Primary pituitary tumours are classified by the World Health Organization as typical adenoma, atypical adenoma, or carcinoma. Information on the incidence and prevalence of these pituitary tumours is limited, and these data in Portugal are scarce, obsolete, or non-existent. Our study evaluates pituitary adenomas (PA) in the population of Lisbon, and it aims to describe the prevalence of all subgroups in order to revise the incidence of the ‘atypical’ histological type and its correlation to tumour subtype, invasion, and recurrence.
Patients and methodsA retrospective, descriptive analysis of patients with PA diagnosed between 2004 and 2013 was performed at Santa Maria University Hospital, a national reference centre.
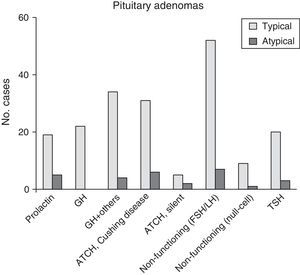
ResultsOf the 220 PA cases diagnosed, 28 (12.7%) fulfilled criteria for atypical lesions, and within that group, 23 were macroadenomas (82.1%) and 13 showed radiological evidence of invasion (46.4%). Ages ranged from 29 to 81 years (mean, 53.4 years). Eleven patients (39.3%) had functional tumours. Sixteen of the 28 patients (57.1%) experienced tumour recurrences; in the 100 adenomas monitored for more than 5 years, the recurrence rate in atypical PA was 7 times higher than in typical PA. Immunohistochemically, 28.6% of the tumours stained positively for ACTH, 25% for gonadotrophins, and 17.9% for prolactin. The proliferation index (Ki67) ranged from 3% and 25% (mean, 6.4%).
ConclusionsAtypical PAs make up 12.7% of all surgically treated PA cases, and they tend to be invasive and recurrent macroadenomas. We found no differences in metastatic potential between typical and atypical PA.
Los tumores hipofisarios primarios son clasificados por la Organización Mundial de la Salud como adenoma típico, adenoma atípico y carcinoma. Existen datos limitados sobre la incidencia y la prevalencia de tumores hipofisarios, siendo en Portugal escasos, obsoletos o inexistentes. Presentamos un estudio que evalúa los adenomas hipofisarios (AH) basado en la población de Lisboa, cuyo objetivo es describir la prevalencia de todos los subgrupos, revisando la incidencia de este tipo histopatológico «atípico» y su correlación con el subtipo de tumor, invasión y recurrencia.
Pacientes y métodosSe realizó un análisis descriptivo retrospectivo de pacientes diagnosticados de AH entre 2004 y 2013, en el Hospital Universitario de Santa Maria (Lisboa), un centro de referencia nacional.
ResultadosDe 220 AH diagnosticados, 28 (12,7%) cumplían criterios de lesiones atípicas, 23 de los cuales (82,1%) fueron macroadenomas y 13 (46,4%) mostraron radiológicamente evidencia de invasión. La edad osciló entre 29-81 años (media 53,4 años). Once pacientes (39,3%) tenían tumores funcionantes. Dieciséis (57,1%) de los 28 pacientes presentaron tumores recurrentes; en 100 de los adenomas diagnosticados, con seguimiento superior a 5 años, se observó una tasa de recurrencia en AH atípicos hasta 7 veces superior. En estudios inmunohistoquímicos destacaron los positivos a ACTH (28,6%), a gonadotrofinas (25%) y a prolactina (17,9%). El índice proliferativo (Ki67) varió entre el 3 y el 25% (media 6,4%).
ConclusionesLos AH atípicos corresponden al 12,7% de los AH resecados, tendiendo a ser macroadenomas, invasivos y recurrentes. No encontramos diferencias entre AH típicos y atípicos en cuanto al potencial metastásico.
Pituitary tumours account for 10% to 15% of all brain tumours.1 Pituitary adenoma (PA) is the most common neoplasm of the sella turcica2 and, from a neurosurgical perspective, the third most common intracranial primary tumour after gliomas and meningiomas.1 Recent studies show that the prevalence of PAs is as much as 4 times higher than was previously believed.3,4 Data on PA incidence are limited, and data from series based on MR images and autopsies contrast with those from surgical series at tertiary hospitals.
PAs are composed of a monoclonal proliferation of anterior pituitary cells. They most frequently occur in women in their third to sixth decade, although they can be found in all age groups.1,5 PAs are not homogeneous; rather, each subtype has its own clinical presentation, hormone secretion profile, tendency towards invasiveness, histopathological characteristics, prognosis, and treatment.6
Since Cushing proposed the first morphological classification system in 1912, there have been numerous other attempts at classifying PAs histologically. Classification is based on: (a) histological criteria. Although tumour classification based on haematoxylin–eosin (HE) stain results does not correlate with functional status, this information is still valuable: it enables differential diagnosis with other entities, permits evaluation of cell atypia or mitotic activity, and reveals any haemorrhages or necrosis. (b) Immunohistochemical criteria. These constitute the gold standard for diagnosis and for analysing the main pituitary hormones (PRL, GH, ACTH, FSH, LH, and TSH), to which we can add the alpha subunit of glycoprotein hormones (FSH, LH, and TSH). (c) Ultrastructural criteria, although electron microscopy is a time-consuming and expensive technique and not routinely performed.7 (d) Clinical and biochemical criteria, such as clinical presentation and pituitary function to determine whether or not the tumour is functioning. (e) Imaging criteria to determine tumour size and sellar/extrasellar extension. (f) Surgical findings.
The most controversial addition to the most recent classification system by the World Health Organization (WHO, 2004)8 is the rating scale for primary endocrine tumours of the pituitary. These tumours are classified as typical pituitary adenomas (ICD 8272/0), atypical pituitary adenomas (ICD 8272/1), and pituitary carcinomas (ICD 8272/3).8 Most PAs are typical, with a bland histological appearance; mitotic figures are rare, and the proliferation index (Ki67) is below 3%. Atypical PAs are borderline or uncertain, with atypical morphological characteristics indicative of aggressive behaviour (such as invasive growth), a high mitotic index, a cellular proliferation index (Ki67) greater than 3%, and extensive nuclear positivity for protein p53. Nevertheless, differences between ‘typical’ and ‘atypical’ adenomas are not clearly defined. There are no morphological criteria for distinguishing locally aggressive atypical PAs from carcinomas when the tumour is limited to the sella turcica.9 While pituitary carcinomas tend to exhibit the usual morphological characteristics associated with malignant neoplasms (hypercellularity, nuclear and cellular pleomorphism, increased mitotic activity, necrosis, and dural/bone invasion), these traits may not be diagnostic. The mechanism by which PAs evolve to become more aggressive and invasive tumours has not yet been fully explained; no studies have demonstrated a continuum from typical adenoma to atypical adenoma and carcinoma. Only rarely does a pituitary adenoma become a carcinoma (malignant transformation), and data describing this process is lacking.10,11
A few studies carried out after 2004 provide clinical experience with the new classification, and some describe the incidence, tumour subtype, and the clinical and pathology features of atypical PAs.5,12,13
The purpose of this study is to determine the incidence, clinical and histopathological characteristics, clinical recurrence, local invasion, and postoperative outcomes of PA cases diagnosed in a Portuguese reference hospital in the last 10 years, especially cases meeting histopathological criteria for atypical tumours.8
Patients and methodsThis retrospective study was conducted at Hospital de Santa Maria in Lisbon, a Portuguese reference hospital with a long-standing neurosurgical tradition. Our population of reference in Lisbon consists of 545245 residents in a greater metropolitan area of 2957.4km2 and 2250533 inhabitants14; this accounts for 27% of Portugal's population. We included patients diagnosed and treated surgically using the endonasal transsphenoidal approach with histological confirmation between 1 January 2004 and 31 December 2013. General criteria for PA surgery at our hospital were as follows: tumours generating acromegaly or Cushing syndrome, clinically non-functioning macroadenomas, especially those causing compressive changes to nearby structures (visual field changes, cranial nerve impingement, headache, etc.), prolactinomas generating compressive symptoms and not responding quickly to medical treatment, or patients with poor tolerance for dopaminergic drugs.
We performed a retrospective review of 235 patients; 15 were excluded due to having non-endocrine tumours, non-adenomatous lesions of the sellar region, and inflammatory processes. This left a total of 220 patients with PA whose medical records, laboratory analyses, and radiology and pathology studies were reviewed. PAs were categorised according to the 2004 WHO classification for endocrine tumours.8 Of the 220 adenoma cases, 28 showed morphological signs indicating greater biological aggressiveness (e.g. nuclear pleomorphism), high mitotic activity, Ki67 proliferation indexes above 3%, and extensive immunopositivity for protein p53, all of which constitute the criteria for atypical adenoma according to this classification. Other proposed parameters include measuring cathepsin B or MMP-9 (matrix metalloproteinase-9),15 evaluating proliferative activity using antiapoptotic markers such as Bcl-2, analysing DNA topoisomerase II-alpha indexes and the expression of cyclooxygenase 2, detecting expression of telomerase, or galectin-3 studies. Unfortunately, none of these parameters has been shown to be more useful as a marker of biological behaviour than histological subtyping based on the hormone content and cell structure, which remain the best independent predictors of aggressive behaviour.16,17 Detecting absence of the p53 gene, decreased expression of the nm23 gene, and anomalies in p27; analysing vascular endothelial growth factor (VEGF), fibroblast growth factor receptor 4 (FGFR4), and pituitary tumour transforming gene (PTTG); identifying deletions in chromosome 11; and profiling micro-RNA expression have also been proposed as measures of tumour aggressiveness, but they are not yet listed as criteria for classifying PAs.15–18
Tumour size was determined by MRI and ranked in 3 categories: microadenomas (≤1cm), macroadenomas (>1 and ≤4cm), or giant adenomas (>4cm). Tumour invasion was defined according to the preoperative MRI findings. This imaging study evaluated invasion of the cavernous sinus according to the Knosp et al. classification.19 That classification is defined by the position of carotid lines with respect to the limits of invasion. These authors propose classifying tumours in 5 grades (from 0 to 4) according to the following criteria: Grade 0 – the tumour does not invade the cavernous sinus and intracavernous anatomical structures remain intact; Grade 1 – tumour extends beyond the medial line (the line connecting the two medial edges of the supra- and intracavernous parts of the internal carotid) without reaching the median line connecting the centres of those parts; Grade 2 – tumour extends beyond the median line (intercarotid), but does not extend beyond or tangent to the lateral line; Grade 3 – tumour extends beyond the lateral line connecting the supra- and intracavernous parts of the carotid; Grade 4 – tumour wraps fully around the intracavernous carotid artery, obliterating all venous compartments. Researchers evaluated patients’ hormone levels before and after surgery, history of prior pituitary surgery, additional treatment, and postoperative recurrence during follow-up (defined as reappearance of the tumour in an MRI study for non-functioning adenomas, and hormonal hypersecretion for functioning adenomas).
Histochemical studies (HE and reticulin) and immunohistochemical studies (PRL, GH, ACTH, FSH, LH, alpha-subunit, TSH, Ki67, and p53) were performed on formalin-fixed paraffin-embedded tissue sections. Researchers cut sections 2 microns thick (for HE) or 4 microns thick (for the immunohistochemical study); sections were then deparaffinised. For the immunohistochemical study, sections underwent antigenic recovery and were incubated with individual antibodies targeting specific pituitary hormones or cell proteins (Table 1). The Ki67 proliferation index was calculated as the percentage of positive nuclei in 500-2000 tumour cells in the areas with the most immunopositive cells, analysed under an optical microscope at 400× magnification. In more difficult cases, the index was also calculated with the help of an image processing programme for immunohistochemical analysis. This method yields results that coincide with the percentage calculated by an experienced pathologist in 89.7% of all cases.20 Since p53 detection may not be reliable and there is no validated cut-off value for prognosis, a positive finding was defined as more than 10 strongly positive nuclei per 10 high-power fields viewed under an optical microscope at 400×. This evaluation is in line with the previous proposal for ‘isolated dispersed positive cells’.21
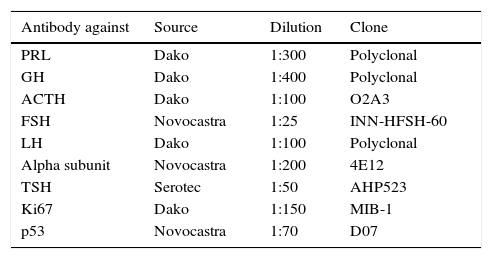
Antibodies used, source, dilution, and clone.
| Antibody against | Source | Dilution | Clone |
|---|---|---|---|
| PRL | Dako | 1:300 | Polyclonal |
| GH | Dako | 1:400 | Polyclonal |
| ACTH | Dako | 1:100 | O2A3 |
| FSH | Novocastra | 1:25 | INN-HFSH-60 |
| LH | Dako | 1:100 | Polyclonal |
| Alpha subunit | Novocastra | 1:200 | 4E12 |
| TSH | Serotec | 1:50 | AHP523 |
| Ki67 | Dako | 1:150 | MIB-1 |
| p53 | Novocastra | 1:70 | D07 |
Statistics were analysed using the software utility GraphPad Prism version 6.05 (GraphPad Software, Inc., CA, USA). We compared categorical data using a 2-tailed Fisher exact test; the unpaired t-test was used to compare subgroups. Statistical significance was set at P<.05.
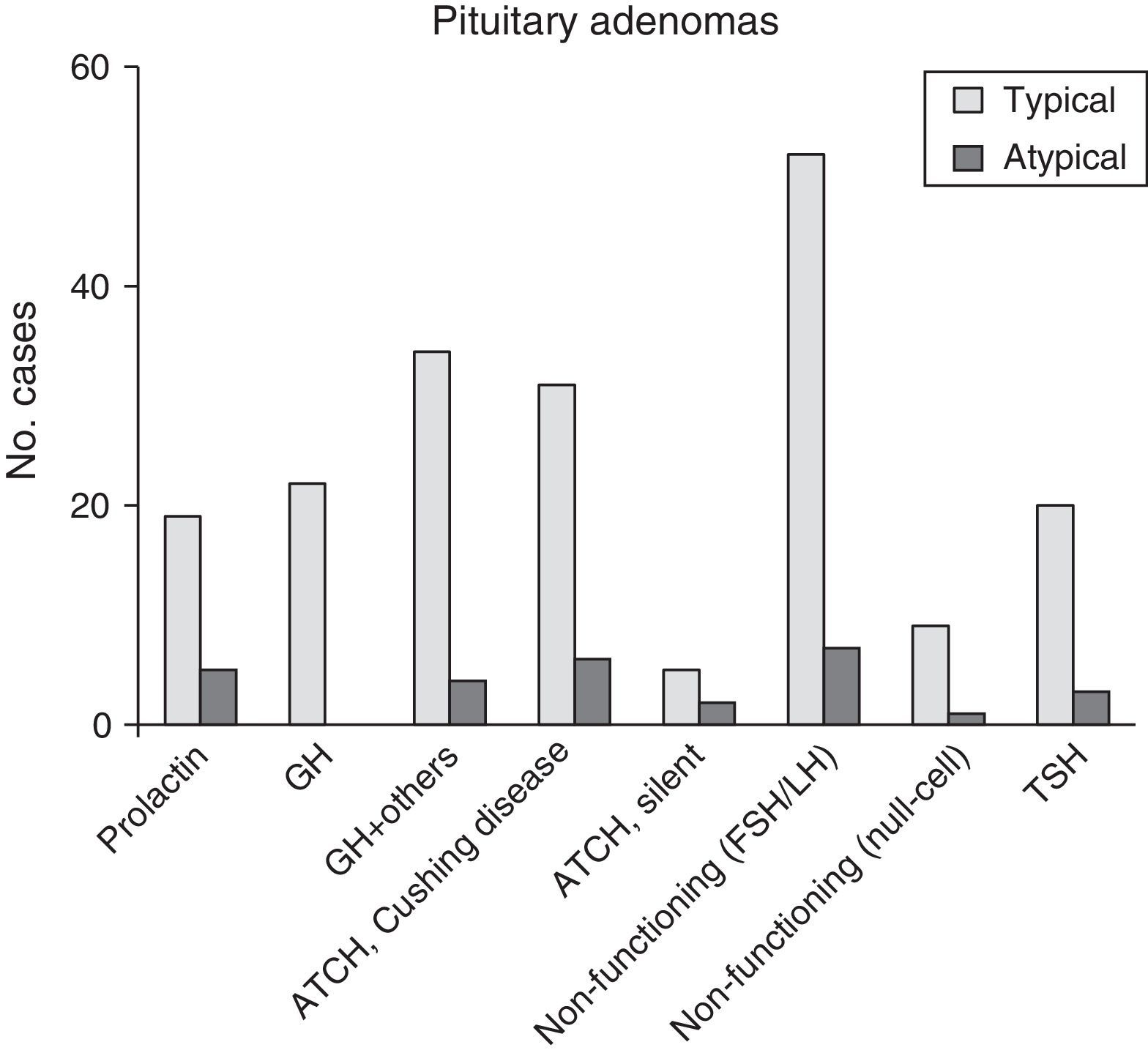
ResultsThe 220 PA patients who underwent endonasal transsphenoidal surgery represented a prevalence of 9.8% and an incidence of 1.24 cases per 100000 inhabitants in 2013. Mean age at diagnosis was 54±10.5 years (range, 13-104), and the total included 124 women and 96 men. According to the WHO classification,8 192 tumours (87.3%) were classified as typical PA (Fig. 1) and 28 tumours (12.7%) as atypical PA (Fig. 2). We did not detect any cases of primary pituitary carcinoma.
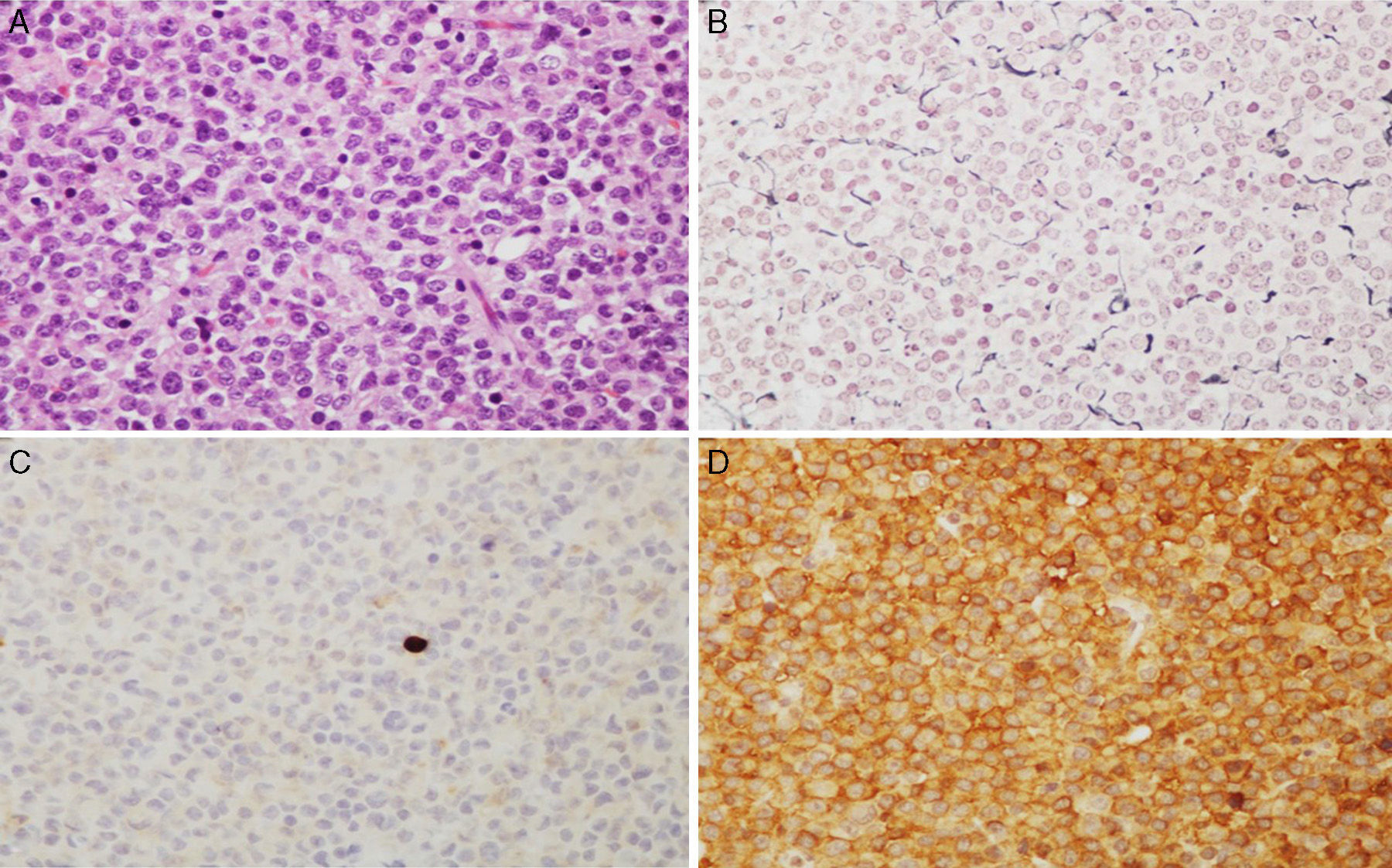
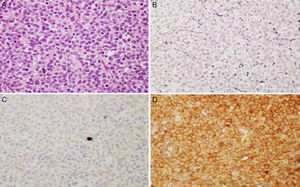
Microscopic images of a typical GH-positive pituitary adenoma. (A) Sheet-like proliferation of monomorphic cells with round or oval nuclei and a moderate amount of eosinophilic cytoplasm (haematoxylin–eosin stain, 200×). (B) The histological technique of reticulin staining demonstrates disruption of the normal acinar pattern of the anterior pituitary (Gomori reticulin stain 200×). (C) The cell proliferation index is low (Ki67<1%, 200×). (D) The adenoma shows strong cytoplasmic immunoreactivity for GH (GH, 200×).
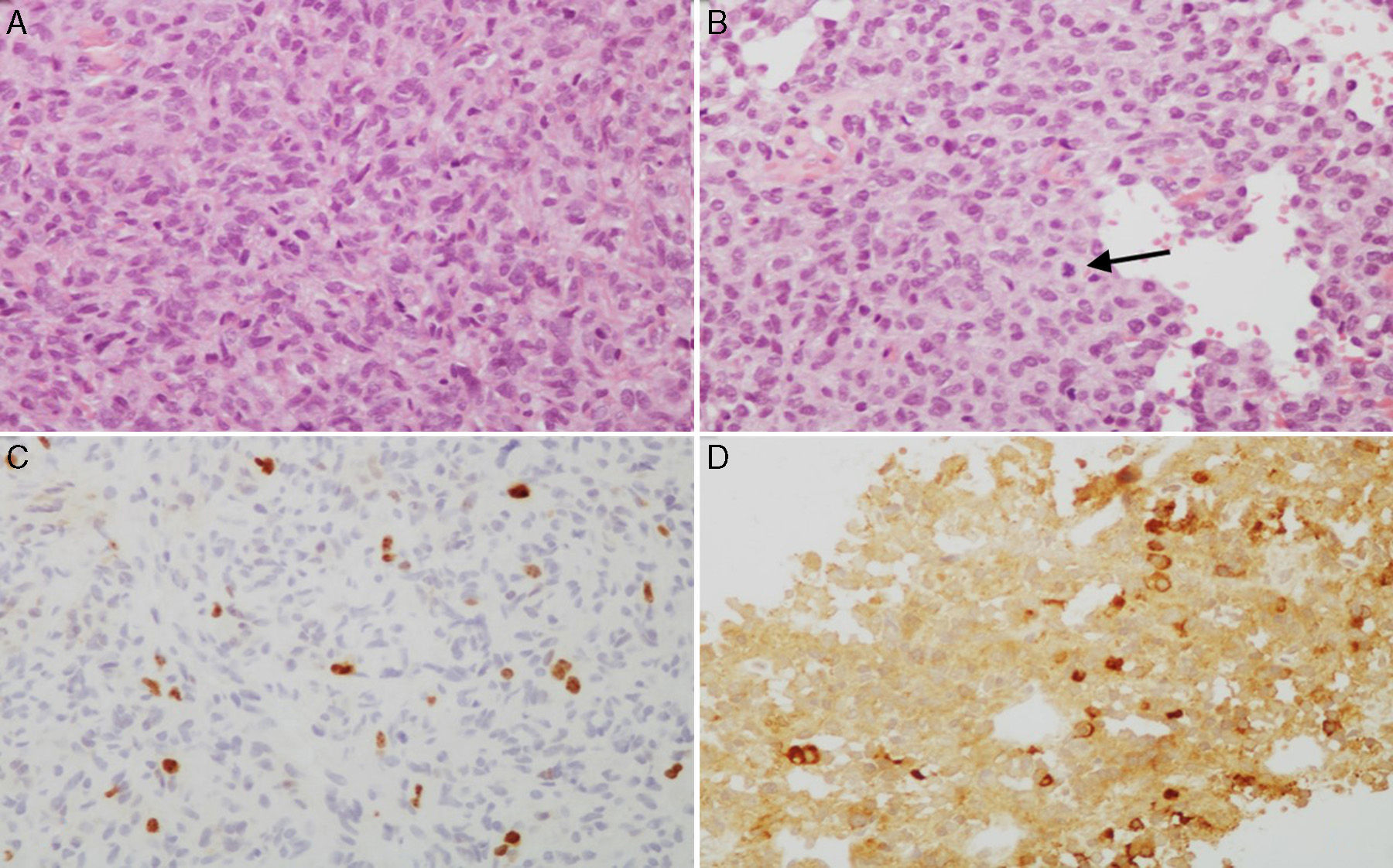
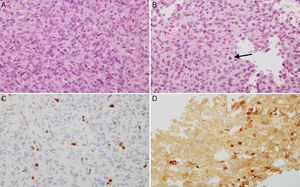
(A and B) Microscopic images of an atypical prolactin-secreting pituitary adenoma. This tumour presents moderate to high cell density with large nuclei; cells may be pleomorphic, with a prominent nucleolus and a moderate amount of pale eosinophilic cytoplasm. Note the occasional mitotic figures (arrow) (haematoxylin–eosin stain, 200×). This adenoma has a high cell proliferation index (4%) (C, Ki67 200×) and cytoplasmic immunoreactivity for prolactin in some cells (D, PRL 200×).
Of the 28 patients with atypical PA, 14 (50%) were women. Their ages ranged between 29 and 81 years (mean, 53.4±9.9 years). Twenty-three patients (82.1%) had macroadenomas, including 13 cases (46.4%) of invasion of neighbouring tissue detected by preoperative neuroradiology studies. Eleven tumours (39.3%) were functioning (4 cases of acromegaly, 6 of Cushing syndrome, and a single tumour secreting TSH with hyperthyroidism). One case presented as pituitary apoplexy. Immunohistochemical analysis showed ACTH positivity in 8 cases (28.6%), comprising 6 with a clinical profile of Cushing disease and 2 that were silent. Seven PAs without clinical secretion were shown to be positive for gonadotropins (25%), whereas 5 tumours were immunohistochemically positive for prolactin (17.9%) (Fig. 3 and Table 2). The Ki67 proliferation index registered between 3% and 25% of the tumour cells, with a mean value of 6.4% (ranging between 3% and 5% in 17; between 5% and 10% in 10; and 20% and 25% in a single patient).
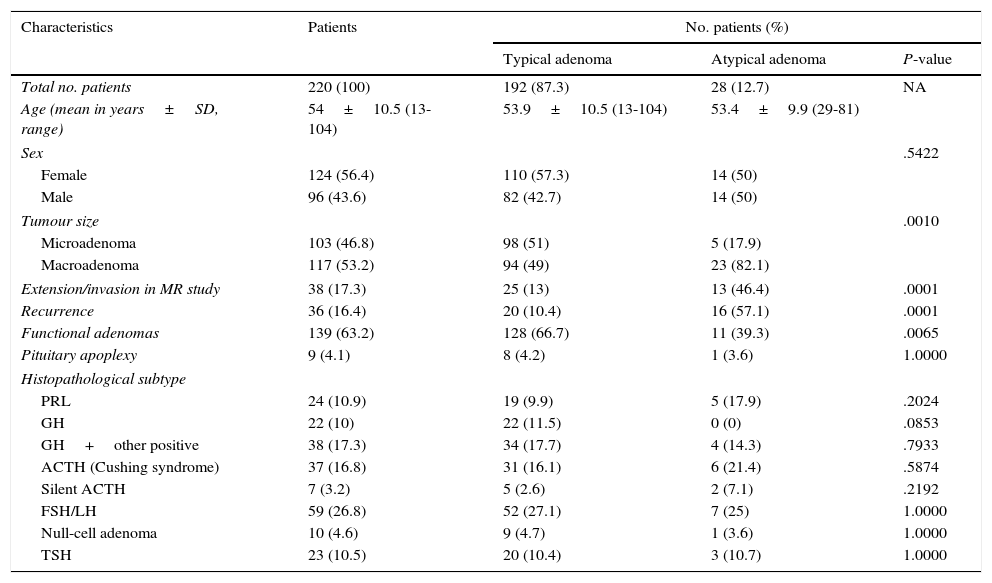
Clinical and histopathology characteristics of 220 patients undergoing transsphenoidal surgery for pituitary adenoma.
| Characteristics | Patients | No. patients (%) | ||
|---|---|---|---|---|
| Typical adenoma | Atypical adenoma | P-value | ||
| Total no. patients | 220 (100) | 192 (87.3) | 28 (12.7) | NA |
| Age (mean in years±SD, range) | 54±10.5 (13-104) | 53.9±10.5 (13-104) | 53.4±9.9 (29-81) | |
| Sex | .5422 | |||
| Female | 124 (56.4) | 110 (57.3) | 14 (50) | |
| Male | 96 (43.6) | 82 (42.7) | 14 (50) | |
| Tumour size | .0010 | |||
| Microadenoma | 103 (46.8) | 98 (51) | 5 (17.9) | |
| Macroadenoma | 117 (53.2) | 94 (49) | 23 (82.1) | |
| Extension/invasion in MR study | 38 (17.3) | 25 (13) | 13 (46.4) | .0001 |
| Recurrence | 36 (16.4) | 20 (10.4) | 16 (57.1) | .0001 |
| Functional adenomas | 139 (63.2) | 128 (66.7) | 11 (39.3) | .0065 |
| Pituitary apoplexy | 9 (4.1) | 8 (4.2) | 1 (3.6) | 1.0000 |
| Histopathological subtype | ||||
| PRL | 24 (10.9) | 19 (9.9) | 5 (17.9) | .2024 |
| GH | 22 (10) | 22 (11.5) | 0 (0) | .0853 |
| GH+other positive | 38 (17.3) | 34 (17.7) | 4 (14.3) | .7933 |
| ACTH (Cushing syndrome) | 37 (16.8) | 31 (16.1) | 6 (21.4) | .5874 |
| Silent ACTH | 7 (3.2) | 5 (2.6) | 2 (7.1) | .2192 |
| FSH/LH | 59 (26.8) | 52 (27.1) | 7 (25) | 1.0000 |
| Null-cell adenoma | 10 (4.6) | 9 (4.7) | 1 (3.6) | 1.0000 |
| TSH | 23 (10.5) | 20 (10.4) | 3 (10.7) | 1.0000 |
Statistical significance: P<.05.
ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinising hormone; NA, not attributable; PRL, prolactin; MR, magnetic resonance; TSH, thyroid-stimulating hormone.
Recurrence affected 36 of the 220 PA cases (16.4%) after a mean of 56.2±31.4 months (range, 3-312 months). Twenty of these cases were typical PAs (20/192, 10.4%). Two of these were clinically non-functioning macroadenomas that were positive for prolactin. There were also 2 cases of acromegaly due to GH-positive macroadenomas, 4 microadenomas leading to Cushing disease and a clinically silent macroadenoma that was also positive for ACTH, 6 non-secreting macroadenomas that were immunohistochemically positive for gonadotropins, and the remaining 5 were non-secreting macroadenomas that were positive for TSH. One of the latter presented as pituitary apoplexy.
Sixteen of the 28 atypical PAs (57.1%) presented recurrences; 12 (75%) were clinically non-secreting, but immunohistochemically positive for prolactin in 3 cases (all macroadenomas), for ACTH in 2 cases (silent macroadenomas), for gonadotropins in 5 macroadenomas, and TSH in one macroadenoma. Another was categorised as a null-cell adenoma, referring to adenomas that lack immunoreactivity for all specific hormonal markers for pituitary cell differentiation. In addition, there were 3 clinical cases of Cushing disease, comprising 2 microadenomas and one macroadenoma with pituitary apoplexy (all positive for ACTH), and a TSH-secreting macroadenoma with hyperthyroidism (Table 3).
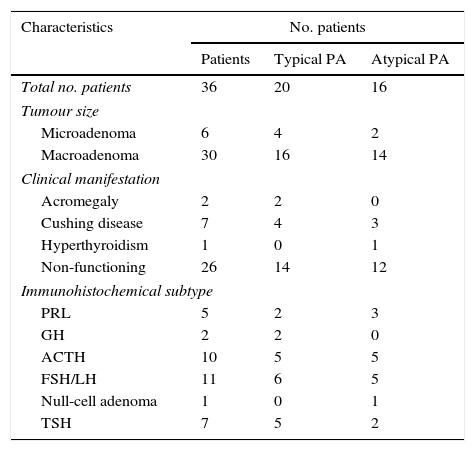
Clinical and immunohistochemical profile of the 36 patients presenting tumour recurrence.
| Characteristics | No. patients | ||
|---|---|---|---|
| Patients | Typical PA | Atypical PA | |
| Total no. patients | 36 | 20 | 16 |
| Tumour size | |||
| Microadenoma | 6 | 4 | 2 |
| Macroadenoma | 30 | 16 | 14 |
| Clinical manifestation | |||
| Acromegaly | 2 | 2 | 0 |
| Cushing disease | 7 | 4 | 3 |
| Hyperthyroidism | 1 | 0 | 1 |
| Non-functioning | 26 | 14 | 12 |
| Immunohistochemical subtype | |||
| PRL | 5 | 2 | 3 |
| GH | 2 | 2 | 0 |
| ACTH | 10 | 5 | 5 |
| FSH/LH | 11 | 6 | 5 |
| Null-cell adenoma | 1 | 0 | 1 |
| TSH | 7 | 5 | 2 |
ACTH, adrenocorticotropic hormone; PA, pituitary adenoma; FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinising hormone; PRL, prolactin; TSH, thyroid-stimulating hormone.
In the group of 100 PAs with more than 5 years of follow-up, we also found more recurrence of atypical PAs than of typical PAs (8/13, 61.5% vs 7/87, 8%; P<.0001).
The preoperative factors correlated with higher probabilities of atypical PA were as follows: tumour size (49% of typical PAs were macroadenomas, vs 82% of the atypical tumours, P=.0010); evidence of tumour invasion in neuroimaging studies (13% for typical tumours vs 46.4% for atypical tumours, P=.0001); and tumours exhibiting clinical secretion (66.7% of typical tumours vs 39.3% of atypical tumours, P=.0065). We observed no differences in age, sex, presentation as pituitary apoplexy, and histological subtype between typical and atypical cases.
Regarding local invasion, 12% of the typical PAs (3/25) exhibited infrasellar invasion (2 GH-positive with acromegaly and one non-secreting macroadenoma testing positive for prolactin). Eighty-eight per cent (22/25) presented suprasellar invasion (1 GH-positive with acromegaly; 21 non-secreting macroadenomas, including 4 immunohistochemically positive for prolactin, 1 positive for ACTH, 12 positive for gonadotropins, and 4 for TSH).
Local invasion in atypical PAs was found in 13 cases, of which 12 (92.3%) were macroadenomas and the other, a microadenoma positive for prolactin. Three of these 13 (23.1%) showed infrasellar invasion with erosion of the base of the sella turcica (1 case of acromegaly positive for GH and TSH, 1 of Cushing disease with ACTH positivity, and 1 of null-cell adenoma). Likewise, 69.2% (9/13) presented suprasellar invasion (1 tumour causing acromegaly and positive for GH, PRL, and TSH; 8 were clinically non-functioning, including 2 positive for prolactin, 2 clinically silent and positive for ACTH, 3 positive for gonadotropins, and 1 positive for TSH). The remaining one was a non-secreting macroadenoma positive for prolactin which had invaded the right cavernous sinus (Fig. 4).
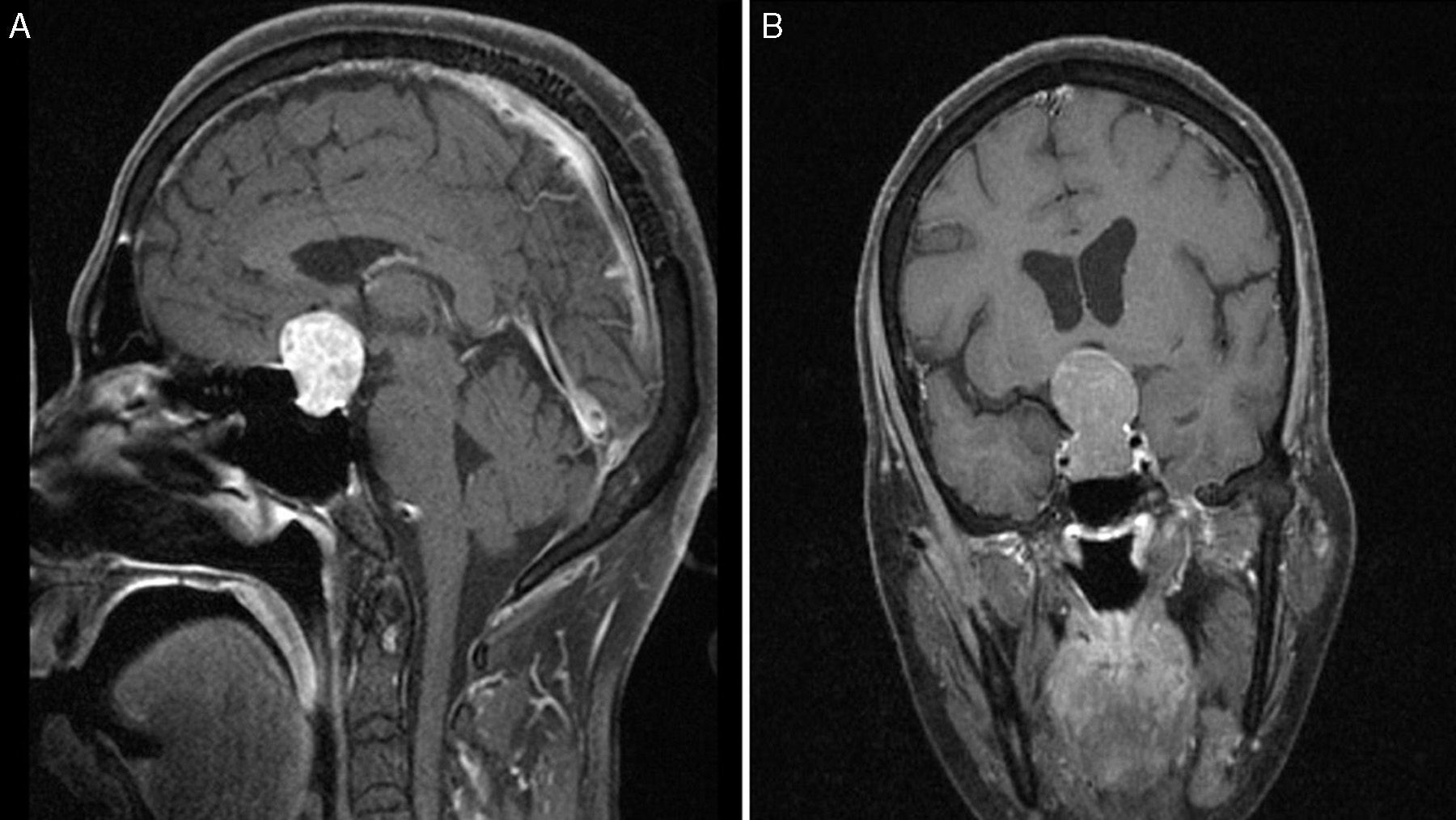
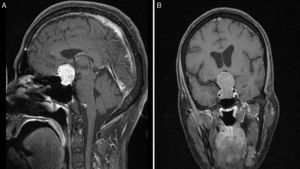
Preoperative T1-weighted MRI after contrast (A, sagittal; B, coronal); images from a patient with atypical macroadenoma, Knosp grade 4. Note the tumour's propensity for suprasellar extension, with bilateral extension to the cavernous sinus, erosion of the dorsum sellae, and hydrocephalus secondary to the tumour.
Although most PAs have a benign phenotype,22 there is also a small subgroup whose presentation and biological activity is borderline between benign and malignant. They show more locally aggressive growth, suprasellar growth, relapses, and ability to invade the sphenoid or cavernous sinuses.
In our series of PAs diagnosed over the last 10 years in a Portuguese reference hospital, the observed incidence of atypical PA was 12.7% in all patients treated surgically (28/220). Of these tumours, 39.3% were hormonally functioning (4 cases of acromegaly, 6 of Cushing disease, and 1 case of hyperthyroidism with a TSH-secreting tumour). Macroadenomas accounted for 82.1% and 46.4% showed signs of invading adjacent structures. Scheithauer et al. (2006) identified 6 cases of atypical PA out of a total of 78 tumours (14.7%); Zada et al. (2011) found 18 cases in a series of 121 PAs (14.8%); and Yildirim et al. (2013) identified 13 cases of atypical PA in a series of 146 total PAs (8.9%).5,12,13 Our study revealed 28 atypical PAs out of 220 total PAs (12.7%); this percentage is in line with data from the literature and confirms that atypical PAs are not as uncommon as was previously believed.23
Broken down by subtype, the most frequent atypical PAs with clinical symptoms were ACTH-secreting tumours (6 cases of Cushing disease) followed by GH-secreting tumours (4 cases of acromegaly). Broken down by histopathology findings, ACTH-positive tumours were again the most frequent, followed by those positive for gonadotropins (25%) and prolactin (17.9%). Together, these made up 71.5% of the total atypical PAs. All of the GH-positive adenomas had elicited acromegaly; they also showed immunoreactivity for other cell lines, especially PRL and TSH. Zada et al. (2011) and Saeger et al. (2007) report that the most common atypical PAs are GH-secreting, silent, and ACTH-secreting; together, these types make up more than 70% of all cases.12,23 These adenomas account for 67.9% of the total in our series.
Ki67, a proliferation antigen, may help us identify a group of adenomas with more locally aggressive behaviour. It tends to show low positivity (<3%).25 According to some authors, higher levels of this antigen are correlated with more rapid tumour growth, increased invasiveness, and recurrence,12 but other studies did not confirm these results.24,25 Three recent articles11,22,26 support the idea that only a high Ki67 proliferation index (more than 20% to 30%), regardless of tumour size and presence or absence of local invasion, would indicate the presence of a carcinoma in situ,27 or a premetastatic pituitary carcinoma in the sellar phase.5
The new system for grading primary pituitary endocrine tumours, proposed by the WHO in 2004, does not clearly establish the differences between typical and atypical tumours. Parameters such as the mitosis count and immunohistochemical findings for p53 positivity lack validated cut-off values. For this reason, some laboratories do not routinely test for Ki67 and p53, since there is not always a clear connection between these results and the clinical behaviour of tumours. In any case, the WHO document requires these tests in order to classify a PA as atypical. We would do well to ask what influence these markers have on the treatment approach. Doctors may adopt a more conservative attitude towards a postsurgical patient with an invasive and actively secreting tumour that cannot be cured surgically, with rare mitotic figures and low Ki67 and p53 indices, than towards a patient whose postoperative neuroradiology reports show complete resection of the tumour and whose histopathology study indicates frequent mitotic figures, a high Ki67 proliferation index, and extensive immunoreactivity for p53. Differentiating between an aggressive benign tumour and a malignant tumour in its initial stages is evidently difficult, as is true of almost all types of endocrine diseases.
The Thapar et al. study from 1996 clearly showed that a Ki67 index above 3% was significant for differentiating between invasive and non-invasive PAs, and this criterion was accepted by the WHO. The study reported a mean threshold for the Ki67 proliferation index of 4.66% in invasive adenomas.28 Zada et al. described a Ki67 index between 3% and 20% (mean value of 7%)12 and Yildirim et al. reported a Ki67 index between 3% and 10% (mean value of 4.7%).13 Our findings are consistent with those from earlier studies, since the Ki67 proliferation index ranged from 3% to 25% with a mean value of 6.4%.
Our study found recurrence in 36 of the 220 PA cases (16.4%); 20 were typical PAs (20/192, 10.4%) and 16 were atypical PAs (16/28, 57.1%). One hundred of the cases of diagnosed PA had more than 5 years of follow-up. In this group, the recurrence rate for atypical PA was 7.6 times higher than for typical PA (atypical, 8/13, 61.5%; typical, 7/87, 8%).
As in other studies, the preoperative factors correlating to a greater probability of atypical PA were larger tumour size, signs of invasion in neuroimaging studies, and having a clinically non-secreting tumour.12,13
As far as we know, this is the first updated, large-scale study to estimate the prevalence of PA in Portugal, as well as the first series of atypical PAs in that country. It is also one of the longest running studies in the world to provide PA classification by histological subtype, the degree of invasiveness, and recurrence of these tumours. It shows that atypical PAs have higher recurrence rates than do typical ones, but the evidence does not suggest that the former are more likely to undergo malignant transformation, or that they have a greater metastatic potential.
FundingNone.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank Dr Yasmin Fernandes for kindly interpreting the MR images and ceding them to us for this study.
Please cite this article as: Tortosa F, Webb SM. Adenomas hipofisarios atípicos: experiencia de 10 años en un centro de referencia de Portugal. Neurología. 2016;31:97–105.