Congenital cytomegalovirus (CMV) infection is an important cause of disability. There is little evidence on the prognostic value of lesions identified in neuroimaging studies.
AimThe study aimed to assess the severity of lesions detected with brain MRI and transfontanellar ultrasound and their relationship with long-term neurological deficits.
Patients and methodsWe performed a retrospective, analytical, observational study of 36 patients with congenital CMV infection. Neuroimaging studies were reviewed and classified according to the modified Noyola’ scale. Imaging findings were compared with neurological alterations in the patients’ most recent follow-up evaluation at the paediatric neurology department.
ResultsThirty-six patients were studied (transfontanellar ultrasound: 30; brain MRI: 29). Twenty of 30 patients showed ultrasound abnormalities; of these, 11 showed alterations on brain MR images (P = .04) and 10 had neurological impairment (P = .008). Transfontanellar ultrasound had a sensitivity of 83.3%, 90% CI: 58-100 and a specificity of 44.4%, 90% CI: 18.7-70.2 for predicting neurological sequelae. Brain MRI displayed abnormalities in 20 of 29 patients, of whom 16 had neurological impairment (P < .001). MRI had a sensitivity of 94%, 95% CI: 80-100 and a specificity of 66.6%, 95% CI: 36-97.5 for predicting neurological sequelae. Modified Noyola’ scale values > 2 were correlated with psychomotor retardation (P < .001).
ConclusionsOur findings validate previous studies reporting a statistical significant correlation between the extension of neuroimaging lesions and severity of neurological deficits.
La infección congénita por citomegalovirus (CMV) supone una importante causa de discapacidad. Existen escasas evidencias acerca del valor pronóstico de las lesiones presentes en los estudios de neuroimagen.
ObjetivoAnalizar la gravedad de las lesiones en la resonancia magnética (RM) y la ecografía transfontanelar y su relación con déficits neurológicos a largo plazo.
Pacientes y métodosSe realizó un estudio observacional analítico retrospectivo de 36 pacientes con infección congénita por CMV. Se revisaron los estudios de neuroimagen y se clasificaron según la escala de Noyola et al. modificada. Se relacionaron los hallazgos de neuroimagen con la afectación neurológica en su última visita en la consulta de neuropediatría.
ResultadosUn total de 36 pacientes fueron estudiados, habiéndose realizado ecografía transfontanelar en 30 y RM cerebral en 29. La ecografía transfontanelar estuvo alterada en 20/30 pacientes, de los cuales, 11 tuvieron alteración en la RM (p = 0,04) y 10 afectación neurológica (p = 0,008). Tuvo una sensibilidad del 83,3%, IC 90%: 58–100 y una especificidad del 44,4%, IC 90%: 18,7–70,2 para la predicción de secuelas neurológicas. La RM estuvo alterada en 20/29 pacientes. Dieciséis de ellos tuvieron afectación neurológica (p < 0,001), teniendo una sensibilidad del 94%, IC 95%: 80–100 y una especificidad del 66,6%, IC 95%: 36–97,5 para la predicción de secuelas neurológicas. Una escala de Noyola et al. ≥ 2 se asoció a retraso psicomotor (p < 0,001)
ConclusiónNuestro trabajo valida los estudios previos en los que se encuentra correlación estadísticamente significativa entre la extensión de las lesiones en neuroimagen y la gravedad de los déficits neurológicos.
Congenital cytomegalovirus (CMV) infection is one of the most frequent prenatal infections in developed countries, affecting between 0.3% and 0.6% of newborns in Europe.1 It is the most frequent cause of delayed psychomotor development and hearing loss of infectious origin.2
Only 10% of newborns with the infection will present symptoms at birth (intrauterine growth retardation, hepatosplenomegaly, thrombocytopaenia, jaundice, petechiae, microcephalus, or abnormal neurological examination findings)3; 90% of neonates are asymptomatic, although 13% of these patients will present sequelae throughout their lives.1
The central nervous system (CNS) is affected in 50% of children with symptomatic infection,1 leading to alterations in psychomotor development, sensorineural hearing loss, and chorioretinitis.3–7
Neuroimaging studies of these patients only detect alterations in two-thirds of cases; the most frequent alterations are presence of cerebral calcifications, ventriculomegaly, periventricular white matter alterations, neuronal migration disorders, cortical atrophy, periventricular cysts, and cerebellar hypoplasia.8,9
Most studies on brain involvement in congenital CMV infection are based on computed tomography (CT) findings; however, this technique causes ionising radiation and is not superior to ultrasound for detecting ventriculomegaly, subependymal cysts, calcifications, or lenticulostriate vasculopathy (LSV).2,7,10–13
Recent studies have aimed to establish the prognostic role of transfontanellar ultrasound and magnetic resonance imaging (MRI) in cerebral involvement of congenital CMV infection.14 However, further studies are needed to determine the precise prognostic role of brain MRI, especially in patients with white matter lesions.2
The aim of this study is to analyse the relationship between imaging alterations (prenatal ultrasound, transfontanellar ultrasound and MRI) and neurological involvement in patients with congenital CMV infection.
Patients and methodsWe conducted an observational, retrospective, analytical study in the paediatric neurology department in collaboration with the paediatric radiology and the paediatric infectious diseases department at a tertiary hospital in the Region of Madrid (Spain).
The study period was from January 2003 to August 2017.
We included patients under follow-up by the paediatric neurology department and diagnosed with congenital CMV infection. This department follows up all patients with confirmed infection, whether symptomatic or asymptomatic. Congenital CMV infection was confirmed by positive PCR or urine culture findings for CMV in the first 15 days of life or positive PCR for CMV in dried blood spots (Guthrie card) from the first 48 hours of life.
Infection was considered symptomatic at birth when patients presented: intrauterine growth retardation (defined as weight at birth more than 2 standard deviations [SD] below the mean), petechiae, hepatomegaly, splenomegaly, microcephalus (defined as head circumference at birth more than 2 SD below the mean), sensorineural hearing loss (altered otoacoustic emissions, confirmed by auditory evoked potentials), chorioretinitis, thrombocytopaenia (less than 100 000 platelets/μL), elevated liver enzymes (alanine transferase > 100 U/L [normal range: 5-50 U/L]), or cholestasis (direct bilirubin > 3 mg/dL [normal range: 0-0.3 mg/dL]).
Neuroimaging studies and scorePathological brain ultrasound findings were defined as intracranial calcifications, ventriculomegaly (axial diameter greater than 10 mm across the atrium at the level of the posterior horns), germinolytic cysts, and/or lenticulostriate vasculopathy. The ultrasound devices used were the Toshiba Xario and Aplio systems.
In the brain MRI study, we obtained sagittal, axial, and coronal slices in T1- and T2-weighted sequences with a 1.5 T scanner. A paediatric radiologist interpreted the images. A second paediatric radiologist reviewed the images, assigning a severity score according to the modified version14 of the Noyola scoring scale,9 as shown in Table 1.
Modified Noyola scale.
| Score | Findings |
|---|---|
| 0 | None of the following abnormalities |
| 1 | Single punctate periventricular calcification, lenticulostriate vasculopathy, caudothalamic germinolysis, ventriculomegalya (excluding severe), and/or focal/multifocal white matter signal abnormality on MRI |
| 2 | Multiple discrete periventricular calcifications, paraventricular germinolytic cysts, severe ventriculomegaly, diffuse white matter signal abnormality, and/or temporal lobe involvement |
| 3 | Extensive calcifications, brain atrophy, abnormal gyration, cortical malformation, dysgenesis of the corpus callosum, and/or cerebellar hypoplasia |
Neurological involvement was assessed in the last consultation and was considered to include the following items: microcephalus (head circumference at birth more than 2 SD below the mean according to the tables published by Fernández et al.15), psychomotor retardation according to the Denver scale (as assessed by an experienced paediatric neurologist), motor alterations (classified as mild, moderate, or severe), behavioural alterations, cognitive alterations, speech impairment, and seizures. Presence of hearing alterations (losses greater than 20 dB) and chorioretinitis was also assessed.
Statistical analysisQualitative variables are expressed as numbers and percentages. Continuous variables are expressed as mean ± SD for normally distributed data and median ± interquartile range for non–normally distributed data. We used the chi-square test or the Fisher exact test to compare qualitative variables and the t test or Mann–Whitney U test to compare continuous variables. Statistical analysis was conducted using SPSS®. P values < .05 were considered significant.
ResultsOf a total of 36 patients, 17 (47.2%) were boys. Six patients (16.7%) were born preterm and 10 (27%) presented low birth weight. Mean birth weight was 2775.6 g ± 622.8 and mean head circumference at birth was 33.1 cm ± 2.0. Eight patients (22%) presented microcephalus at birth.
Congenital CMV infection was suspected before birth in 24 patients (66.7%). We observed alterations in prenatal ultrasound studies of 13 patients (36%); the main findings were intrauterine growth retardation and ventriculomegaly observed during the third trimester.
Twenty patients (55.6%) presented symptoms compatible with congenital CMV infection at birth; Table 2 lists the most frequent clinical manifestations.
Clinical symptoms at birth.
| Hypoacusia | 13 patients (36.1%) |
| IUGR | 9 patients (25%) |
| Microcephalus | 5 patients (13.9%) |
| Thrombocytopaenia | 4 patients (11.1%) |
| Petechiae | 3 patients (8.3%) |
| Hepatosplenomegaly | 3 patients (8.3%) |
| Hepatitis | 1 patient (2.8%) |
| Hyperbilirubinaemia | 1 patient (2.8%) |
| Chorioretinitis | 1 patient (2.8%) |
IUGR: intrauterine growth retardation.
Antiviral treatment (oral valganciclovir) was administered to 20 patients (55.6%). Sixteen of the 24 patients in whom CMV infection was suspected before birth (66.7%) started treatment before one month of life.
The most recent follow-up consultation in the paediatric neurology clinic was performed when patients had a mean age of 49.6 ± 40.0 months (4 years). Eighteen patients (50%) were asymptomatic and the remaining 18 presented neurological sequelae: 15 patients (41.7%) showed psychomotor retardation, which was severe in only 2 cases. Eleven patients (30%) presented cognitive alterations and 12 (33%) presented speech impairment. Only 2 patients (5.6%) presented seizures. Twelve patients (33.3%) had hearing alterations, with 3 (25%) requiring cochlear implants. Two patients (5.6%) presented visual disturbances due to chorioretinitis. None of the 36 patients died during the study period.
Fifteen of the patients with neurological alterations in the last follow-up consultation (83.3%) presented symptoms at birth (P = .001). Ten (55.6%) presented alterations in the transfontanellar ultrasound performed in the first months of life (P = .013) and 16 (88.9%) presented abnormal brain MRI findings (P < .001).
Neuroimaging techniquesAll patients underwent at least one imaging study (transfontanellar ultrasound, brain MRI, or both).
The main neuroimaging findings are detailed in Table 3.
Neuroimaging findings.
| Transfontanellar ultrasound; n = 30 (normal, n = 10; alterations, n = 20) | |
|---|---|
| White matter alterations | 8 patients (22.2%) |
| Lenticulostriate vasculopathy | 8 patients (22.2%) |
| Ventriculomegaly | 6 patients (16.7%) |
| Calcifications | 5 patients (13.9%) |
| Cortical atrophy | 1 patient (2.8%) |
| Brain MRI, n = 29 (normal, n = 9; alterations, n = 20) | |
|---|---|
| White matter alterations | 18 patients (50%) |
| Cysts | 9 patients (25%) |
| Ventriculomegaly | 7 patients (19.4%) |
| Vasculopathy | 6 patients (16.7%) |
| Cortical atrophy | 4 patients (11.1%) |
| Calcifications | 3 patients (8.3%) |
| Cerebellar atrophy | 1 patient (2.7%) |
| Neurological symptoms in patients with brain MRI alterations (n = 20) | |
|---|---|
| Delayed psychomotor development | 13 patients (65%) (P = .007) |
| Hearing loss | 11 patients (55%) (P = .004) |
| Cognitive alterations | 10 patients (50%) (P = .009) |
| Speech impairment | 10 patients (50%) (P = .046) |
| Seizures | 1 patient (5%) (P = .495) |
| Chorioretinitis | 1 patient (5%) (P = .548) |
MRI: magnetic resonance imaging.
Thirteen patients presented anomalies compatible with congenital CMV infection in the prenatal ultrasound. Of these patients, 11 (84.6%) presented symptoms at birth (P = .008). Ten (79.6%) subsequently presented alterations in the brain MRI study (P = .07). Twelve patients with alterations compatible with congenital CMV infection in the prenatal ultrasound (92.3%) presented neurological alterations in the most recent neurology follow-up consultation (P < .001).
Four patients underwent a prenatal MRI study, with 3 showing alterations (ventriculomegaly, cortical atrophy, and/or white matter alterations).
Transfontanellar ultrasoundTransfontanellar ultrasound studies were performed in 30 patients (83.3%), and alterations were detected in 20 (66%).
Eleven patients (55%) in the latter group had presented symptoms compatible with congenital CMV infection at birth (P = .024). Also, 11 (55%) of these patients presented brain MRI anomalies (P = .044), which were severe in 50%. Eight of the 10 patients with normal transfontanellar ultrasound findings presented normal psychomotor development (P = .003). Ten of the 20 patients showing alterations in the transfontanellar ultrasound presented delayed psychomotor development (P = .001).
LSV was observed in 8 of the 30 transfontanellar ultrasounds. No association was identified between presence of LSV and symptoms at birth (P = .108); of the 8 patients with LSV, 5 (62.5%) presented delayed psychomotor development (P = .045).
As a predictor of neurological sequelae, transfontanellar ultrasound presented a sensitivity of 83.3%, (90% CI, 58-100), specificity of 44.4% (90% CI, 18.7-70.2), positive predictive value of 50% (90% CI, 25.6-74.4), and a negative predictive value of 80% (90% CI, 50.2-100).
Brain MRIBrain MRI studies were performed in 29 patients (80%), at a mean age of 19.25 months (0.23-111.41). MRI detected alterations in 19 patients (70%); the most frequent alterations are detailed in Table 3 (Fig. 1).
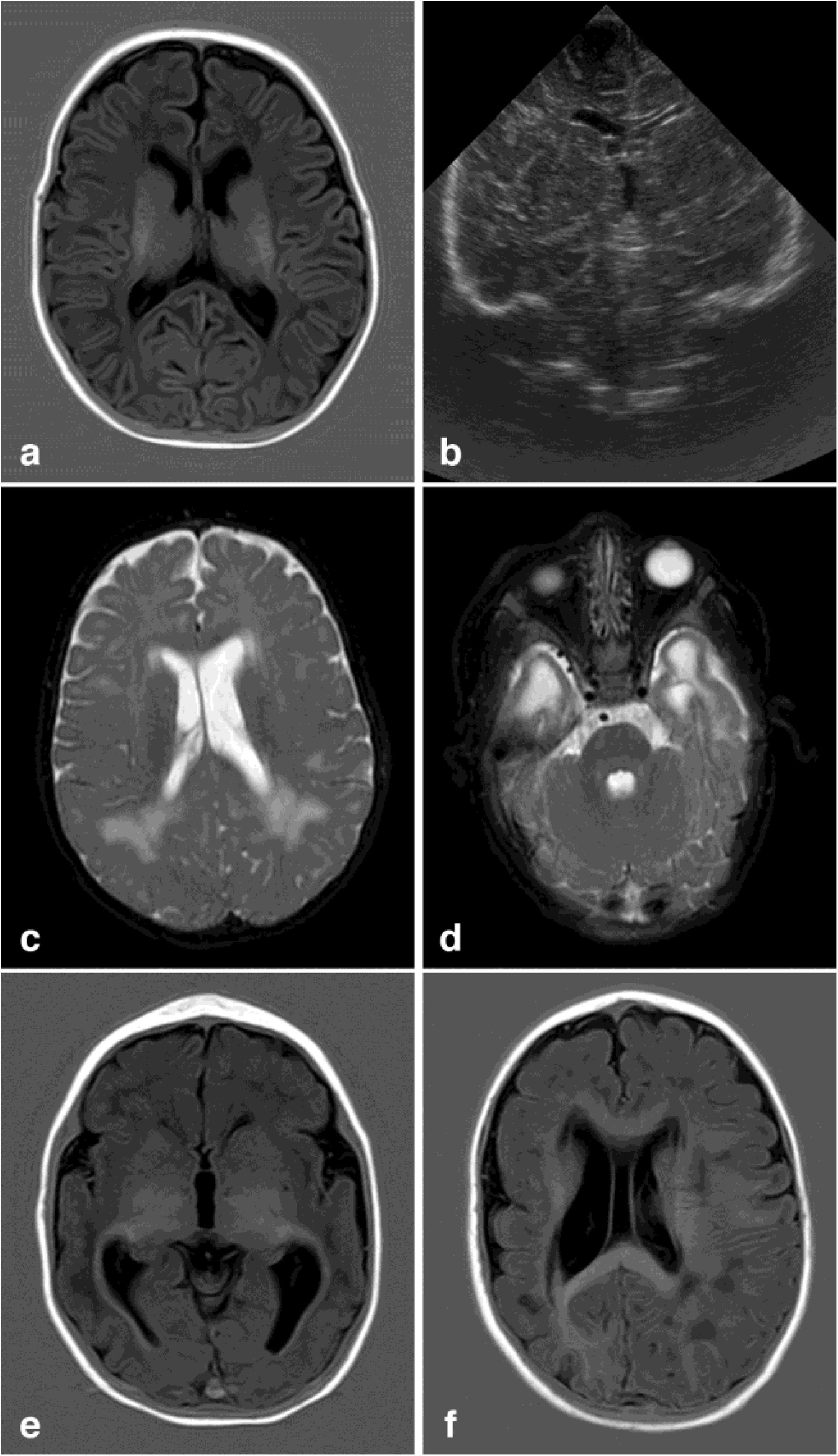
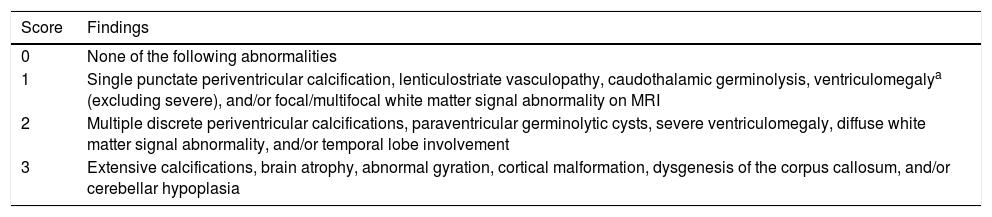
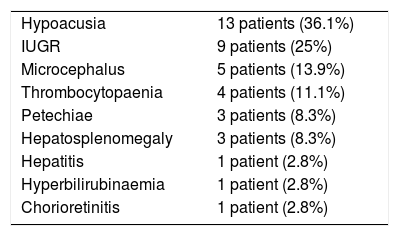
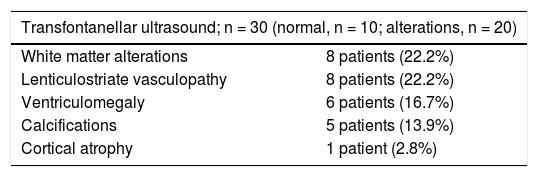
Neuroimaging findings corresponding to the stages of the modified Noyola classification; (a and b) axial slices from T1-weighted brain MRI and transfontanellar ultrasound studies of a 43-day-old patient, scoring 1 on the classification. It shows the presence of a 6-mm germinolytic cyst in the left caudothalamic groove, as well as mild ventriculomegaly of the left lateral ventricle; (c and d) axial slices from T2-weighted brain MRI of a 2-month-old patient, with lesions scoring 2. Multicystic encephalopathy; (e) axial slice from a T1-weighted brain MRI scan of a 4-month-old patient, with a lesion scoring 3. Extensive loss of cortico-subcortical differentiation of the cortical perisylvian grey matter. Hypoplasia of the corpus callosum. Anomalies of neuronal migration and/or band heterotopia. Increased signal in the subcortical white matter of both temporal poles due to gliosis or malacia. Ventricles in the higher threshold of normality; (f) axial slice from a brain MRI FLAIR sequence of a 6-month-old patient, with a lesion scoring 3. Patchy signal in the white matter of both hemispheres and hemispheric asymmetry, with the right hemisphere being smaller in size and showing associated ventriculomegaly. Loss of cortico-subcortical differentiation and increased sulcation, compatible with polymicrogyria.
A positive correlation was identified between brain MRI alterations, presence of symptoms at birth, and the presence of neurological alterations. Thirteen patients with brain MRI alterations compatible with congenital CMV infection (68.4%) had presented symptoms at birth (P = .25). Sixteen patients with brain MRI alterations (84.2%) presented neurological alterations in the last follow-up consultation at the paediatric neurology clinic (P < .001); Table 3 lists the main neurological deficits detected.
According to the modified Noyola classification, 14 patients (38.9%) presented alterations scoring 0, 6 patients (16.6%) had alterations scoring 1, 12 patients (33.3%) had alterations scoring 2, and 4 patients (11.1%) had alterations scoring 3; a positive correlation was observed between presence of severe alterations in neuroimaging studies and neurological involvement: all 4 of the patients with alterations scoring 3 presented delayed psychomotor development (P = .002) and cognitive alterations (P = .001).
MRI alterations scoring 2 or 3 were observed in 12 of the 18 patients (66%) with delayed psychomotor development (P < .001), in all 6 patients with behavioural alterations (P = .003), 10 of the 11 patients (90%) with cognitive alterations (P < .001), 10 of the 12 patients (83%) with speech impairment (P = .001), and 10 of the 12 patients (83%) with hearing loss (P = .001).
MRI showed a sensitivity of 94% (95% CI, 80-100), specificity of 66.6% (95% CI, 36-97.5), a positive predictive value of 80% (95% CI, 60-100), and a negative predictive value of 88% (95% CI, 68-100).
DiscussionInfection with CMV during the prenatal period may cause a wide variety of neurodevelopmental alterations, which differ according to the trimester in which the infection occurred. Infection early in gestation may give rise to significant CNS malformations including lissencephaly, polymicrogyria, schizencephaly, calcifications, cerebellar hypoplasia, and/or hypoplasia/agenesis of the corpus callosum. Infection during the last months of gestation provokes less prominent alterations, such as white matter alterations or myelination disorders.16,17
Although head CT studies are useful for detecting some alterations associated with neurodevelopmental retardation in these patients,8,16,18 they do not constitute the best tool for detecting neuronal migration disorders or white matter alterations; patients are also exposed to high levels of ionising radiation.2 Head CT scans were not performed in any of the patients of our study; ours is the first study in which all patients diagnosed with congenital CMV infection were studied with transfontanellar ultrasound, MRI, or both.
In the neonatal period, MRI and ultrasound are the techniques of choice for detecting such brain alterations as periventricular cysts, cerebellar hypoplasia, and hippocampal dysplasia.10 There is growing interest in the prognostic role of MRI for congenital CMV infection.3,11,14,19,20 Establishing a precise, early diagnosis of the risk of neurodevelopmental alterations helps us to correctly advise parents and to personalise the follow-up and treatment of each patient.11
Transfontanellar ultrasound is a very useful tool for assessing the typical findings of brain involvement in congenital CMV infection, such as ventriculomegaly, periventricular cysts, and cerebral calcifications.2 Furthermore, it does not expose patients to radiation or require sedation.2,10,20 The study by Alarcón et al.11 confirms that transfontanellar ultrasound is comparable to CT in terms of its capacity to detect most of the cerebral lesions caused by CMV, and shows that the Noyola classification, originally designed for CT scans, is also applicable to transfontanellar ultrasound findings. As in our study, the presence of alterations scoring 2-3 presented a high predictive value for association with alterations in psychomotor development.
The role of transfontanellar ultrasound findings, and especially the presence of LSV, has been widely studied.21–23 In our study, transfontanellar ultrasound was performed in 83.3% of patients; we observed that most patients with normal findings presented adequate neurodevelopment, whereas neurodevelopmental alterations were observed in approximately half of patients presenting alterations in the transfontanellar ultrasound. These findings contrast with those obtained by Capretti et al.,2 who identified a correlation between neuroimaging and neurological assessment findings in 40 patients with congenital CMV infection, with transfontanellar ultrasound showing a sensitivity of 57%. However, this may be explained by the fact that such findings as mild ventriculomegaly or LSV were not considered pathological.
Furthermore, the role of LSV as an ultrasound finding in congenital CMV infection is debated. In the study by Giannattasio et al.,20 LSV was not associated with poor prognosis, with neurodevelopmental alterations reported in 39% of patients with LSV and 54% of those who did not present this alteration.20 These data are consistent with the results of our study.
The prospective study by Alarcón et al.14 included 26 patients with congenital CMV, and concluded that normal transfontanellar ultrasound findings are sufficient to predict favourable neurological development. However, our study included 2 patients with normal transfontanellar ultrasound findings and neurodevelopmental alterations; therefore, brain MRI was necessary to rule out brain involvement in patients diagnosed with congenital CMV infection.
Some studies have shown that MRI provides additional information in 20% of cases, especially in the diagnosis of brain developmental disorders14 such as temporal lobe lesions, cortical malformations, and white matter alterations.2 However, few studies have demonstrated the association between neurological prognosis and transfontanellar ultrasound and MRI alterations in patients with congenital CMV infection.
White matter alterations are frequently the only manifestation of CMV infection, and may be related to psychomotor development alterations.9,24,25 In our study, 75% of patients underwent MRI studies, with white matter alterations being the most frequent alteration detected, followed by periventricular cysts. Of these patients, 84.2% presented neurological alterations in the last follow-up consultation at the paediatric neurology clinic.
The study by Kwak et al.26 found that the volume of white matter affected in the brain MRI scan performed at 2 years was associated with intelligence quotient scores. However, white matter lesions are sometimes difficult to distinguish from delayed myelination.10,27
Periventricular cysts are suggestive of viral infection due to the vulnerability of the germinal matrix to infection: in our study, 25% of patients undergoing MRI presented germinolytic cysts.
Our results are similar to those reported by Alarcón et al.,14 showing a positive correlation between the severity of neuroimaging alterations and neurological involvement. Furthermore, the presence of alterations scoring 2-3 on the Noyola classification presented a high predictive value for the association with alterations in psychomotor development.
Establishing a precise, early diagnosis of the risk of neurodevelopmental alterations helps us to correctly advise parents and to personalise the follow-up and treatment of each patient.14
The strength of our study lies in the inclusion of both symptomatic and asymptomatic patients, with imaging studies performed in all cases. Furthermore, patients were followed up for a long period of time (the oldest patient was 14.3 years old). The main limitations were the exclusion of patients with congenital CMV infection who were not followed up at the paediatric neurology clinic, which may have led to bias, with the series including patients with more severe neurological involvement. Furthermore, as brain MRI was not performed at a specific age, some lesions may have been misinterpreted as immature myelination. Lesion progression could not be observed as serial MRI studies were not conducted.
ConclusionsBased on our findings, we can establish a series of recommendations for the follow-up of these patients. It would be recommendable to perform transfontanellar ultrasound studies at birth and to complement the study with brain MRI and start treatment if alterations are detected. If transfontanellar ultrasound findings are normal, an MRI scan should be performed at 4 weeks, with a further brain MRI scan at 2 years to be considered in all patients with congenital CMV infection. Follow-up should continue during the school stage.
FundingThis study received no public or private funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Escobar Castellanos M, de la Mata Navazo S, Carrón Bermejo M, García Morín M, Ruiz Martín Y, Saavedra Lozano J, et al. Asociación entre neuroimagen y secuelas neurológicas en pacientes con infección congénita por citomegalovirus. Neurología. 2022;37:122–129.
This study was presented as an oral communication at the 41st Annual Meeting of the Spanish Society of Paediatric Neurology (SENEP) in Girona, 14–16 June 2018.