache unit and a primary care centre, finding higher rates (19.8%) than those observed in the general population. A large population study conducted in the United States reported a prevalence of PTSD in the previous year of 2%–5%, depending on the setting and age group,12 whereas in Spain the overall prevalence is 0.6%.31 The frequency of PTSD in our sample of patients with migraine is similar to those reported by studies into PTSD in this population (25%),6,8 a rate approximately twice as high as that observed in controls without migraine.8 The existing research was conducted mainly in the United States, where the prevalence of PTSD is higher than in Spain; this potential bias should be taken into account when interpreting our results. However, our study found similar rates in our patients with migraine and also shows that these 2 conditions frequently co-occur in our setting.
We observed a trend toward higher prevalence of PTSD in the group of patients with CM (25%) than in those with EM (8.6%), although differences were not statistically significant. This difference has been described by other authors, with previous studies reporting rates of up to 43% in patients with CM7; this suggests that PTSD may play a role in migraine transformation. The small size of our sample may have limited the statistical power of our study.
The most frequent traumatic event in our sample was unexpected death of a loved one; this is consistent with the results of previous epidemiological studies.10,31 This traumatic event has a low conditional risk of PTSD,31 as our results also reflect. Some traumatic events, such as serious threat or harm to a relative or close friend, physical abuse, and rape, were more frequent among patients with PTSD, which suggests that they present a higher conditional risk of PTSD. We found no significant differences in the number of patients with CM or EM reporting at least one traumatic event over the course of their lives; this rate is consistent with those reported in previous studies,7 and is similar to that observed in the general population (approximately 50%).31 Likewise, no significant differences were observed in the frequency of any type of traumatic event between patients with CM and those with EM. However, history of rape was more frequent among patients with RM (26%). This rate is significantly higher than that observed in the Spanish general population. Previous population studies report a prevalence rate of history of sexual violence (harassment, abuse, and rape) of 3.4% (more specifically, the prevalence of rape is 0.4%), showing that this is the traumatic event with the highest conditional risk of PTSD in women (16.5%).31 In our study, this event occurred at a significantly younger mean age than the remaining traumatic events. Although the association between traumatic events and migraine risk is unclear, the effects of trauma are known to be more severe when it occurs at young ages, and there seems to be an association between traumatic events in childhood and the development of some psychiatric disorders and chronic pain conditions in adulthood.32 In this line, some retrospective studies have found an association between childhood traumatic events and risk of migraine.13,14 Women with migraine more frequently report traumatic events (physical, psychological, or sexual abuse; family or domestic trauma) than those without migraine, and scores for adverse childhood experiences correlate with migraine frequency, particularly CM.14
In our study, patients with PTSD in the previous month scored higher for depression, anxiety, and fatigue, and lower for quality of life; this is consistent with previous studies,6,9 with the exception of the results on fatigue, which has not previously been evaluated. In a study classifying patients with migraine according to the set of comorbidities they presented, the authors observed that patients with anxiety and depression had more frequently experienced sexual, physical, or emotional abuse, and that this group scored higher on scales assessing headache-related disability and lower on quality of life scales.13 In our study, in turn, patients with PTSD did not present more headache days or higher disability and migraine impact scores than patients without PTSD, which suggests that this patient group may present greater disease burden due to number of comorbidities and impact on quality of life, rather than due to specific migraine parameters. However, headache frequency in our study was measured with a variable derived from the MIDAS, which may have introduced bias as it is based on patient-reported data.
Likewise, no significant differences in interictal serum levels of CGRP, VIP, or PACAP were found between patients with and without PTSD; this suggests that PTSD has no direct impact on migraine biomarkers. No previous study has evaluated the consequences of PTSD on serum levels of CGRP and VIM, although the literature does include studies into the association with PACAP level. This parasympathetic peptide plays a pivotal role in neuronal circuits mediating stress response33; plasma levels of PACAP have shown a positive correlation with the severity of PTSD symptoms in women.34 Unlike in the previously cited article, our analyses were not stratified by sex, and PTSD was evaluated qualitatively (presence/absence) rather than quantitatively; our study found no differences in baseline PACAP levels.
Patients with PTSD were found to have higher numbers of cranial autonomic symptoms. Some of these, such as increased facial skin temperature35 and changes in heart rate and blood pressure, are frequent in PTSD.33 According to our results, these patients may also present higher numbers of cranial autonomic symptoms linked to headache than patients with migraine but without PTSD. However, these symptoms are highly prevalent in CM.36 Therefore, the high percentage of patients with CM in the PTSD group in our study (87%) may have biased our results. In fact, serum levels of VIP and PACAP, 2 parasympathetic peptides involved in the regulation of autonomic response,33,37 were correlated with the number of autonomic symptoms; while baseline levels of these 2 peptides were not elevated in patients with PTSD, they were elevated in those with CM.
Our study has several limitations: 1) Due to the lack of a control group, results were compared against prevalence rates of PTSD and traumatic events in the general population without migraine, which vary depending on the setting and may not be extrapolatable to our region. In any case, our results are consistent with those of previous studies. 2) Despite the possibility of a participation bias resulting in an overestimation of cases of PTSD and traumatic events, the retrospective assessment of traumatic events is subject to recall bias,32 which may at least partially compensate for the previous limitation. 3) Our sample was small, which limits the interpretation of results. However, some of our observations may constitute a starting point for future research. 4) The techniques for estimating serum neuropeptide levels are widely known to have a number of methodological issues.38 In any case, we took significant steps to minimise sample processing times, and used the same ELISA kits as those used previously by other authors. One of the strengths of this study is the fact that we recruited some patients from a primary care centre, with a view to minimising selection bias (patients attended at headache units usually have more severe migraine).
In conclusion, this study provides novel data suggesting that PTSD is frequent among patients with migraine, especially CM, also in our setting. According to our results, history of sexual abuse is surprisingly frequent among patients with RM. Although the presence of PTSD does not modify specific migraine parameters, it does have a negative impact on prognosis, as it is associated with greater numbers of comorbidities and poorer quality of life. Therefore, headache units should screen for and specifically address PTSD. Future studies with larger samples should aim to analyse the association between severe migraine and PTSD and traumatic events.
FundingThis study was funded by Instituto de Salud Carlos III, Madrid (grant FISS PI15/01285) and Instituto de Investigación Marqués de Valdecilla (IDIVAL), Santander.