Understanding alterations to brain anatomy and cognitive function associated with neurodegenerative diseases remains a challenge for neuroscience today. In experimental neuroscience, several computerised tests have been developed to contribute to our understanding of neural networks involved in cognition. The Attention Network Test (ANT) enables us to measure the activity of 3 attentional networks (alertness, orienting, and executive function).
ObjectivesThe main aim of this review is to describe all the anatomical and functional alterations found in diverse neurological diseases using the ANT.
Material and methodsWe collected studies published since 2010 in the PubMed database that employed the ANT in different neurological diseases. Thirty-two articles were obtained, addressing multiple sclerosis, epilepsy, and Parkinson’s disease, among other disorders.
ConclusionsSome of the anatomical structures proposed in the 3 attentional networks model were confirmed. The most relevant structures in the alertness network are the prefrontal cortex, parietal region, thalamus, and cerebellum. The thalamus is also relevant in the orienting network, together with posterior parietal regions. The executive network does not depend exclusively on the prefrontal cortex and anterior cingulate cortex, but also involves such subcortical structures as the basal ganglia and cerebellum and their projections towards the entire cortex.
Comprender las alteraciones en la anatomía y función del cerebro en los procesos cognitivos para las enfermedades neurodegenerativas es aún un desafío para la neurociencia actual. Desde la neurociencia experimental, algunos tests computarizados han sido desarrollados para mejorar nuestro conocimiento de las redes neurales involucradas en la cognición. El Attention Network Test (ANT) permite medir la activad de las tres redes atencionales (alerta, orientación y función ejecutiva).
ObjetivosEl principal objetivo de esta revisión fue describir todas las alteraciones anatómicas y funcionales encontradas en diversas enfermedades neurológicas usando el ANT.
Material y métodosUn protocolo de revisión fue aplicado seleccionando estudios desde 2010 en la base de datos PubMed que involucraban al Attention Network Test en diferentes enfermedades neurológicas. Se obtuvieron treinta y dos artículos para esclerosis múltiple, epilepsia o Parkinson entre otras patologías.
ConclusionesSe confirman algunas de las estructuras anatómicas propuestas para el modelo de tres grandes redes atencionales. Las estructuras más relevantes para la red de alerta son la corteza prefrontal, regiones parietales, tálamo y el cerebelo. El tálamo es también relevante para la red de orientación, junto a regiones parietales posteriores. Respecto a la red ejecutiva, no depende exclusivamente de la corteza prefrontal y corteza cingulada anterior, sino también de estructuras subcorticales como los ganglios basales y el cerebelo y sus proyecciones hacia toda la corteza.
The Attention Network Test (ANT) is a computer-based test that has been used in over 300 studies since its creation in 2001.1 According to most of these studies, it is useful for evaluating the activity of different attention networks in humans. The attention system involves 3 main neural networks.2 The first of these is the alerting network, which maintains an adequate level of alertness to prepare the body to respond. This network is located mainly in the right hemisphere, in frontal and parietal regions, and involves the noradrenergic system.3–5 The second network is the orienting network, which enables the brain to selectively focus on specific characteristics of a task (selecting objects by colour, locating in space, etc). The main neurotransmitter in this network is acetylcholine. The orienting network is mainly located in posterior parietal regions, the pulvinar nucleus of the thalamus, the superior colliculus, and the frontal eye fields, predominantly in the right hemisphere.5–7 Lastly, the executive network is responsible for the management of any potential conflicts in cognitive processing that the task may generate.8 The main anatomical structures involved in this network are the anterior cingulate cortex, the dorsolateral prefrontal cortex, and the dorsomedial prefrontal cortex, and the main neurotransmitter involved is dopamine.5
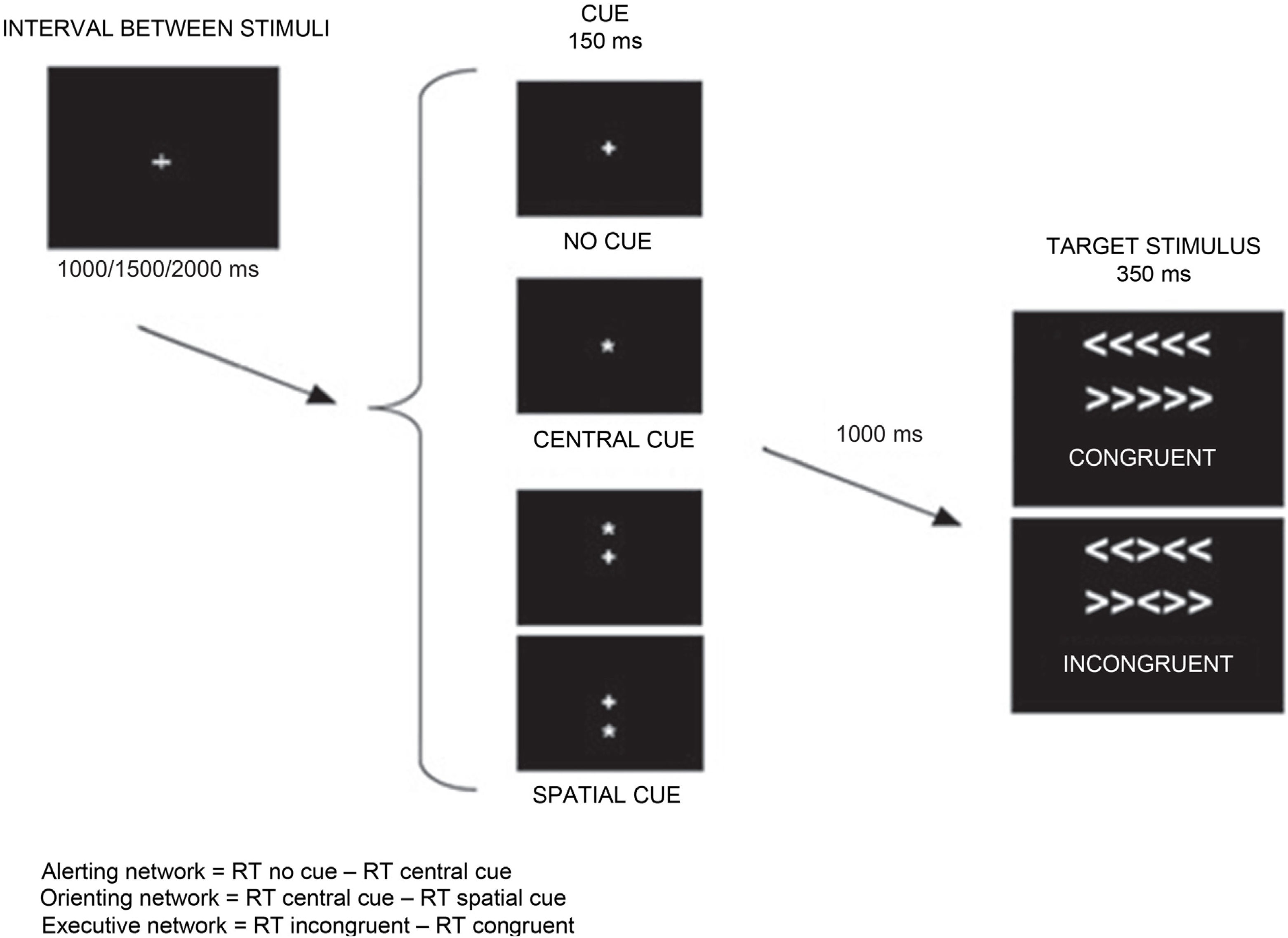
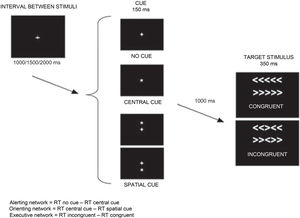
This review is not intended to provide a detailed description of the test (studies addressing this issue can be found in the references section); however, we do describe the central idea of the ANT and how to interpret its results. The main purpose of the ANT is to evaluate the activity of the 3 neural networks according to behavioural responses (mainly reaction time) under different experimental conditions. The test subject must indicate the direction (left or right) of a central arrow (target stimulus) surrounded by distracting arrows that may point in the same direction (congruent stimulus) or in a different direction (incongruent stimulus). Before the target stimulus is displayed, the subject may be presented with several cues: a central cue (does not indicate where the arrow will appear, but indicates that the target stimulus will appear at a fixed time interval) or a spatial cue (indicates both when and where the arrow will appear). In some cases, the stimulus may not be preceded by any cue. Attention network activity may be calculated based on these conditions (cued, congruent, or incongruent) (Fig. 1).9 Several research groups have adapted the test for patients with different characteristics or for different patient populations (eg, children).10–14
Example of one of the modalities of the Attention Network Test.
RT: reaction time.
Adapted from Vázquez-Marrufo et al.17
Understanding the anatomical and functional basis of cognitive impairment in neurodegenerative diseases is one of the major challenges of neuroscience. Studies combining such computer-based tests as the ANT with neuroimaging data may help us to describe the functional structure of different cognitive processes, including attention. The main purpose of this study was to analyse the available evidence on the application of the ANT in different neurodegenerative diseases with a view to increasing our understanding of cognitive processing and cognitive impairment.
Material and methodsWe gathered scientific articles from the PubMed database using the following keywords: “attention network test,” “attentional network test,” “attention network task,” and “neurodegenerative disease.” A search with the keywords “attention network test” and “neurodegenerative disease” yielded no results. Therefore, we decided to use as keywords only the possible variants of the name of the ANT, and subsequently selected those studies focusing on neurodegenerative diseases of interest for this review (multiple sclerosis [MS], epilepsy, Alzheimer disease [AD], Parkinson’s disease [PD], Lewy body dementia [LBD]).
The earliest article reviewed is a study of the ANT in multiple sclerosis published in 2010. We should point out that the key concept “attention network task” is more general than ANT; however, our review found that all articles using the former term focused on the use of the ANT.
ResultsA total of 31 studies met the criteria listed in the previous section. One study on Parkinson’s disease was found to be a protocol study not presenting any results, and was therefore excluded from our review.
Multiple sclerosisThe first study of patients with MS reported alterations in the alerting network, but not in the orienting or the executive networks (Table 1).15 The authors suggested that patients had difficulty remaining alert without warning cues, and that the thalamus plays a key role, although they do not provide anatomical or functional data to support this hypothesis. A subsequent study found alerting network dysfunction, which resulted in abnormal executive network scores due to the interaction between both networks.16 These authors also pointed to the thalamus as a key structure in this network. Another study reported alterations in the alerting and orienting networks in patients with MS.17 The researchers also found decreased amplitude of the evoked potential component known as contingent negative variation interval (time interval between a cue and a target stimulus). This points to alterations in different regions responsible for contingent negative variation in the cortex of patients with MS.
Main results from the studies into multiple sclerosis included in this review.
| Authors | Patients | Results |
|---|---|---|
| Urbanek et al.15 (2010) | N = 114 (57 healthy controls and 57 RRMS) | Alerting network dysfunction |
| Crivelli et al.16 (2012) | N = 54 (27 healthy controls and 27 RRMS) | Alerting and executive network dysfunction |
| Wojtowicz et al.18 (2013) | N = 61 (30 healthy controls and 31 RRMS) | Executive network dysfunctionGreater intraindividual variability in test responses |
| Vázquez-Marrufo et al.17 (2014) | N = 52 (26 healthy controls and 26 RRMS) | Alerting and orienting network dysfunctionPhysiological correlate: decreased contingent negative variation amplitude |
| Wojtowicz et al.19 (2014) | N = 22 (11 healthy controls and 11 RRMS) | Intraindividual variability as a stable measure to estimate the degree of impairment during disease progression |
| Roth et al.21 (2015) | N = 80 (40 healthy controls, 20 SPMS, and 20 RRMS) | Executive or alerting network dysfunction (depending on the scoring system used) |
| Ayache et al.20 (2016) | N = 16 (8 RRMS with tDCS and 8 RRMS without tDCS) | tDCS applied to the dorsolateral prefrontal cortex decreased pain but had no influence on the efficiency of attention networks. |
RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; tDCS: transcranial direct current stimulation.
Using a modified version of the ANT, Wojtowicz et al.18 found no alterations in the alerting or orienting networks, but did find changes in the executive network, which suggests impairment of top-down attentional control mechanisms, although the authors did not mention any specific anatomical region. Furthermore, a subsequent study by the same research group concluded that intraindividual variability in task performance, used in their previous study, constitutes an ideal measure for the longitudinal follow-up of attentional alterations.19
The ANT may also be useful for evaluating whether a potential treatment may cause alterations to any of the 3 attention networks. For instance, a recent study showed that transcranial direct current stimulation of the dorsolateral prefrontal cortex modulates pain but has no observable effect on attention network function.20 This lack of changes in attention network function reflects the complexity of these networks (cortico-cortical and cortico-subcortical); stimulation of the dorsolateral prefrontal cortex alone is insufficient to achieve observable effects. Lastly, using several scores of the effects on attention networks, another study group found deficits in the alerting network in some cases and in the executive network in others.21
Parkinson’s diseaseA study analysing the presence of attention deficits in patients with mild and moderate PD found alterations in the orienting network, which was attributed to alterations in the cholinergic system (Table 2).22 A subsequent study reports dysfunction in all 3 attention networks in patients with PD; this was attributed to degeneration of the different neurotransmitter systems involved in each network.23 A more recent article reported that patients with PD and fatigue exhibited more severe impairment of the executive network than patients without fatigue, and hypothesised that fatigue and the executive network may share elements of the striato-thalamo-prefrontal loop.24
Main results from the studies addressing Parkinson’s disease included in this review.
| Authors | Patients | Results |
|---|---|---|
| Lou23 (2009) | N = 25 (9 healthy controls and 16 PD) | Alerting network dysfunctionOrienting network dysfunctionExecutive network dysfunction |
| Vandenbossche et al.25 (2011) | N = 32 (10 healthy controls, 11 PD without dementia with FOG, and 11 PD without dementia or FOG) | Executive network dysfunction in patients with PD and FOGAntipsychotics did not improve executive network function. |
| Zhou et al.22 (2012) | N = 72 (28 healthy controls and 44 PD without dementia) | Orienting network dysfunction |
| Cristinzio et al.28 (2013) | N = 27 (13 healthy controls and 14 PD without dementia) | The ANT-interaction revealed slower response times in the patient group. |
| Hall et al.26 (2016) | N = 53 (25 PD without dementia with hallucinations and 28 PD without dementia or hallucinations) | Lower accuracy rates in patients with PD and hallucinations, particularly in conflict resolution tasks (executive network) |
| Pauletti et al.24 (2017) | N = 75 (37 healthy controls and 32 PD without dementia, 15 with and 17 without fatigue) | Executive network dysfunctionDamage in the striato-thalamo-prefrontal loop (right frontal eye field, temporal parietal junction, and right superior parietal lobe) |
| Boord et al.27 (2017) | N = 46 (21 healthy controls and 25 PD without dementia) | Overactivation of frontoparietal networks in patients with PD (neural compensatory mechanism) |
ANT: attention network test; FOG: freezing of gait; PD: Parkinson’s disease.
Another symptom analysed due to its potential link with attention networks is freezing of gait (FOG).25 The executive network involves frontoparietal and mesocorticolimbic pathways; dysfunction at this level would play a major role in the development of FOG. However, alterations to such executive function components as conflict resolution and mental flexibility were not correlated with presence of FOG. This lack of correlation was attributed to insufficient sensitivity of the neuropsychological instruments used. Furthermore, antipsychotic medication did not improve executive network function despite increasing dopamine levels.
A study including patients with psychotic symptoms, which may occur in PD, observed poorer executive network function, which the authors attribute to deficits in the frontostriatal pathway.26 Another study, which performed a functional connectivity analysis, detected increased activation of the frontoparietal networks, particularly the right frontal eye field, bilateral intraparietal sulcus, and right superior parietal lobule, during highly demanding executive tasks. The authors hypothesised that overactivation of several structures was necessary for patients to perform adequately in the ANT (compensation with other neural resources).27 Lastly, in a study using the ANT-interaction, patients with PD were found to be slower than controls; however, acoustic cues substantially improved response times.28 This approach has been suggested in other rehabilitation studies using acoustic stimulation (music, tones, etc).29,30
EpilepsyIn a study including children with generalised idiopathic epilepsy, executive network alterations were found to be associated with alterations in the prefrontal lobe and anterior cingulate cortex.31 The fact that the alerting and orienting networks were unaffected was attributed to the lack of thalamic atrophy (Table 3). In another study of epilepsy with centrotemporal spikes, the researchers observed alterations in the orienting network, which suggests that centrotemporal spikes may interfere with this network.32 In the case of temporal lobe epilepsy, several research groups have used fMRI to study the anatomical, functional, and connectivity alterations associated with the disease.33–38 Although not all studies report alerting network dysfunction, they do all report alterations in resting-state thalamic functional connectivity with cortical and subcortical structures, which, the authors hypothesise, are explained by the excitotoxic damage caused by epileptic discharges. Likewise, several studies propose that the cerebellum and parietal regions may be relevant structures for the integrity of the alerting network. Table 4 provides detailed information on specific regions and their potential association with attention networks.
Main results from the studies of epilepsy included in this review.
| Authors | Patients | Results |
|---|---|---|
| Tian et al.31 (2010) | N = 74 (37 healthy controls and 37 IGE without medication) | Executive network dysfunction |
| Zheng et al.33 (2012) | N = 20 (8 healthy controls and 12 TLE) | In TLE, a negative correlation was observed between reaction and response times in the right occipital lobe and cerebellum, as well as in such other regions as the right frontal lobe, temporal lobe, and brainstem.The lack of differences in network function between patients and controls was attributed to neural compensatory mechanisms. |
| Chen et al.34 (2015) | N = 32 (16 healthy controls and 16 TLE with medication) | Negative correlation between functional connectivity (thalamus–anterior cingulate cortex) and alerting network performance |
| Yang et al.32 (2015) | N = 180 (90 healthy controls and 90 BECTS without medication) | Orienting network dysfunction, parietal lobe alterations |
| Zheng et al.39 (2015) | N = 22 (11 healthy controls and 11 TLE with medication) | Poorer overall performance in patients with TLE was attributed to altered activity in prefrontal and parietal regions and other areas involved in executive processes necessary for conflict resolution. |
| Liu et al.35 (2016) | N = 63 (20 healthy controls and 43 TLE) | Alerting network dysfunction, associated with a decrease in fractional anisotropy in the bilateral parahippocampal gyrus, especially in patients with right TLE |
| Li et al.36 (2016) | N = 38 (18 healthy controls and 20 TLE with medication) | Patients presented differences in functional connectivity of structures associated with the alerting network. |
| Gao et al.37 (2018) | N = 81 (34 healthy controls and 47 right TLE with medication) | Results revealed deficits in intrinsic and phasic alertness. However, no significant differences between patients and controls were observed in alerting network performance. |
| Li et al.41 (2018) | N = 40 (20 healthy controls and 20 TLE with medication) | Executive network dysfunctionLower theta-band coherence and power spectral density in the prefrontal and frontal lobes |
| Zhou et al.38 (2019) | N = 46 (23 healthy controls and 23 TLE) | Abnormal topological organisation of the default mode network, which may explain executive dysfunction. |
| Zhou et al.40 (2019) | N = 60 (30 healthy controls and 30 right TLE) | Cerebellar–cerebral network disruption negatively affects attention.Positive correlation between executive control effect and functional connectivity in the right dentate nucleus and the left precentral and postcentral gyri |
BECTS: benign epilepsy with centrotemporal spikes; IGE: idiopathic generalised epilepsy; TLE: temporal lobe epilepsy.
Main regions involved in epilepsy, as observed with functional magnetic resonance imaging and diffusion tensor imaging.
| Healthy controls | Patients | Findings | |
| Zheng et al.33 (2012) | Alerting network:- Right occipital lobe- Cerebellum- Right frontal lobe- Right limbic lobe- Left superior temporal gyrus- Brainstem | Alerting network:- Right occipital lobe- Cerebellum- Right thalamus- Right medial frontal gyrus- Left inferior frontal gyrus- Cingulate gyrus- Left limbic lobe | Patients with TLE show significantly lower brain activation overall.Patients present hypoactivity in the cerebellum, right occipital lobe, right frontal lobe, and brainstem, and slight right-sided lateralisation. |
| Chen et al.34 (2015) | Normal resting-state FC of the bilateral thalamus with:- Frontal lobe- Temporal lobe- Insula- ACC- Thalamus- Basal ganglia- Amygdala- Brainstem- Cerebellum | Decreased resting-state FC of the right thalamus with:- Bilateral ACC- Brainstem- Left thalamus- Cerebellum- Bilateral putamen- Right caudate nucleus- Amygdala | Decreased FC between the thalamus ipsilateral to the lesion and the ACC is associated with deficient allocation of cognitive resources, but not with any specific attention network.Alterations in FC with the brainstem may explain alerting network dysfunction (noradrenaline). |
| Zheng et al.39 (2015) | Areas activated in conflict resolution tasks (executive network):- Bilateral inferior temporal gyrus- Right hippocampus- Right posterior cingulate gyrus- Right superior frontal lobe- Right parietal lobe | Areas activated in conflict resolution tasks (executive network):- Right prefrontal lobe- Right frontal lobe- Left superior temporal lobe- Right anterior cerebellum | Considering the attention network function as a whole, decreased activation is observed in the right prefrontal lobe, right parietal lobe, right posterior cingulate gyrus, left medial temporal gyrus, and left cerebellar peduncle in conflict resolution tasks (executive network). |
| Liu et al.35 (2016) | The study does not include a control group. | Resting-state FC:- Left TLEIncreased in angular gyrus, inferior occipital gyrus, and superior parietal gyrusDecreased in supplementary motor area, inferior parietal lobe, medial temporal gyrus, and medial superior frontal gyrus- Right TLEIncreased in inferior occipital gyrus, parahippocampal gyrus, and cerebellumDecreased in medial temporal gyrus, precentral gyrus, and inferior frontal gyrus | Patients with right TLE present decreased FA in interhemispheric fibres connecting the parahippocampal gyrus. |
| Li et al.36 (2016) | Alerting network:- Right dorsolateral prefrontal cortex- Medial frontal gyrus- Parietal lobe- Temporal lobe- Right cerebellum | Decreased FC in the right frontoparietal network in patients with TLE:- Right inferior parietal lobe and angular gyrus partially isolated from the networkIn patients with TLE, increased FC of the following structures in the right frontoparietal network:- Right inferior frontal gyrus- Medial frontal gyrus- Dorsolateral superior frontal gyrus- Superior occipital gyrus- Cuneus- Rolandic operculum | Decreased FC in the areas mentioned may affect the detection of salient new events, sustained attention, and phasic alertness.Negative correlation between right cuneus FC and the alertness effect |
| Gao et al.37 (2018) | FC of the thalamus (alerting network):- Superior frontal cortex- Right precuneus- Right medial temporal gyrus- Right medial frontal gyrus- Right inferior temporal gyrus | Decreased FA: right insula, parahippocampal gyrus, amygdala, right superior temporal pole, and right superior temporal gyrusDecreased FN: right superior temporal gyrus and right superior temporal poleDecreased FL: right parahippocampal gyrus and right amygdalaDecreased FC (with thalamus): amygdala, insula, right inferior parietal lobe | Decreased FA, FN, FL, and thalamic FC with other structures and regions, associated with alerting network dysfunction. However, test results did not reflect alterations.Negative correlation between FA in the right insula–superior temporal pole (which reflects alerting network dysfunction) and disease duration |
| Zhou et al.38 (2019) | DMN comprising:- Medial superior frontal gyrus- Inferior parietal gyrus- Posterior cingulate gyrus- Precuneus- Right angular gyrusWithin the DMN, decreased connectivity between the bilateral medial superior frontal gyrus and the left medial orbital superior frontal gyrus | Increased nodal strength of the right inferior parietal gyrus (interpreted as a compensatory mechanism for executive dysfunction) | |
| Zhou et al.40 (2019) | Normal FC of the bilateral dentate nucleus:- Prefrontal cortex- Primary motor cortex- Supplementary motor cortex- Occipital cortex- Parietotemporal cortex- Cingulum- Basal ganglia- Thalamus- Brainstem- Cerebellum | Decreased FC of the right dentate nucleus:- Left calcarine gyrus- Left postcentral gyrus- Left precentral gyrus- Left cuneus | Increased FC: right cerebellar crus I with right inferior parietal lobe (positively correlated with executive control effect) |
ACC: anterior cingulate cortex; DMN: default mode network; FA: fractional anisotropy; FC: functional connectivity; FN: fibre number; FL: fibre length; TLE: temporal lobe epilepsy.
Regarding the executive network and functional connectivity, several studies have reported differences in network activity between patients and healthy controls (Table 2).39,40 Poorer executive performance in temporal lobe epilepsy is due to decreased activation or abnormal activity in prefrontal, frontal, and parietal areas, as well as other areas also involved in executive attention processes. Zhou et al.40 observed an increase in functional connectivity between the right cerebellar crus I and the right inferior parietal lobule, 2 areas that play a major role in executive function; this was interpreted as a compensatory mechanism. Other studies have observed a decrease in theta band power spectral density in the frontal lobe41; due to the important role of this area in the executive network, its impairment hinders recruitment of cognitive resources.
Alzheimer disease and Lewy body dementiaSeveral studies have detected different patterns of attention network dysfunction in patients with AD and with LBD (Table 5).42,43 AD is associated with decreased efficiency of the orienting network due to deficiencies in the alerting network. It has also been suggested that changes in occipital brain volume play a role in the development of alerting and orienting network deficiencies. In patients with LBD, alerting network dysfunction affects both the orienting and executive networks. Orienting network dysfunction in LBD is associated with degeneration of the cholinergic system and loss of occipital white matter volume. The executive network may also present alterations in both types of dementia due to alterations in the frontostriatal circuit.43 The lack of changes in volume of the brain structures in some cases may suggest that cognitive performance is affected by functional or microstructural alterations.
Main results from the studies addressing Alzheimer disease and Lewy body dementia included in this review.
| Authors | Patients | Pathological results |
|---|---|---|
| Fuentes et al.42 (2010) | N = 49 (18 healthy controls, 13 LBD, and 18 AD) | Both diseases were associated with alerting network dysfunction.LBD is associated with orienting and executive network dysfunction. |
| Zhang et al.49 (2015) | N = 43 (15 healthy controls, 12 MCI, and 16 AD) | Behavioural test results reflect alterations in all 3 attention networks in patients with AD, and executive network dysfunction in patients with MCI.Hypo- and hyperconnectivity between different structures in both types of dementia |
| Kobeleva et al.48 (2017) | N = 71 (21 healthy controls, 30 LBD, and 20 AD) | Patients with LBD present disconnection of the DAN and VAN, affecting executive performance.Excessive deactivation of the anterior DMN in LBDHyperconnectivity of the posterior DMN, especially the posterior cingulate cortex, during the task was observed in patients with AD; this was interpreted as a compensatory mechanism or a disturbance of transition between networks (from DMN activation during rest to DMN deactivation during task).Increased FC between DMN and DAN in AD |
| Cromarty et al.43 (2018) | N = 105 (21 healthy controls, 30 LBD, and 20 AD) | Alerting network dysfunction in LBD and AD, associated with damage to the locus coeruleusOrienting network dysfunction in LBD is associated with degeneration of the cholinergic system and loss of occipital white matter.Executive network dysfunction in LBD and AD, attributed to frontostriatal dysfunction |
| McDonough et al.51 (2019) | Literature review | Patients with amnestic MCI present executive network dysfunction.In AD, neurodegeneration progressively affects all 3 attentional networks. |
| Schumacher et al.50 (2019) | N = 103 (22 healthy controls, 48 LBD, and 33 AD) | Slow reaction times in AD are correlated with loss of grey and white matter volume.These alterations seem to be linked to cortical atrophy in AD and to microstructural damage in LBD. |
AD: Alzheimer disease; DAN: dorsal attention network; DMN: default mode network; FC: functional connectivity; LBD: Lewy body dementia; MCI: mild cognitive impairment; VAN: ventral attention network.
Furthermore, the attention model that distinguishes 2 pathways (the dorsal [DAN] and ventral attention networks [VAN])44–47 may complement the attention model evaluated by the ANT. Both pathways may be seen as components of the orienting network. LBD is associated with hypoconnectivity between the DAN and VAN due to decreased activation of the VAN, and reduced connectivity of both attention networks with the executive network; this affects information exchange between them and hinders coordination of the DAN and the VAN by the executive network.48 In a previous study, degeneration of the DAN was found to be correlated with executive dysfunction. Furthermore, connectivity between the VAN and the frontal eye field increases in early stages of AD; this has been interpreted as a possible compensatory mechanism for executive network deficits in the context of mild cognitive impairment.49
Other studies have focused on such structures as the default mode network (DMN), reporting hyperconnectivity between the posterior DMN and the DAN, and particularly with the posterior cingulate cortex, in patients with AD.48 This seems to be due to compensatory mechanisms or to errors in transitions and changes of functional state of the DMN. LBD, in turn, displays hypoconnectivity of the anterior DMN during task initiation, which is attributed to less efficient distribution of cognitive resources.48
A study focusing on reaction times observed slower overall times in patients with AD, due to a greater number of extremely slow responses in these patients, which was correlated with grey and white matter loss. In LBD, however, the slow overall reaction times were explained by a general increase in mean response times.50 The alterations observed in AD seem to be linked to cortical atrophy, whereas in LBD they are explained by microstructural changes. A recent review reports executive dysfunction in amnestic mild cognitive impairment and alterations in all 3 attention networks secondary to degeneration in patients with AD.51
DiscussionOur review of the available evidence on the ANT suggests that the wide range of neurological diseases reflects the complexity of the structure, function, and connectivity of the 3 attention networks. In the case of MS, the most severely affected network is the alerting network, although alterations may also be observed in the orienting and executive networks. Several studies suggest that the thalamus is responsible for the attention deficits evaluated by the ANT. However, other studies have detected cortical changes related to attentional dysfunction.
Most studies into PD suggest that the executive network is the most severely impaired. Research has been conducted into the relationship between attention network and specific symptoms, including fatigue, FOG, and hallucinations. Studies suggest that the frontostriatal pathway is the most severely impaired, although severe alterations have also been observed in the frontoparietal pathway. Interestingly, while dopamine is the neurotransmitter responsible for adequate executive function, dopaminergic medication does not modulate deficits in the executive network.
Attention networks have been studied in patients with different types of epilepsy. Patients with generalised idiopathic epilepsy show executive network alterations linked to prefrontal cortex damage. Epilepsy with centrotemporal spikes is associated with attention deficits linked to orienting network dysfunction. However, the most widely studied type of epilepsy is temporal lobe epilepsy. Studies evaluating these patients with fMRI and diffusion tensor imaging (DTI) propose the same structures and regions as being the most relevant in the alerting network: the medial frontal gyrus, cuneus, inferior parietal lobe, superior temporal gyrus, right occipital lobe, thalamus, brainstem, and cerebellum. In spite of the connectivity changes observed in these structures, some patients do not present impaired responses, which may be explained by compensatory mechanisms or greater sensitivity of neuroimaging studies, which may detect abnormalities before alterations can be detected with the ANT. Furthermore, the prefrontal cortex, anterior cingulate cortex, parietal cortex, and cerebellum have been proposed as essential components of the executive network. From a physiological perspective, an association has been described between alterations in the frontal theta band and executive network deficits, as the former interfere with the recruitment of cognitive resources during the executive task.
AD, together with LBD, is the most extensively studied dementia; patients with both conditions present deficits in all 3 attention networks, although with different patterns of impairment. In LBD, the executive network is most affected, whereas patients with AD present more global alterations. These are attributed mainly to degeneration of the frontoparietal and frontostriatal pathways.
We found extensive evidence that attention networks are altered in all neurodegenerative diseases. However, each disease presents a distinct pattern of dysfunction as a result of different structural and/or functional alterations. Furthermore, it is widely accepted that all 3 attention networks are affected in parallel with disease progression.
In the light of these findings, we may conclude that the ANT may help in the evaluation of attention network function in patients with neurological diseases, due to its ease of administration and its accuracy as a general indicator of impairment; it is also useful for early detection of attention dysfunction before standard neuropsychological tests can be used.
We should nonetheless point out that some studies report diverging, or even contradictory, results. This may be due to the heterogeneity of cognitive impairment itself. However, we cannot rule out the possibility that these differences may be due to issues with sample size, the patients’ degree of impairment, or the methodology used. Validation studies are needed to confirm the replicability of the test in the clinical setting, as is the case with other computer-based tasks used in the study of cognitive impairment.52
The main neuroimaging techniques used in the studies of the ANT are fMRI and DTI. Functional MRI is a non-invasive neuroimaging technique providing metabolic data. It uses the magnetic susceptibility of oxyhaemoglobin and deoxyhaemoglobin to obtain images of in vivo activity of the brain regions activated by the experimental task.53 DTI, in contrast, uses the diffusion properties of water molecules to obtain colour-coded images of the white matter, which enables evaluation of the integrity of neural connections.54
None of the studies on MS included in this review combined the ANT with neuroimaging techniques. Therefore, the interpretations of the neuroanatomical basis of alterations in different attention networks constitute suggestions from the authors, based on previous studies on the neural substrate of these networks. Functional MRI reveals increased frontoparietal activation in patients with PD; this is interpreted as a compensatory mechanism due to executive dysfunction.27 In line with this finding, the ANT shows that the executive network is the most severely affected.
All studies into epilepsy applying neuroimaging techniques included patients with temporal lobe epilepsy. The most frequently used technique was fMRI, with both task-based resting-state studies. These studies have also detected increases and decreases in functional or structural connectivity between certain structures. Increases were interpreted as a neural compensation mechanism, whereas decreases were associated with neurodegeneration.
Lastly, studies into AD and LBD have reported both functional hyperconnectivity (linked to neural compensatory mechanisms) and hypoconnectivity (attributed to neurodegeneration of the pathways connecting certain structures or regions).48,49 Voxel-based morphometry has detected white and grey matter loss in both types of dementia.43,50
ConclusionThis literature review, including studies of different neurological diseases and reporting different attention deficits and anatomic and functional alterations, seems to confirm some of the structures proposed for the model of 3 attention networks. The most relevant structures in the alerting network are the prefrontal cortex, parietal regions, thalamus, and cerebellum. The thalamus is also an important element of the orienting network, together with posterior parietal regions. The executive network involves not only the prefrontal cortex and anterior cingulate cortex, but also such subcortical structures as the basal ganglia and cerebellum and their projections to the cortex.
FundingThis study was funded by the Spanish Ministry of Science, Innovation, and Universities (project code PSI2016-78133-P).
Conflicts of interestThe study was designed in accordance with ethical guidelines and standards. All authors meet the authorship criteria. The authors have no conflicts of interest to declare.