Glycaemic variability (GV) refers to variations in blood glucose levels, and may affect stroke outcomes. This study aims to assess the effect of GV on acute ischaemic stroke progression.
MethodsWe performed an exploratory analysis of the multicentre, prospective, observational GLIAS-II study. Capillary glucose levels were measured every 4 hours during the first 48 hours after stroke, and GV was defined as the standard deviation of the mean glucose values. The primary outcomes were mortality and death or dependency at 3 months. Secondary outcomes were in-hospital complications, stroke recurrence, and the impact of the route of insulin administration on GV.
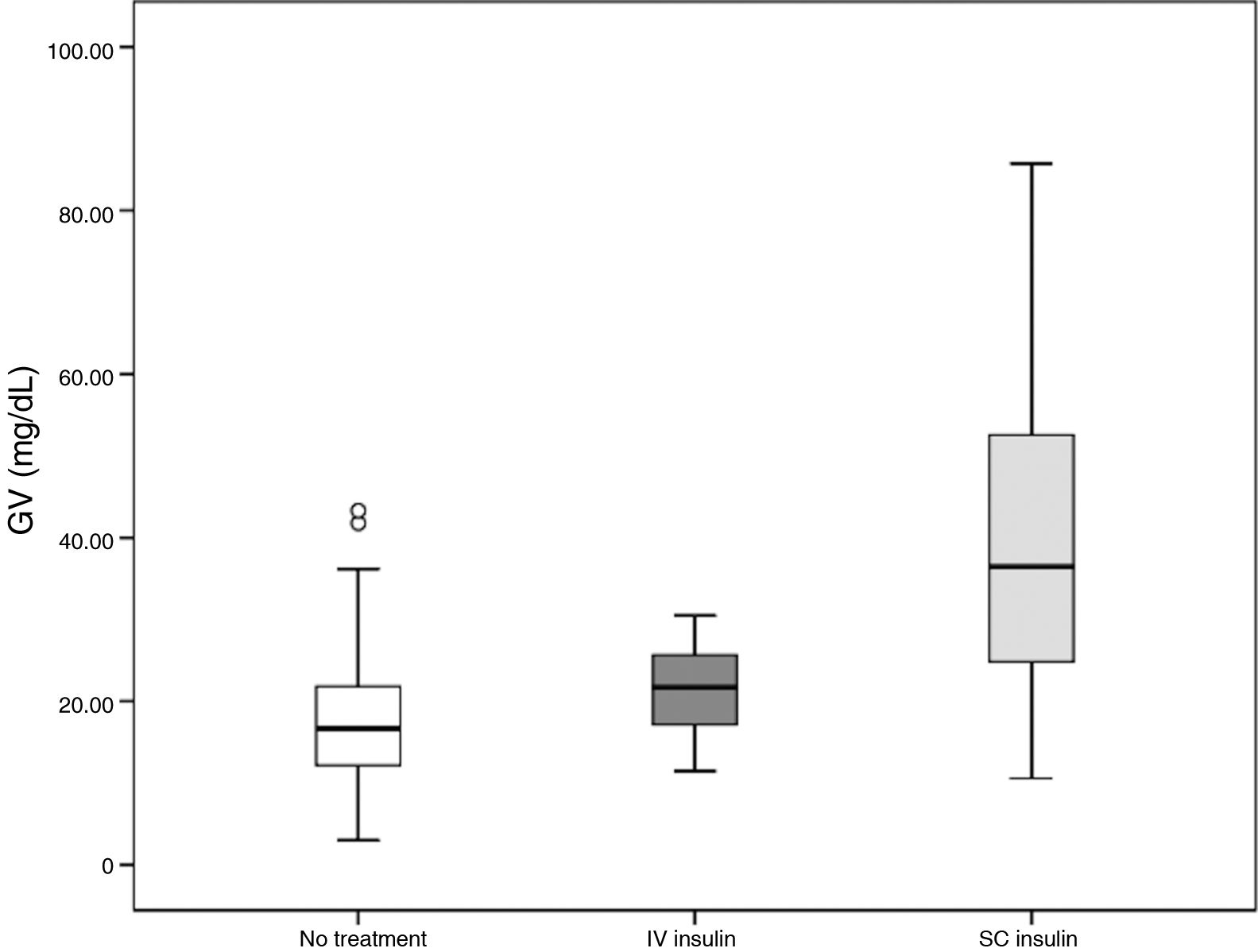
ResultsA total of 213 patients were included. Higher GV values were observed in patients who died (n = 16; 7.8%; 30.9 mg/dL vs 23.3 mg/dL; p = 0.05). In a logistic regression analysis adjusted for age and comorbidity, both GV (OR = 1.03; 95% CI, 1.003-1.06; p = 0.03) and stroke severity (OR = 1.12; 95% CI, 1.04-1.2; p = 0.004) were independently associated with mortality at 3 months. No association was found between GV and the other outcomes. Patients receiving subcutaneous insulin showed higher GV than those treated with intravenous insulin (38.95 mg/dL vs 21.34 mg/dL; p < 0.001).
ConclusionsHigh GV values during the first 48 hours after ischaemic stroke were independently associated with mortality. Subcutaneous insulin may be associated with higher VG levels than intravenous administration.
La variabilidad glucémica (VG) hace referencia a las oscilaciones en los niveles de glucosa en sangre y podría influir en el pronóstico del ictus. Objetivo: Analizar el efecto de la VG en la evolución del infarto cerebral agudo (IC).
MétodosAnálisis exploratorio del estudio GLIAS-II (multicéntrico, prospectivo y observacional). Se midieron los niveles de glucemia capilar cada cuatro horas durante las primeras 48 horas y la VG se definió como la desviación estándar de los valores medios. Variables principales: mortalidad y muerte o dependencia a los tres meses. Variables secundarias: porcentaje de complicaciones intrahospitalarias y de recurrencia de ictus, e influencia de la vía de administración de insulina sobre la VG.
ResultadosSe incluyeron 213 pacientes. Los pacientes que fallecieron (N = 16;7,8%) presentaron mayores valores de VG (30,9 mg/dL vs. 23,3 mg/dL; p = 0,05). En el análisis de regresión logística ajustado por edad y comorbilidad, tanto la VG (OR = 1,03; IC del 95%: 1,003-1,06: p = 0,03) como la gravedad del IC (OR = 1,12; IC del 95%: 1,04-1,2; p = 0,004) se asociaron de forma independiente con la mortalidad a los tres meses. No se encontró asociación entre la VG y las demás variables de estudio. Los pacientes que recibieron tratamiento con insulina subcutánea mostraron una mayor VG que los tratados con insulina intravenosa (38,9 mg/dL vs. 21,3 mg/dL; p < 0,001).
ConclusionesValores elevados de VG durante las primeras 48 horas tras el IC se asociaron de forma independiente con la mortalidad. La administración subcutánea de insulina podría condicionar una mayor VG que la vía intravenosa.
The concept of glycaemic variability (GV) refers to fluctuations in blood glucose levels. The development of continuous glucose monitors (CGM) has expanded our understanding of the dynamics of glucose levels in patients with diabetes mellitus (DM).1 A recent meta-analysis showed an association between high GV and microvascular damage in patients with DM; however, evidence of the role of GV in macrovascular damage is inconsistent.2
In recent years, research has focused on the influence of GV on the prognosis of patients with DM in different clinical settings, analysing whether its effects are independent of hyperglycaemia or glycosylated haemoglobin (HbA1c) levels. Furthermore, an association has been found between greater GV and higher morbidity rates in patients attended at intensive care units, as well as longer hospital stays in non–critically ill patients,3,4 regardless of the disease. On the other hand, some authors have suggested that, in addition to post-stroke hyperglycaemia, GV may act as a predictor of stroke outcomes, showing an association with increased cardiovascular mortality and early neurological deterioration.5,6 According to one retrospective study, GV may be associated with mortality in patients treated with intravenous thrombolysis (IVT) after stroke.7
There is extensive evidence that post-stroke hyperglycaemia is a poor prognostic factor, even in patients treated with IVT and mechanical thrombectomy.8-14 However, clinical trials have failed to demonstrate the benefits of tight glycaemic control for patients with stroke, which suggests that these patients’ poor prognosis may be influenced by other parameters.15
This study aimed to analyse the influence of GV on acute stroke prognosis in patients admitted to stroke units. We also analysed the influence of the route of insulin administration on GV.
MethodsWe conducted an exploratory analysis of the Glycemia in Acute Stroke II (GLIAS II) study. The full details of the study design are described elsewhere.14 The GLIAS II study is a multicentre, prospective, observational, academic cohort study involving the neurology departments of 9 Spanish healthcare centres. The study included patients aged 18 to 85 years with acute stroke of less than 24 hours’ progression. The authors gathered demographic characteristics, medical history data (including prior diagnosis of DM and metabolic syndrome), previous comorbidities according to the Charlson Comorbidity Index,16,17 and stroke characteristics, among other data. Stroke severity was measured with the National Institutes of Health Stroke Scale (NIHSS) at admission and on day 7 or at discharge (whichever occurred first).
Capillary blood glucose levels were determined every 4hours during the first 48hours. Post-stroke hyperglycaemia is defined as a blood glucose level > 155 mg/dL.18 To determine GV, we calculated the mean (standard deviation) glucose level over the first 48hours for each patient.19 HbA1c level, determined at admission in all patients, is an indicator of glycaemic control over the previous 3 months.20 Treatment and feeding of patients with post-stroke hyperglycaemia complied with each hospital's protocol. Data were also gathered on all treatments prescribed to correct hyperglycaemia; participation in the GLIAS II study did not influence the dose or administration route of insulin.
The primary variables analysed were mortality and dependence (modified Rankin Scale [mRS] score: 3-6) at 3 months. Secondary variables were complications during hospitalisation (symptomatic cerebral haemorrhage, cerebral oedema, early neurological deterioration, pneumonia, and urinary tract infection) and stroke recurrence at 3 months. Early neurological deterioration was defined as an increase ≥ 4 points on the NIHSS on day 7 or at discharge (whichever occurred first) as compared to baseline. Lastly, we also analysed the impact of the route of insulin administration (subcutaneous, intravenous, no correction treatment) on GV.
Statistical analysisStatistical analysis was performed by the biostatistics platform at Hospital La Paz Research Institute (IdiPAZ) using the SPSS statistics software, version 12.0 for Windows (SPSS Inc.; Chicago, IL, USA). Categorical variables are expressed as percentages, and proportions in each group were compared using the chi-square test or the Fisher exact test for dichotomous variables. Continuous variables were expressed as mean and standard deviation (SD) or median and quartiles 1 and 3 (Q1-Q3). We used the t test to analyse the relationship between GV and demographic variables or stroke subtype, and linear regression analysis to study its association with age.
Analyses were exploratory, without making any assumptions. Firstly, we regarded GV as a continuous variable and used the t test or the Mann-Whitney U test, as appropriate. To compare insulin prescription and administration route between groups, we used one-factor ANOVA with post-hoc Bonferroni correction.
The effect of GV level on stroke prognosis was analysed using a stepwise model. Firstly, we conducted a forward stepwise logistic regression analysis including those variables showing a significance level of P < .1 in the comparison of means, adjusting for age, baseline NIHSS score, and Charlson Comorbidity Index. Results are expressed as odds ratios (OR) with 95% confidence intervals (95% CI).
Secondly, we analysed the ROC curve to determine the predictive value of the area under the curve and the optimal cut-off point for GV that best discriminated between favourable and unfavourable outcomes. The cut-off point was the value for which the sum of specificity and sensitivity was highest, with equal weight given to false positives and false negatives. Lastly, we calculated ROC curves to predict the sensitivity of the models that were found to be statistically associated with the primary objectives of the study. All tests were two-tailed. Statistical significance was set at P < .05.
Research ethicsAll patients or their families gave informed consent for participation in the study. The study was approved by the clinical research ethics committee at Hospital Universitario La Paz (PI-855) and classified as an observational study by the Spanish Agency of Medicines and Medical Devices.
ResultsThe GLIAS II study included 213 patients. Baseline clinical characteristics and laboratory data are summarised in Table 1. Nearly half of patients presented post-stroke hyperglycaemia, with a mean GV value of 23.83 mg/dL. Greater GV was observed in patients with history of DM (36.3 vs 18.4 mg/dL; P < .001) and metabolic syndrome (31.9 vs 22.1 mg/dL; P < .001). No significant differences were observed in GV values as a function of age, sex, history of arterial hypertension, history of dyslipidaemia, or stroke subtype.
Demographic and stroke-related data.
| GLIAS II study cohort (N = 2013) | |
|---|---|
| Age in years, mean (SD) | 71.24 (10.83) |
| Men, n (%) | 128 (60.1) |
| Arterial hypertension, n (%) | 151 (70.9) |
| Dyslipidaemia, n (%) | 97 (45.5) |
| Metabolic syndrome, n (%) | 18 (34) |
| Charlson Comorbidity Index, median (Q1-Q3) | 2 (1-3) |
| DM, n (%) | 64 (30) |
| Baseline NIHSS, median (Q1-Q3) | 5 (3-11) |
| IVT, n (%) | 82 (38) |
| Stroke subtype, n (%) | |
| Large-vessel disease with stenosis > 50% | 24 (11.3) |
| Large-vessel disease with stenosis < 50% | 16 (7.5) |
| Cardioembolic | 74 (34.7) |
| Small-vessel disease | 54 (25.4) |
| Other determined cause | 6 (2.8) |
| Cryptogenic | 39 (18.3) |
| Systolic blood pressure at admission, mean (SD) | 156.94 (27.99) |
| Diastolic blood pressure at admission, mean (SD) | 83.75 (15.67) |
| Body temperature at admission, mean (SD) | 36.09 (0.53) |
| Oxygen saturation at admission, mean (SD) | 96.49 (2.21) |
| Capillary blood glucose at admission (mg/dL), mean (SD) | 134 (54.07) |
| HbA1c (%; mmol/mol) at admission; mean (SD) | 6.16; 43 (1.23) |
| Episodes of post-stroke hyperglycaemia > 155 mg/dL within 48 hours of stroke, n (%) | 97 (45.5) |
| Glycaemic variability (mg/dL), mean (SD) | 23.83 (15.11) |
DM: diabetes mellitus; IVT: intravenous thrombolysis; NIHSS: National Institutes of Health Stroke Scale; SD: standard deviation.
A total of 69 patients received insulin treatment during the first 48 hours: 6 were treated exclusively with intravenous insulin and 63 with subcutaneous insulin, following different treatment strategies (sliding-scale insulin alone [30 patients], basal insulin alone [2], sliding-scale insulin plus intravenous insulin [8], sliding-scale insulin plus basal insulin [14], intravenous insulin plus basal insulin [1], sliding-scale insulin plus basal insulin plus intravenous insulin [8]).
Patients treated exclusively with intravenous insulin showed lower GV values than those receiving subcutaneous insulin; values in the latter group were nearly 20 mg/dL higher (P < .001). Furthermore, GV in the group receiving intravenous insulin was similar to that observed in patients not receiving treatment for hyperglycaemia. These results are summarised in Table 2 and Fig. 1.
A total of 203 patients (95.3%) were followed up for 3 months. Data on mortality, death or dependence, and complications of hospitalisation are summarised in Table 3. A total of 16 patients (7.8%) died: 6 (37.5%) due to complications directly linked to stroke (cerebral oedema or malignant infarction), 4 (25%) due to non-vascular causes, 3 (18%) due to vascular causes unrelated to stroke, and 3 (18%) due to unknown causes. No differences were found in the frequency of deaths between stroke subtypes (P = .7).
Analysis of primary and secondary variables.
| Primary variables (n = 203) | Mean glycaemic variability (SD), mg/dL | 95% CI | P |
|---|---|---|---|
| 3-month mortality | |||
| Yes (n = 16) | 30.9 (16.1) | ||
| No (n = 187) | 23.3 (15.9) | –15.4 to 0.16 | .05 |
| mRS 3-6 at 3 months | |||
| Yes (n = 57) | 23.2 (14.9) | ||
| No (n = 146) | 25.5 (16.1) | –2.4 to 6.9 | .34 |
| Stroke recurrence at 3 months | |||
| Yes (n = 8) | 24.0 (8.1) | ||
| No (n = 195) | 23.9 (15.5) | –11.0 to 10.7 | .97 |
| Secondary variables: complications during hospitalisation (n = 213) | |||
| Urinary tract infection | |||
| Yes (n = 5) | 19.2 (9.8) | ||
| No (n = 208) | 23.9 (15.2) | –8.7 to 18.2 | .49 |
| Pneumonia | |||
| Yes (n = 8) | 24.2 (16.6) | ||
| No (n = 205) | 23.8 (15.0) | –11.1 to 10.3 | .94 |
| Cerebral oedema | |||
| Yes (n = 10) | 25.0 (18.1) | ||
| No (n = 203) | 23.7 (14.9) | –10.9 to 8.3 | .78 |
| Symptomatic haemorrhagic transformation | |||
| Yes (n = 2) | 19.7 (20.4) | ||
| No (n = 211) | 23.8 (15.1) | –17.0 to 25.3 | .7 |
| Early neurological deterioration | |||
| Yes (n = 21) | 26.7 (15.0) | ||
| No (n = 192) | 23.5 (15.1) | –10.1 to 3.5 | .35 |
CI: confidence interval; mRS: modified Rankin Scale; SD: standard deviation.
In the unadjusted analysis, we observed a trend toward higher GV in patients who died at 3 months (30.9 vs 23.3 mg/dL; P = .05); no significant differences were found for death, dependence, or complications during hospitalisation.
In the age- and comorbidity-adjusted logistic regression analysis, both stroke severity (OR = 1.12; 95% CI, 1.04-1.2; P = .004) and GV (OR = 1.03; 95% CI, 1.003-1.06; P = .03) were independently associated with mortality.
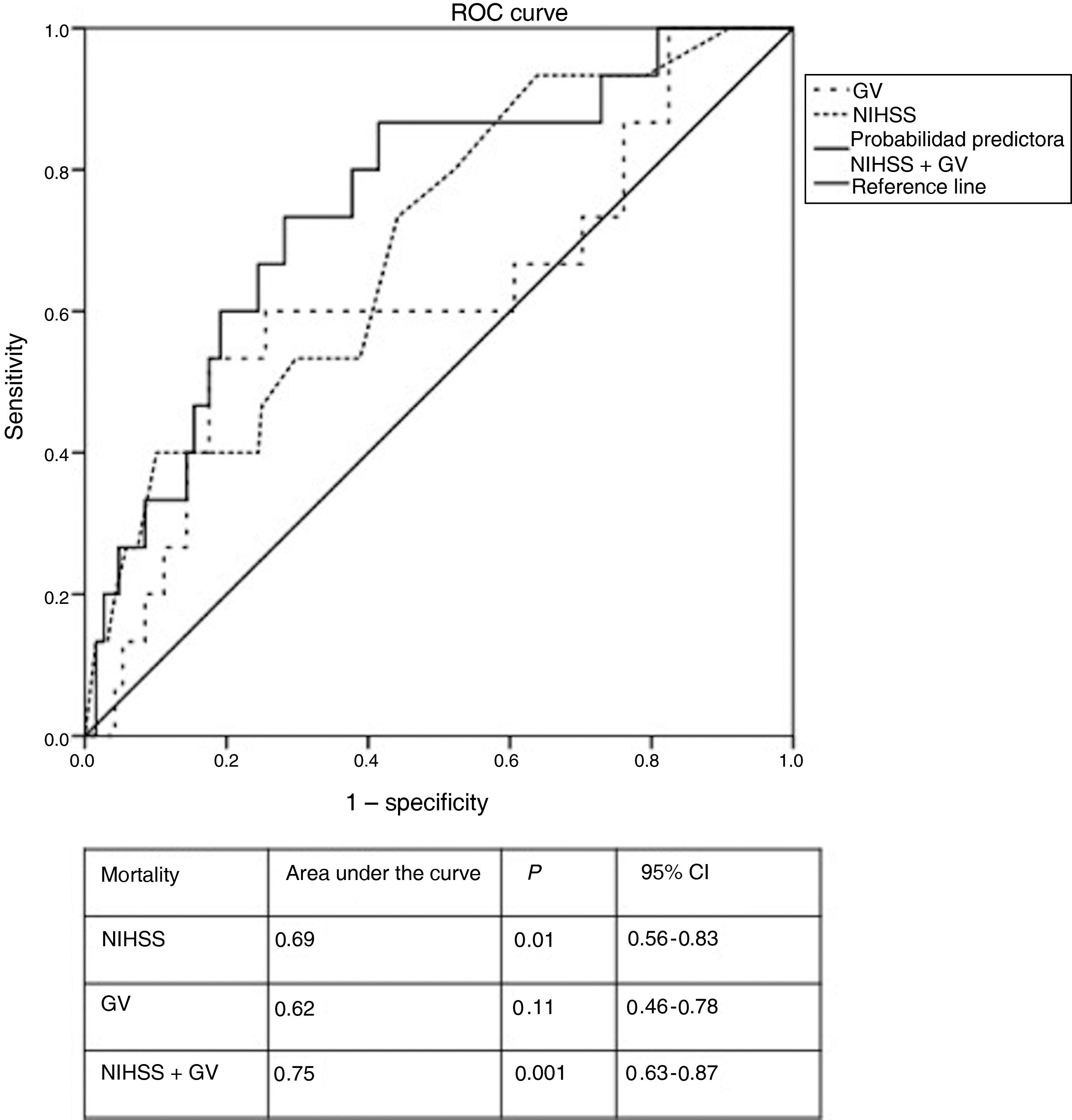
In the ROC curve for GV, the optimal cut-off value for predicting mortality was 32.5 mg/dL, nearly 10 mg/dL higher than the mean value for the cohort. Furthermore, patients treated with subcutaneous insulin displayed a GV above that value. Fig. 2 shows the ROC curve for the logistic regression model. The combination of stroke severity and GV showed 75% probability of predicting mortality, whereas stroke severity and GV separately showed probability rates of 69% and 62%, respectively. Elevated GV values increased the probability of 3-month mortality by 6%.
DiscussionIn the GLIAS II study cohort, patients who died presented higher GV in the 48hours after stroke than survivors; after adjusting for age and comorbidities, we found both GV and stroke severity to be independently associated with mortality.
Few studies have analysed the impact of GV on stroke prognosis, with diverging results. A recent study aiming to identify the blood glucose threshold that best predicts poor stroke prognosis after treatment with IVT found no association between GV and poor outcomes.7 However, Hui et al.6 showed that patients with elevated GV more frequently presented early neurological deterioration. Other studies including patients with DM type 2 have reported an association between elevated GV and higher cardiovascular mortality rates in patients with stroke.5 In this exploratory analysis of the GLIAS II study cohort, we included patients with and without history of DM and patients treated with IVT. After adjusting for age and comorbidities, we observed that GV and baseline stroke severity acted as independent predictors of mortality, with a cut-off value of 32.5 mg/dL in the ROC curve analysis; these findings are similar to those reported by previous studies evaluating the influence of GV on other diseases.3,21 Stroke subtype may also have an influence on mortality.22,23 However, no significant differences between stroke subtypes were observed in GV in the acute phase of stroke or in mortality rates, which suggests that the effect of GV is independent of stroke aetiology. Future studies specifically focusing on this issue will surely provide definitive conclusions on the prognostic value of GV in different stroke subtypes.
As there currently is no gold standard for quantifying GV for research or clinical purposes, each research group uses a different method. Some of the most widely used parameters are the standard deviation of the mean, range, and mean amplitude of glycaemic excursion.24 In the GLIAS II study, as in other studies including patients with stroke,6 GV was defined as the standard deviation of the mean glucose level of each patient. A total of 8 to 10 measurements are considered necessary for the standard deviation of the mean glucose level to correctly reflect GV.19 In the GLIAS II study, blood glucose level was measured every 4hours, up to a total of 8 measurements per patient. However, most studies using the standard deviation of the mean glucose level report lower numbers of measurements, ranging from 2 to 6 per patient,3,7 whereas one study measured glucose levels more than 10 times.6 These differences in the parameter used to calculate GV may explain the differences observed between studies. At present, the use of CGMs provides an opportunity to better understand the prognostic value of GV in stroke, as well as the factors influencing blood sugar fluctuations in the first days after stroke. To our knowledge, few studies have used CGMs in patients with stroke,8,25-27 and only one has found an association between GV and infarct volume growth.26
This exploratory analysis also revealed that the administration route of insulin used to correct post-stroke hyperglycaemia has an impact on GV. Intravenous administration was associated with lower GV, whereas treatment with subcutaneous insulin was associated with GV values above 32.5 mg/dL, the cut-off value identified in our study for higher mortality risk. During acute cerebral ischaemia, the brain's metabolic demand increases, leading to a greater need for energy substrates. In the context of hypoxia, this increased need for glucose leads to an increase in anaerobic metabolism, resulting in higher levels of lactate and pyruvate. Adequate glucose supply to the brain is only possible when glucose homeostasis is maintained. On the one hand, hyperglycaemia has been shown to increase oxidative stress, promote inflammatory cytokine release, and increase intracellular accumulation of lactic acid, causing mitochondrial dysfunction, which ultimately leads to energy failure. On the other, hypoglycaemia may lead to neuroglycopenia and cerebral metabolic crises due to the brain's limited capacity to compensate for hypoglycaemia: lack of glucose in hypoxic brain tissue may induce mitochondrial dysfunction due to the generation of oxygen free radicals, alterations to transmembrane ion gradients, and activation of apoptosis signalling pathways.28 Therefore, glucose level fluctuations, with episodes of hyperglycaemia and episodes of hypoglycaemia secondary to pharmacological treatment of hyperglycaemia, may worsen prognosis; these fluctuations underlie the concept of GV.
The American Diabetes Association recommends treating hyperglycaemia with intravenous insulin in hospitalised patients; the target blood glucose level for critically ill patients ranges from 140 to 180 mg/dL (7.7-10 mmol/L).20 However, these recommendations are based on weak evidence, given that none of the clinical trials conducted to date has been able to demonstrate the superiority of rapid correction of hyperglycaemia over standard treatment in patients with stroke.15 The most recent clinical trial on this topic also failed to demonstrate that intensive treatment for hyperglycaemia is superior to standard treatment in patients with stroke.29 However, there is little consensus regarding the standard treatment for post-stroke hyperglycaemia among the clinical trials published to date, and none of them accounted for glucose level fluctuations (ie, GV). Considering the tight glycaemic control needed to maintain normal metabolism in hypoxic cerebral tissue, the deleterious effects of hyper- and hypoglycaemia in stroke patients may explain the negative results of trials of treatments for aggressive correction of hyperglycaemia.30 Unfortunately, none of these trials has analysed GV.
The main findings of this exploratory study are as follows: 1) GV is independently associated with increased risk of mortality in patients with acute stroke; 2) the combination of GV and stroke severity presents better predictive capacity for poor prognosis than stroke severity alone; 3) subcutaneous insulin administration is associated with higher GV values than intravenous insulin. If the latter finding is confirmed in prospective studies, it may have an impact on treatment protocols for post-stroke hyperglycaemia, which may include GV as a marker of glucose level fluctuations, as has previously been suggested by other authors.28,30,31
Our study presents certain limitations. Firstly, it includes a small number of patients with poor prognosis, which limits our ability to interpret the data, especially regarding the route of insulin administration; further prospective studies should specifically analyse this question, as is the case with the GLIAS III study (N C T 04001049), which started recruiting patients in the first semester of 2020. Secondly, the standard deviation of the mean blood glucose value, though frequently used in other studies, may not be the best measure of GV; such other parameters as mean amplitude of glycaemic excursion and coefficient of variation should also be explored.19 Lastly, the cut-off value for GV identified in the ROC curves as a predictor of mortality did not reach statistical significance when analysed in isolation, although it did when combined with NIHSS score. This exploratory analysis of the GLIAS II study may be considered a hypothesis generator; future prospective studies using CGMs in patients with acute stroke should seek to validate the threshold GV value associated with greater risk of mortality. The main strengths of the GLIAS II study are its multicentre prospective design and the fact that it was specifically designed to study the influence of glucose parameters on stroke prognosis.
In conclusion, high GV within 48hours of stroke significantly increases the risk of mortality, after adjusting for age, comorbidity, and severity. The use of subcutaneous insulin seems to be associated with significantly higher GV values than intravenous insulin. Further studies are needed to better characterise the influence of GV on stroke prognosis, as this may have implications for the treatment of post-stroke hyperglycaemia, with a view to preventing extreme blood glucose levels and to assist in selecting the most appropriate treatment.
FundingThis study was funded by Instituto de Salud Carlos III and the European Regional Development Fund (PI 09/01781). It was promoted by Project Stroke of the Spanish Society of Neurology's Stroke Study Group and the cerebrovascular disease research networks RETICS, INVICTUS, and INVICTUS Plus (RD12/0014/0006, RD16/0019/0005).
Conflicts of interestThe authors have no conflicts of interest to declare.
Researchers collaborating in the GLIAS II study: Borja E. Sanz-Cuesta, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Patricia Martínez-Sánchez, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. María Gutiérrez-Fernández, Neurology and Cerebrovascular Disease Research Laboratory, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Berta Rodríguez-Frutos, Neurology and Cerebrovascular Disease Research Laboratory, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Jaime Ramos-Cejudo, Neurology and Cerebrovascular Disease Research Laboratory, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Laura Otero-Ortega, Neurology and Cerebrovascular Disease Research Laboratory, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Daniel Prefasi, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. María Ángeles Mangas-Guijarro, Neurology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Luis Felipe Pallardo, Endocrinology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Remedios Frutos, Radiology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Andres Fernández-Prieto, Radiology Department. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Rosario Madero, Biostatistics Service. Hospital Universitario La Paz, IdiPAZ Biomedical Research Institute. Madrid, Spain. Juan Manuel García-Sánchez, Neurology Department. Hospital de Basurto. Bilbao, Spain. Nuria Aymerich, Neurology Department. Hospital General de Navarra. Pamplona, Spain. Miguel Blanco†, Neurology Department. Hospital Clínico Universitario. Santiago de Compostela, A Coruña, Spain. Noemí Díez-González, Neurology Department. Hospital de Donostia. Gipuzkoa, Spain. Ana de Arce, Neurology Department. Hospital de Donostia. Gipuzkoa, Spain. Félix González, Neurology Department. Hospital de Donostia. Gipuzkoa, Spain. Joan Martí-Fábregas, Neurology Department. Hospital de la Santa Creu i Sant Pau. Barcelona, Spain. Mireya Fernández-Fournier, Neurology Department. Hospital Universitario Ramón y Cajal. Madrid, Spain. Fernando Díaz-Otero, Neurology Department. Hospital Universitario Gregorio Marañón. Madrid, Spain. Juan Álvarez-Linera, Neuroradiology Unit. Hospital Ruber Internacional. Madrid, Spain.