Paraneoplastic neurological syndromes (PNS) constitute a heterogeneous group of immunopathogenic disorders caused by tumours located outside the nervous system. Before the diagnosis is established, we must rule out neurological complications resulting directly from the tumour or its treatment.1 From a pathophysiological perspective, PNS are explained by the presence of common antigens in tumour cells and in some structures of the nervous system, with the result that the antitumour immune response also affects healthy cells.2,3 According to the PNS diagnostic criteria, detection of both the onconeuronal antibodies and the primary tumour are 2 of the most useful factors when establishing diagnosis. However, not all patients present circulating antibodies4: these may go undetected in up to 50% of patients,5 as in our case.
Our patient is a 68-year-old woman who presented progressive dyspnoea associated with pulmonary thromboembolism and deep vein thrombosis, as well as confusional syndrome and secondarily generalised partial seizures. After a 2-month improvement period, she once again presented confusional syndrome, gait ataxia, and recurrent seizures leading to status epilepticus.
The blood analysis displayed normal results (including thyroid and parathyroid hormone levels, vitamins, folic acid, CEA, and Ca 15.3); results for angiotensin-converting enzyme, long-chain fatty acids, serum and urine protein test, and immunofixation were also normal. The autoimmunity study (anti-DNA antibodies, IgM and IgG anti-cardiolipin antibodies, anti-Cenp-B antibodies, anti-histone antibodies, anti-Jo-1/HRS antibodies, anti-nucleosome antibodies, anti-PCNA antibodies, anti-PM/Scl antibodies, anti-ribosomal P antibodies, anti-topo I (Scl-70) antibodies, anti-Sm antibodies, anti-RNP/Sm antibodies, anti-SSA/Ro60 antibodies, anti-SS-A/Ro52 antibodies, anti-SS-B/La antibodies, antimicrosomal antibodies, TSH receptor antibodies, anti-thyroglobulin antibodies) yielded normal results, with the exception of those for anti-nuclear antibodies. Serology tests for syphilis, HIV, and JC virus returned negative results. The CSF analysis only revealed high protein levels (118mg/dL), with negative results for Gram staining, cultures, and cytology study. No intracellular onconeural antibodies or surface antigen antibodies were detected in the serum or the CSF (anti-Hu, Yo, Ri, CV2, PNMA 2 [Ma2/Ta], amphiphysin, recoverin, Sox1, titin, Zic 4, GAD65, and Tr [DNCR]).
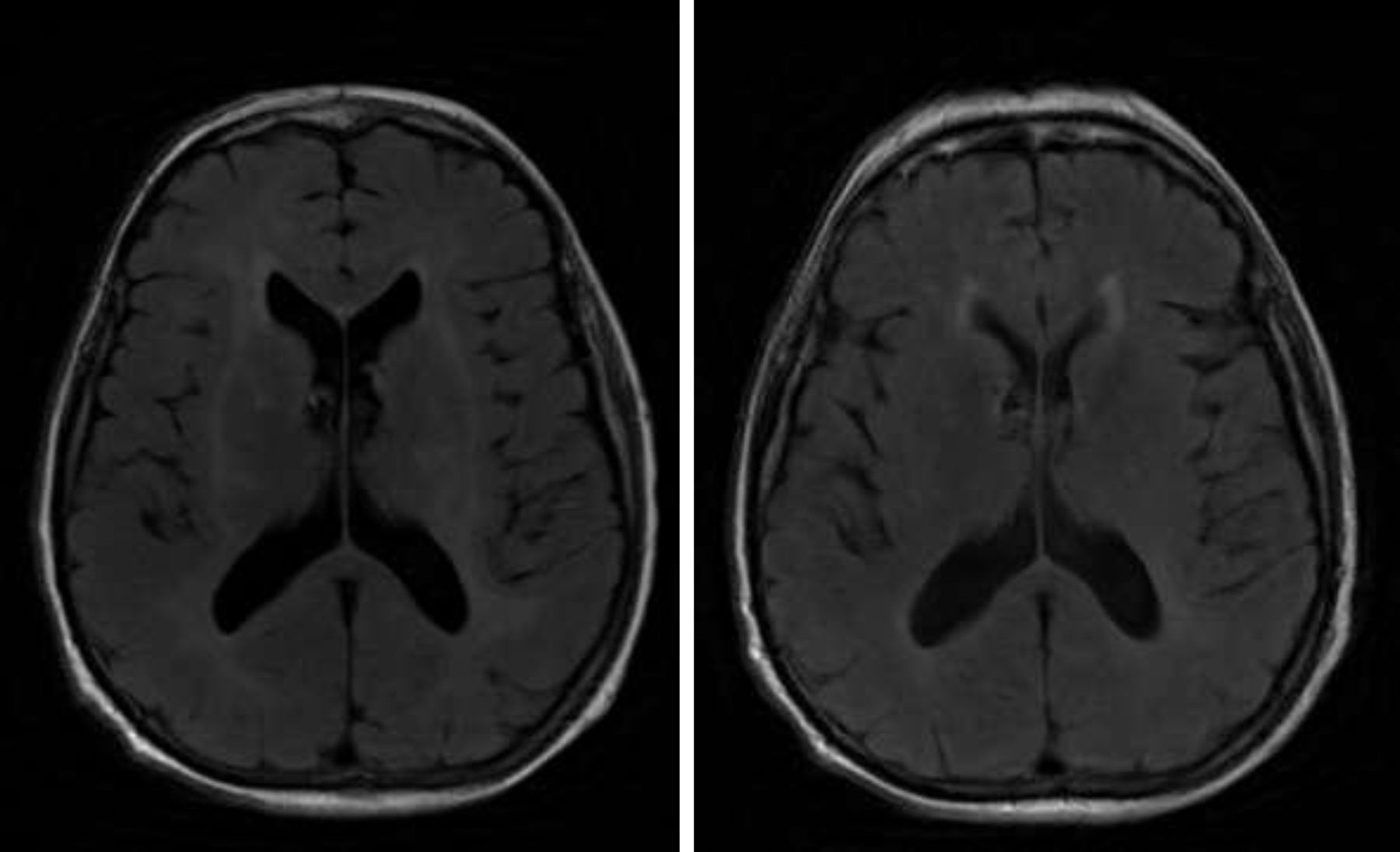
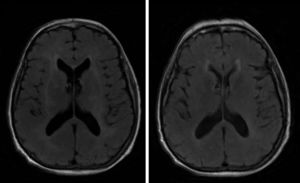
A brain MRI scan revealed bilateral supratentorial leukoencephalopathy, with no gadolinium contrast enhancement or specific findings (Fig. 1). Several electroencephalography (EEG) studies revealed diffuse slowing with occasional epileptiform activity. An electromyography study revealed an asymmetrical demyelinating/axonal sensorimotor polyneuropathy of distal predominance, which was more pronounced in the lower limbs.
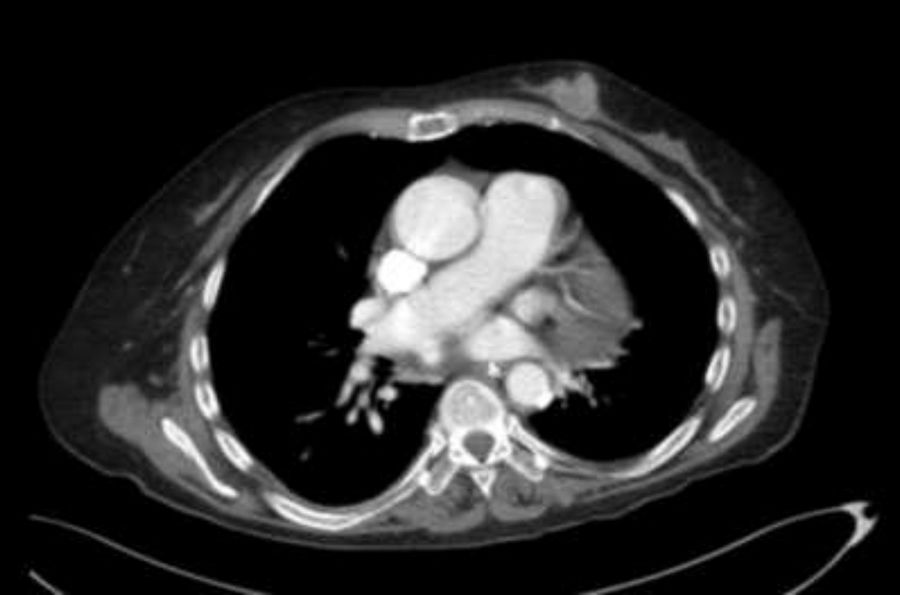
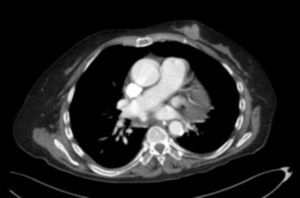
We ruled out thiamine deficiency, toxic-metabolic, inflammatory, and infectious encephalopathy. Given suspicion of atypical PNS, complementary tests were performed, revealing an invasive ductal carcinoma in the left breast (Fig. 2), with no signs of metastasis. After surgical resection of the tumour and hormone therapy with exemestane and radiation therapy, the patient progressed favourably, showing normal results in a neurological examination and EEG study at 3 months. A brain MRI scan revealed that leukoencephalopathy had significantly decreased and was barely visible (Fig. 1).
Having ruled out other causes and in the absence of a better explanation, we diagnosed the patient with atypical PNS (acute encephalopathy, secondarily generalised partial seizures, and asymmetric demyelinating/axonal sensorimotor polyneuropathy). The favourable clinical outcome and the normal MRI, EEG, and CSF findings after tumour treatment (without immunosuppressants) suggests that a coincidental association is unlikely and points to a definitive diagnosis of PNS.
We stress the importance of maintaining a high level of suspicion of PNS (a potentially curable disease) in cases of neurological alterations of unknown cause, once other conditions have been ruled out, even in the absence of onconeuronal antibodies, since this does not rule out the diagnosis.6
Although cases of PNS with no known associated autoantibodies have been described, cases with such complex clinical symptoms are rare. The clinical heterogeneity of our case represents a contribution to the existing knowledge on atypical PNS. Furthermore, it demonstrates the need for studies revealing improvement, which is mandatory in the absence of autoimmune markers.
Author contributionsStudy design, drafting, and approval of the final version: E. Casas Peña.
Approval of the final version: M.A. Martín Santidrián and J. González Fernández.
Drafting: M.V. Castrillo Fraile.
Please cite this article as: Casas Peña E, Martín Santidrián MA, González Fernández J, Castrillo Fraile MV. Síndrome paraneoplásico atípico sin anticuerpos onconeuronales detectables: a propósito de un caso. Neurología. 2019;34:207–209.