Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays an important role in the modulation of plasma levels of low density lipoprotein cholesterol (LDLC). PCSK9 binds to the LDL receptor (LDLR), disrupts its endocytic recycling itinerary and directs it to lysosomal degradation. Activation of PCSK9 can thus decrease the expression of LDLR in the liver and inhibit LDL uptake, which leads to hypercholesterolaemia.
DevelopmentCurrently we now know that different polymorphisms of PCSK9 are associated with the occurrence of ischaemic stroke. On the other hand, PCSK9 inhibitors prevent binding of PCSK9 to LDLR and inhibit degradation of LDLR, which results in increased hepatic uptake of LDL and lower LDL levels in blood.
Different phase 2 and 3 studies, including OSLER and ODYSSEY LONG-TERM, have demonstrated the efficacy and safety of the new monoclonal antibodies against PCSK9 such as evolucumab and alirocumab, and the first exploratory analyses have shown evidence of their efficacy in decreasing vascular events, including stroke.
ConclusionsAlthough few strokes have been reported by these studies, new ongoing trials examining the cardiovascular effects of evolucumab (FOURIER study), alirocumab (ODYSSEY OUTCOMES study), and bococizumab (SPIRE-1 and SPIRE-2 studies) will reveal the true potential of these drugs, particularly for the prevention of stroke.
La proproteína convertasa subtilisina kexina tipo 9 (PCSK9) desempeña un papel importante en la modulación de los niveles plasmáticos de colesterol unido a las lipoproteínas de baja densidad (LDLC). La PCSK9 se une al receptor del LDLC (LDL[R]) perturba su reciclado endocítico y lo dirige a la degradación lisosomal. Por tanto, la activación de PCSK9 disminuye la expresión de los LDL(R) a nivel hepático e inhibe la captación de LDLC, lo que provoca hipercolesterolemia.
DesarrolloHoy se sabe que diferentes polimorfismos de la PCSK9 se asocian a la aparición de ictus isquémico. Por otra parte, los fármacos inhibidores de PCSK9 inhiben la unión de PCSK9 con el LDL(R) y evitan la degradación del LDL(R) por lo que aumentan la captación hepática de LDLC, disminuyendo sus niveles en sangre.
Diferentes estudios con anticuerpos monoclonales en fase 2 y 3 como el OSLER y el ODYSSEY LONG TERM han demostrado la eficacia y seguridad de los nuevos anticuerpos monoclonales contra PCSK9 como el evolucumab y alirocumab, y los primeros análisis exploratorios ya evidencian su eficacia en la disminución de eventos vasculares, incluidos los ictus.
ConclusionesAunque el número de ictus recogidos en estos estudios ha sido bajo, en la actualidad existen varios ensayos centrados en resultados cardiovasculares en curso con evolocumab (estudio FOURIER), con alirocumab (estudio ODYSSEY OUTCOMES) y con bococizumab (estudios SPIRE-1 y SPIRE-2) que nos desvelarán el auténtico potencial de estos fármacos en la enfermedad cardiovascular y, en particular, en la prevención del ictus.
First approved by the US Food and Drug Administration (FDA) in 2015 and subsequently by the European Medicines Agency, the new proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors alirocumab and evolocumab reduce levels of low-density lipoprotein (LDL) cholesterol by 60%-70%, offering a new approach in the treatment of dyslipidaemia and cardiovascular disease, and particularly stroke, the main cause of vascular mortality globally.1 However, reaching this point has been a long journey and we have had to debunk many widespread myths.
For years, most epidemiological and observational studies and the first meta-analyses2 found no clear association between high cholesterol levels and the risk of stroke. This may be explained by the heterogeneity of patients with stroke, as well as methodological problems.3
Furthermore, statins were believed for years to be the only lipid-lowering therapy able to reduce stroke risk; low-fat diet or other drugs were thought to be ineffective.4 This belief followed from the idea that the decreases observed in stroke incidence in statin trials were not due to decreases in LDL cholesterol levels but to pleiotropic effects of these drugs.3
However, the recently published IMPROVE-IT trial5 showed that combined therapy with simvastatin and ezetimibe, a non-statin drug that reduces intestinal cholesterol absorption, reduced LDL cholesterol levels, leading to a significant decrease in rates of cardiovascular events, including ischaemic stroke (hazard ratio [HR]=0.79; P=.008).
A recent meta-analysis including the results of the IMPROVE-IT trial and analysing data from over 266000 patients observed that the 1% decrease in total cholesterol, regardless of the treatment used, was associated with a 0.8% decrease in the relative risk of stroke.6
In fact, the first phase II and phase III clinical trials of PCSK9 inhibitors have shown that these lipid-lowering agents also reduce the incidence of cardiovascular events, including stroke, and may therefore play a key role in stroke prevention in the short to medium term.
Action mechanism of PCSK9 inhibitorsWhen PCSK9 was first described, in 2003, it was regarded as an important protein for liver regeneration and neuronal differentiation, and was therefore named neural apoptosis-regulated convertase 1.7 We now know that PCSK9 belongs to a family of secretory serine proteases and plays a major role in the proteolytic maturation of such secretory proteins as neuropeptides, growth factors, cytokines, and prohormones.8 Members of the PCSK family cleave amino acid residues and modulate the activity of precursor proteins. Furthermore, some of these proteins (PCSK1, PCSK3, PCSK5, PCSK6, and PCSK9) regulate the activity of important modulators of lipid metabolism.9
PCSK9 plays a crucial role in modulating plasma LDL cholesterol levels via a post-transcriptional mechanism by inhibiting the LDL receptor (LDL[R]). PCSK9 binds to the repeat domain of the epidermal growth factor-A of the LDL(R) on the surface of hepatocytes. The PCSK9-LDL(R) complex is transported to the lysosomal system of hepatocytes for LDL(R) destruction, preventing the receptor from returning to the cell surface and consequently decreasing the population of hepatic LDL(R).
A lower number of LDL(R) on the surface of hepatocytes results in increased levels of LDL cholesterol in the blood, causing hypercholesterolaemia.8,9
PCSK9 is expressed in several organs, particularly the liver and also in the intestine and the kidney. The PCSK9 gene, located on chromosome 1p32.3, contains 12 exons and codes for an inactive glycoprotein of 692 amino acids.8
PCSK9 inhibitors impede the binding of PCSK9 to LDL(R), preventing LDL(R) degradation; this increases LDL cholesterol uptake by the liver and decreases serum LDL cholesterol levels.3,8
Therefore, PCSK9 inhibitors and statins ultimately have the same action mechanism as they increase LDL(R) activity on the surface of hepatocytes. Statins, however, increase numbers of LDL(R) on the surface of hepatocytes by inhibiting the enzyme 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase; PSCK9 levels also increase to regulate the numbers of LDL(R), limiting the efficacy of statins in reducing LDL cholesterol levels and increasing the efficacy of PCSK9 inhibitors combined with statins.3
Role of PCSK9 inhibitors. Association between PCSK9 and ischaemic strokeSeidah et al.7 identified the ninth member of the PCSK family, PCSK9, in 2003. The same year, PCSK9 was found to play a crucial role in cholesterol metabolism after 2 gain-of-function mutations were identified in PCSK9 in 2 French families diagnosed with heterozygous familial hypercholesterolaemia, with no detectable mutations in the genes coding for LDL(R) and apolipoprotein B.10 In 2005, PCSK9 inhibition was also found to reduce LDL cholesterol levels when the first loss-of-function mutation was detected in PCSK9, associated with decreased LDL cholesterol levels.11 More importantly, the Atherosclerosis Risk in Communities (ARIC) study demonstrated that decreased levels of LDL cholesterol were associated with a 28%-47% decrease in the risk of coronary heart disease,12 that is, they showed a strong correlation with cardiovascular disease. This suggested that suppression of PCSK9 activity may be a promising therapeutic target for hypercholesterolaemia.
In the summer of 2015, 12 years after the protein was discovered and after numerous research projects, the FDA approved the first PCSK9 inhibitors.
Over the past 10 years, numerous studies have shown a clear correlation between various PCSK9 polymorphisms and the risk of ischaemic stroke. Abboud et al.13 were the first to suggest the involvement of the E670G polymorphism (rs505151) of PCSK9 in cerebrovascular disease. This study included 237 middle-aged patients (aged 45-60 years) with small-vessel occlusion and large-vessel atherosclerosis and 326 matched controls with no history of stroke. In the multivariate analysis, presence of the minor allele (G) was found to be a predictor of large-vessel atherosclerosis (OR=3.52, 95% CI, 1.25-9.85; P=.017). Furthermore, in a series of 604 consecutive autopsies from Finnish men and women (mean age 62.5 years), G allele carriers usually had more severe atherosclerosis in the circle of Willis and its branches; the authors concluded that PCSK9 was associated with increased risk of atherothrombotic ischaemic stroke secondary to large-vessel atherosclerosis and that risk was determined by the severity of intracranial atherosclerosis.
These results have been confirmed in other populations, such as a Han Chinese population, where the PCSK9 polymorphisms rs1711503 and rs2479408 were correlated with ischaemic stroke.14 In a Tunisian cohort, the G670 allele of PCSK9 also seemed to be an independent risk factor for ischaemic stroke.15
A recent meta-analysis16 of the rs505151 polymorphism of PCSK9, including 3 studies (759 patients and 906 controls), concluded that the rs505151 A>G variant of PCSK9 (amino acid substitution from glutamate to glycine at position 670 [p.E670G] in exon 12, resulting in altered PCSK9 activity) may contribute to susceptibility to ischaemic stroke, increasing the risk by 36% (OR=1.36, 95% CI, 1.01-1.58).
PCSK9 gene polymorphisms may therefore constitute not only risk factors for ischaemic stroke but also predictive markers of the risk of ischaemic stroke.
Clinical trialsAt present, 2 PCSK9 inhibitors, alirocumab and evolocumab (Table 1), are commercially available, with around 10 monoclonal antibodies in different development stages; numerous phase II and phase III studies are underway, in addition to several ongoing post-marketing studies to be published in 2017 and 2018.
Comparison of the PCSK9 inhibitors currently available.
| Alirocumab (Praluent©) | Evolocumab (Repatha©) | |
|---|---|---|
| Type of antibody | Human IgG1 | Human IgG2 |
| Bioavailability | 85% | 72% |
| Half-life | 17-20 days | 11-17 days |
| Metabolism | Degradation via small peptides and amino acids. Does not affect cytochrome P450 | Degradation via small peptides and amino acids. Does not affect cytochrome P450 |
| Elimination | Low concentration: saturable binding to PCSK9 High concentration: non-saturable proteolysis | Low concentration: saturable binding to PCSK9. High concentration: non-saturable proteolysis |
| Drug-drug interactions | None | None |
| Dosage | 75mg every 2 weeks 150mg every 2 weeks | 140mg every 2 weeks or 420mg once a month |
| Route of administration | Subcutaneous Injection with pre-filled pen or syringe | Subcutaneous Injection with Pushtronex system |
| Follow-up | Lipid profile 4-8 weeks after treatment onset | Lipid profile 4-8 weeks after treatment onset |
To date, the longest phase II and phase III studies of these antibodies are the OSLER studies (Open Label Study of Long Term Evaluation Against LDL-C Trial)17 for evolocumab (marketed in Spain as Repatha© [Angen]), which include the phase II OSLER-1 study and the phase III OSLER-2 study; and the ODYSSEY Long Term study (Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy)18 for alirocumab, marketed as Praluent® (Sanofi).
The OSLER-1 and OSLER-2 studies17 included 4465 patients who had previously participated in evolocumab “parent trials,” randomly allocated to receive either standard treatment alone (n=1489) or a combination of standard treatment plus subcutaneous evolocumab (n=2976) once every 2 weeks at a dose of 140mg or once a month at a dose of 420mg. The primary endpoint was the occurrence of treatment-related adverse events. Secondary endpoints were changes in LDL cholesterol levels and cardiovascular outcomes, which were regarded as pre-established and exploratory. The results of both OSLER studies were analysed as a whole.
The researchers observed a 61% decrease in LDL cholesterol levels (from 120mg/dL to 48mg/dL) in the patients receiving evolocumab. Decreases persisted throughout the follow-up period (mean duration of 11 months). The researchers also observed a decrease in the rate of cardiovascular events among the patients receiving evolocumab. At one year of treatment, the rate of cardiovascular events was over 50% lower in the group receiving evolocumab than in the group receiving the standard treatment only (0.95% vs. 2.18%; HR=0.47; 95% CI, 0.28-0.78; P=.003).
Although some studies have shown significantly lower levels of LDL cholesterol after 8-12 weeks of treatment with alirocumab plus statins, the researchers of the ODYSSEY Long Term trial18 decided to evaluate the effects of the treatment over a longer period. The trial included 2341 patients (63.2% men; mean age 60 years) with high cardiovascular risk, LDL cholesterol levels ≥70mg/dL, and receiving the maximum tolerable dose of statins. Patients were randomly allocated to receive either a 150-mg subcutaneous injection of alirocumab every 2 weeks (n=1553) or placebo (n=788). The primary endpoint was the change observed in LDL cholesterol levels from baseline to week 24. Over the study period, LDL cholesterol levels were observed to decrease by 61% in the alirocumab group, compared to 0.8% in the placebo group (P<.001). At 24 weeks, mean absolute levels of LDL cholesterol were 48mg/dL in the alirocumab group and 119mg/dL in the placebo group. Decreases persisted throughout the 78-week follow-up period. As in the evolocumab study, patients receiving alirocumab presented fewer cardiovascular events than those in the placebo group (1.7% vs 3.3%; HR=0.52; P=.02). In addition to muscle pain and local skin reactions at the injection site (3% and 4%, respectively), both PCSK9 inhibitors caused neurocognitive alterations (evolocumab: 0.9% vs 0.3% for placebo; alirocumab: 1.2% vs. 0.5% for placebo). These included amnesia and memory impairment, and they did not vary significantly according to the level of LDL cholesterol.17,18 The effects of PCSK9 inhibitors on cognitive function are currently being evaluated by the EBBINGHAUS trial (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects),19 a substudy of the FOURIER evolocumab trial using the analysis of the ODYSSEY OUTCOMES alirocumab trial.20
However, despite the OSLER and ODYSSEY Long Term trials finding that PCSK9 inhibitors also decreased the risk of cerebrovascular events, the number of stroke patients was small in both studies: in the OSLER studies, there were 3 strokes and one transient ischaemic attack (TIA) in the active treatment group vs. 2 strokes and 5 TIAs in the standard treatment group, and in the ODYSSEY study, there were 2 ischaemic strokes in the alirocumab group vs 9 in the control group.17,18
A recent meta-analysis21 of the effect of PCSK9 inhibitors on stroke, which included the 6806 patients from the OSLER and ODYSSEY studies, found no clear reduction in the risk of stroke (risk ratio [RR]=1.43; 95% CI, 0.45-4.57; P=.55) or any significant differences in the risk of cerebrovascular events including TIAs (RR=0.65; 95% CI, 0.25-1.68; P=.37). This lack of association is probably explained by the small number of strokes, the very short follow-up period (mean of 1.5 years), and the fact that most patients (over 75%) were already taking statins.
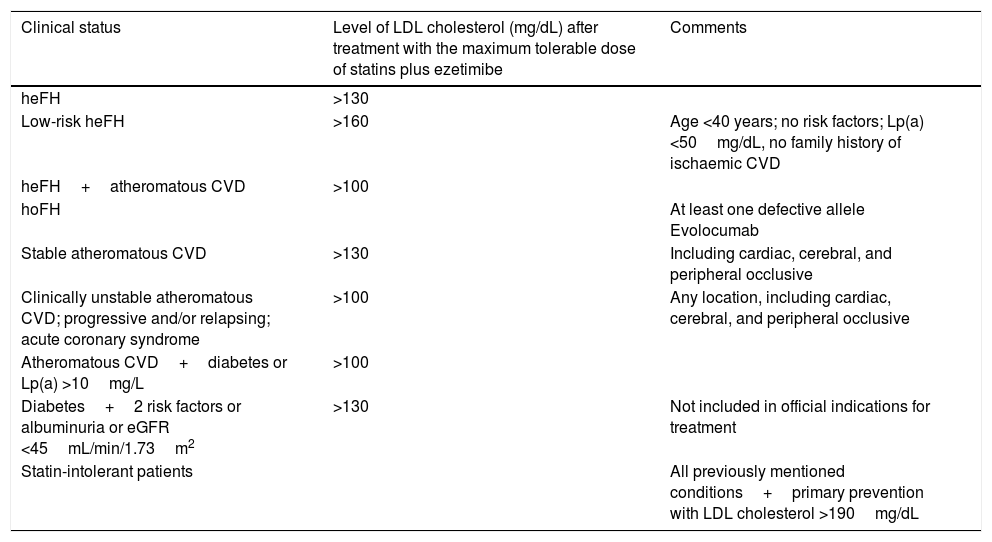
Conclusions. Present and future role of PCSK9 inhibitors in strokeVarious sets of guidelines and recommendations are currently available for the use of PCSK9 inhibitors, such as the guidelines of the Spanish Society of Arteriosclerosis (Table 2).22 These drugs seem to play a central role in the treatment of 3 main patient groups: individuals with familial hypercholesterolaemia, patients with history or a high risk of cardiovascular events that cannot be successfully managed with high-dose statins, and patients intolerant to statins.
Indications for PCSK9 inhibitors according to the consensus document published by the Spanish Society of Arteriosclerosis.
| Clinical status | Level of LDL cholesterol (mg/dL) after treatment with the maximum tolerable dose of statins plus ezetimibe | Comments |
|---|---|---|
| heFH | >130 | |
| Low-risk heFH | >160 | Age <40 years; no risk factors; Lp(a) <50mg/dL, no family history of ischaemic CVD |
| heFH+atheromatous CVD | >100 | |
| hoFH | At least one defective allele Evolocumab | |
| Stable atheromatous CVD | >130 | Including cardiac, cerebral, and peripheral occlusive |
| Clinically unstable atheromatous CVD; progressive and/or relapsing; acute coronary syndrome | >100 | Any location, including cardiac, cerebral, and peripheral occlusive |
| Atheromatous CVD+diabetes or Lp(a) >10mg/L | >100 | |
| Diabetes+2 risk factors or albuminuria or eGFR <45mL/min/1.73m2 | >130 | Not included in official indications for treatment |
| Statin-intolerant patients | All previously mentioned conditions+primary prevention with LDL cholesterol >190mg/dL |
CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; heFH: heterozygous familial hypercholesterolaemia; hoFH: homozygous familial hypercholesterolaemia; Lp(a): lipoprotein(a).
Taken from Masana et al.22
The cost-effectiveness of PCSK9 inhibitors is much debated: assuming an annual cost of $10000, some authors state that the annual price of these drugs should be reduced by 50%-60% in order for them to be cost-effective.23 This is a major limitation of PCSK9 inhibitors; treatment onset and continuation must therefore be adjusted to strict criteria.
The real value of PCSK9 inhibitors should be measured in terms of their clinical effects on cardiovascular events. The prospective exploratory analysis of studies of PCSK9 inhibitors has shown a decrease in major cardiovascular events, including stroke. Although few strokes have been recorded, several trials are currently underway into cardiovascular outcomes, including trials of evolocumab (FOURIER24; NCT01764633), alirocumab (ODYSSEY OUTCOMES20; NCT01663402), and bococizumab25 (SPIRE-1; NCT01975376 and SPIRE-2; NCT01975389). The results of these studies will shed light on these drugs’ potential for cardiovascular diseases in general, and for stroke prevention in particular.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Castilla-Guerra L, Fernández-Moreno MC, Rico-Corral MA. Colesterol e ictus: papel de los inhibidores de la proproteína convertasa subtilisina/kexina tipo 9. Neurología. 2019;34:198–203.